Abstract
Rationale & Objectives
Hyperglycemia is frequently observed early after transplantation and associated with development of post-transplant diabetes mellitus (PTDM). Here, we assessed continuous subcutaneous insulin infusion (CSII) targeting afternoon hyperglycemia.
Study Design
Open-label randomized parallel 3-arm design.
Settings & Participants
In total, 85 kidney transplant recipients without previous diabetes diagnosis were randomized to postoperative CSII therapy, basal insulin, or control.
Interventions
Insulin was to be initiated at afternoon capillary blood glucose level of ≥140 mg/dL (7.8 mmol/L; CSII and basal insulin) or fasting plasma glucose level of ≥200 mg/dL (11.1 mmol/L; control).
Outcomes
Hemoglobin A1c (HbA1c) levels at 3 months post-transplant (primary endpoint). PTDM assessed using oral glucose tolerance test at 12 and 24 months.
Results
CSII therapy lasted until median day 18 and maximum day 88. The median HbA1c value at month 3 was 5.6% (38 mmol/mol) in the CSII group versus 5.7% (39 mmol/mol) in the control group (P = 0.70) and 5.4% (36 mmol/mol) in the basal insulin group (P = 0.02). At months 12 and 24, the odds for PTDM were similar compared with the control group (odds ratios [95% confidence intervals], 0.80 [0.18-3.49] and 0.71 [0.15-3.16], respectively) and the basal insulin group (0.96 [0.18-5.68] and 1.51 [0.24-12.84], respectively). Mild hypoglycemia events occurred in the CSII and the basal insulin groups.
Limitations
This study is limited by outdated insulin pump technology, frequent discontinuations of CSII, a complex protocol, and concerns regarding reliability of HbA1c measurements.
Conclusions
CSII therapy was not superior at reducing HbA1c levels at month 3 or PTDM prevalence at months 12 and 24 compared with the control or basal insulin group.
Index Words: American Diabetes Association, basal insulin, capillary blood glucose, continuous glucose monitoring, continuous subcutaneous insulin infusion, intention-to-treat, kidney transplant recipients, oral glucose tolerance test, per-protocol, post-transplant diabetes mellitus, two-hour plasma glucose
Transplantation is the optimal treatment for eligible individuals who require kidney replacement therapy, but post-transplant diabetes mellitus (PTDM) is a common consequence and incurs elevated risks for cardiovascular disease and mortality.1, 2, 3, 4, 5, 6, 7 Hyperglycemia may affect the large majority of patients early after kidney transplantation and is a strong predictor of subsequent PTDM development.5,8
In the early post-transplant period, steroid-induced hyperglycemia may require potent glucose-lowering agents, insulin thus being the recommended treatment.9 However, insulin carries a considerable risk of hypoglycemia.10 Continuous subcutaneous insulin infusion (CSII) is an advanced technology for insulin administration, has been shown to improve glycemic control and risk for hypoglycemia in the nontransplanted general population, and may therefore be beneficial in transplanted individuals.11 In sensor-augmented insulin pump therapy, interstitial glucose levels are measured continuously to additionally provide real-time feedback to the patients.12, 13, 14
Afternoon hyperglycemia following steroid administration in the morning inspired the use of an intermediate acting insulin dosing scheme post kidney transplantation. In 2 previous randomized controlled trials, conceptualized in 2008 and 2012, we showed that early initiation of basal insulin on elevated afternoon glucose levels (>140 mg/dL) could prevent sustained PTDM after kidney transplantation.15,16 The studies foresaw tight glucose control during the early post-transplant period that provides an opportunity for PTDM prevention.17,18 In the latter multicenter trial, a third study arm was opened at one study center, comprising a single-center randomized trial with 3 treatment arms. The CSII therapy algorithm has previously been published.19 Here, we assessed long-term glucose control in patients treated with CSII therapy versus standard-of-care and subcutaneous once-daily basal insulin therapy using HbA1c levels at month 3 after kidney transplantation and during the 2-year follow-up, hypothesizing that glucose control may be improved using early postoperative CSII therapy.
Methods
Study Design
This study was an investigator-initiated, open-label, randomized single-center clinical trial with an unblinded end point evaluation performed at the Medical University of Vienna between April 2013 and November 2017. Adult kidney transplant recipients without previous diabetes diagnosis and who were treated with triple immunosuppression consisting of once-daily tacrolimus, mycophenolic acid, and glucocorticosteroids were eligible. Participants were randomized 1:1:1 to the CSII group, standard-of-care group, and basal insulin group (BI group). The study was externally monitored and undertaken in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients after approval from the institutional review board (EK#10/2012). The study was registered with ClinicalTrials.gov (Identifier: NCT01680185). A more detailed description of the study methods can be found in the Supplemental Methods (Item S1) and the published study protocol.19
Study Intervention
Study interventions were introduced face-to-face by nephrologists familiar with the used systems. Participants in the CSII group received Medtronic MiniMed Paradigm Veo® 754 insulin pumps with a Paradigm Quick-set® (Medtronic, Inc). Patients were instructed to perform 4-point capillary blood glucose (CBG) measurements using glucometers and test strips (Contour Link, Bayer HealthCare Diabetes Care). Starting at day 1-2 post-transplant, basal insulin infusion was to be initiated when preprandial afternoon CBG levels reached 140 mg/dL (7.8 mmol/L), targeting 110 mg/dL. Average infusion rates over the day using CSII were previously published.19 Additionally, patients were to receive real-time continuous glucose monitoring (CGM) using Enlite sensors with MiniLink transmitters (Medtronic, Inc). However, after the inclusion of 4 patients, it was decided to discontinue the use of the glucose sensors because they were too complicated to handle for patients and staff (main difficulties involved the alarming system of the real-time transmitter).
In the control group, in addition to fasting plasma glucose measurements, 4-point CBG measurements were performed as part of the center’s routine. If fasting glucose measurements persistently exceeded 200 mg/dL (11.1 mmol/L) short-acting insulin was to be introduced, aiming at pre-lunch and afternoon capillary glucose levels of <200 mg/dL (11.1 mmol/L) (Supplemental Methods, Item S1). For maintenance therapy, sulfonylureas were recommended.
Participants in the BI group were trained to use intermediate acting insulin injections administered in the morning (human insulin isophane, Humulin N [Eli Lilly]) and to perform CBG measurements in analogy to the CSII group. Insulin was to be initiated when preprandial afternoon CBG levels reached 140 mg/dL (7.8 mmol/L), aiming at afternoon CBG levels of 110 mg/dL (6.1 mmol/L). BI titration and mealtime short-acting insulin followed a predefined tapering scheme (Supplemental Methods, Item S1).16
Outcome Measures
Primary Endpoint
The level of hemoglobin A1c (HbA1c) at 3 months post-transplantation in patients treated with CSII compared with the standard-of-care control group constituted the primary endpoint. The safety of the intervention was evaluated by the number of hypoglycemia episodes, confirmed by CBG levels below 60 mg/dL (3.3 mmol/L). For the main analysis set, a per-protocol (PP) analysis was intended; however, in light of the available data and the influence of protocol adherence in our previous trial, the intention-to-treat (ITT) analysis was presented as the primary analysis.16
Secondary Endpoints
Secondary endpoints included the following: (1) level of HbA1c at 3 months in the CSII group compared with the BI group; (2) course of HbA1c (specifically, increase of ≥0.5% at month 3 and stability after 3 months); (3) PTDM development at 6, 12, and 24 months; (4) CBG measurements; and (5) kidney function. PTDM was defined as use of glucose-lowering medication, oral glucose tolerance test (OGTT)-derived two-hour plasma glucose (2hPG) level of ≥200 mg/dL (11.1 mmol/L) and HbA1c level of ≥6.5% (48 mmol/mol).
Additional Details Regarding the Outcome Measures
Consistent with the ITP-NODAT study, a PP analysis included patients with completed follow-up.16 Patients misclassified or not properly treated were excluded (BI was not weaned in one patient and not initiated in a second patient). We also conducted sex-specific analyses and descriptive analyses of CSII treatment duration and discontinuations. For the analysis by sex, we used biological sex, as was documented in the patient records.
Post hoc Analysis in Participants with High Risk versus Low Risk for PTDM
Following literature review and our previous analysis, participants were considered at high risk for PTDM if any of the following criteria applied at baseline: (1) family history of diabetes; (2) polycystic kidney disease; (3) age ≥ 60 years; (4) age ≥ 45 years and triglyceride level ≥ 200 mg/dL; (5) age ≥ 45 years, triglyceride level ≥150 mg/dL, and body mass index ≥ 27 kg/m2; (6) age ≥ 45 years, triglyceride level ≥ 150 mg/dL and high-density lipoprotein level ≥ 40 mg/dL (men) or ≥50 mg/dL (women).16,20, 21, 22, 23
Statistical Analysis
The sample size calculation revealed a minimal group size of 25, and the sample size calculations of the ITP-NODAT study revealed that 26 participants would be needed to detect a 0.7% difference in the HbA1c level with a power of 80% (Supplemental Methods, Item S1).19 Categorical outcomes were presented as frequencies and proportions, whereas continuous variables were presented as means ± standard deviations (SD) or medians and interquartile ranges (IQR), depending on their distribution. P values were reported according to two-tailed analyses, and P values < 0.05 were considered statistically significant. For independent samples, the t test or the Mann–Whitney U test was used and for paired data, and the paired t test or Wilcoxon signed-rank test was used depending on the data distribution. Given the explorative nature of the secondary endpoints, P values were not adjusted for multiple testing. Calculations were performed using Microsoft Excel 2020 for macOS (Microsoft Corporation), IBM SPSS Statistics for macOS Version 27.0 (IBM), and R 4.1.2 (R Core Team).
Results
Flow and Characteristics of the Trial Participants
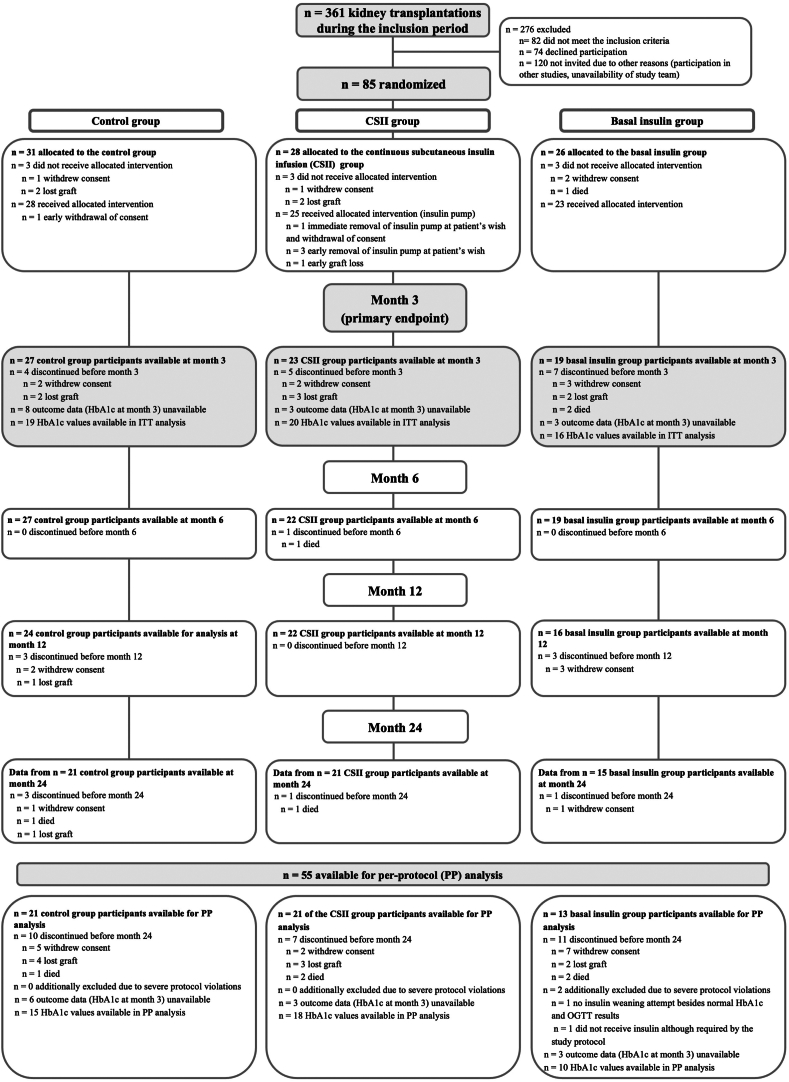
In total, 85 kidney transplant recipients were randomized into the control group (N = 31), CSII group (N = 28) and BI group (N = 26), respectively (Fig 1). In 3 patients, CSII was discontinued early, and few patients experienced pain or local reactions related to the pump as previously reported in detail by Werzowa et al.19 Hospitalization after transplant lasted a median (IQR) of 20 (15-37) days and was similar between groups (Fig S1). In total, 69 participants reached the primary endpoint at month 3, and 55 participants were included in the PP analysis. Baseline patient characteristics have partly been published previously (Table 1).19 CSII group participants had a tendency toward higher comorbid conditions or risk in several variables compared with the control group (male sex, current smoker, 3 or more antihypertensives, family history of diabetes, repeat transplant, polycystic kidney disease, or cardiovascular comorbid condition). Glomerular disease as primary condition was most frequent in the control group.
Figure 1.
Flow chart. Adapted consolidated standards of reporting trial (CONSORT) flow chart of participants available for intention-to-treat (ITT) analysis. Exclusions for the per-protocol (PP) analysis are listed at the bottom of the figure. Number of patients receiving glucose-lowering medication at the respective time point are listed in Figure 3. Abbreviations: CSII, continuous subcutaneous insulin infusion; OGTT, oral glucose tolerance test.
Table 1.
Participant Characteristics at Baseline
| Characteristic | Control, N = 31a |
N Missing |
CSII, N = 28a |
N Missing |
Basal insulin, N = 26a |
N Missing |
|---|---|---|---|---|---|---|
| Demographic and anthropometric characteristics | ||||||
| Female | 15 (48.4%) | 0 | 10 (35.7%) | 0 | 10 (40.0%) | 1 |
| Recipient age (y) | 53.3 ± 15.2 | 0 | 52.8 ± 11.6 | 0 | 55.8 ± 12.9 | 0 |
| Recipient age ≥ 60 y | 12 (38.7%) | 0 | 6 (21.4%) | 0 | 10 (38.5%) | 0 |
| Weight (kg) | 75.5 (68.8-85.1) | 3 | 76.8 (70.0-86.8) | 0 | 76.7 (70.5-85.7) | 3 |
| BMI (kg/m2) | 26.1 (22.5-28.5) | 3 | 24.8 (22.9-29.0) | 0 | 25.6 (23.4-30.1) | 3 |
| BMI ≥ 30 kg/m2 | 6 (21.4%) | 3 | 7 (25.0%) | 0 | 6 (26.1%) | 3 |
| Smoking status | 10 | 5 | 5 | |||
| Current | 3 (14.3%) | 6 (26.1%) | 5 (23.8%) | |||
| Former | 9 (42.9%) | 5 (21.7%) | 4 (19.0%) | |||
| Never | 9 (42.9%) | 12 (52.2%) | 12 (57.1%) | |||
| Antihypertensive medications | 4 | 2 | 3 | |||
| None | 5 (18.5%) | 4 (15.4%) | 4 (17.4%) | |||
| 1 or 2 | 14 (51.9%) | 9 (34.6%) | 8 (34.8%) | |||
| 3 or more | 8 (29.6%) | 13 (50.0%) | 11 (47.8%) | |||
| Transplant-related risk factors | ||||||
| Family history of diabetes | 2 (8.7%) | 8 | 5 (19.2%) | 2 | 5 (23.8%) | 5 |
| Chronic hepatitis C | 1 (3.6%) | 3 | 0 (0.0%) | 0 | 0 (0.0%) | 4 |
| CMV antibody positive | 16 (57.1%) | 3 | 20 (71.4%) | 0 | 13 (56.5%) | 3 |
| CMV high risk | 5 (18.5%) | 4 | 5 (17.9%) | 0 | 7 (30.4%) | 3 |
| PRA highest ≥ 10% | 2 (7.1%) | 3 | 4 (14.8%) | 1 | 2 (8.7%) | 3 |
| Number of mismatches | 2.4 ± 1.4 | 3 | 2.9 ± 1.1 | 1 | 3.0 ± 1.4 | 3 |
| Repeat transplant | 4 (14.3%) | 3 | 6 (21.4%) | 0 | 5 (21.7%) | 3 |
| Living donor | 1 (3.6%) | 3 | 0 (0.0%) | 0 | 0 (0.0%) | 3 |
| Primary kidney disease | 5 | 0 | 3 | |||
| Glomerular disease | 12 (46.2%) | 6 (21.4%) | 4 (17.4%) | |||
| Vascular disease | 6 (23.1%) | 6 (21.4%) | 7 (30.4%) | |||
| Polycystic kidney disease | 3 (11.5%) | 5 (17.9%) | 5 (21.7%) | |||
| Tubulointerstitial disease | 1 (3.8%) | 1 (3.6%) | 0 (0.0%) | |||
| Unknown | 4 (15.4%) | 10 (35.7%) | 7 (30.4%) | |||
| Comorbid conditions | 4 | 0 | 3 | |||
| Cardiovascular | 10 (37.0%) | 16 (57.1%) | 10 (43.5%) | |||
| Respiratory | 0 (0.0%) | 5 (17.9%) | 4 (17.4%) | |||
| Urinary | 1 (3.7%) | 2 (7.1%) | 1 (4.3%) | |||
| Endocrinologic | 3 (11.1%) | 6 (21.4%) | 1 (4.3%) | |||
| Neurologic | 0 (0.0%) | 0 (0.0%) | 2 (8.7%) | |||
| Psychiatric | 0 (0.0%) | 0 (0.0%) | 1 (4.3%) | |||
| Other | 0 (0.0%) | 1 (3.6%) | 0 (0%) | |||
| Maintenance immunosuppression early after transplantation | ||||||
| Tacrolimus | 31 (100.0%) | 0 | 28 (100.0%) | 0 | 26 (100.0%) | 0 |
| Mycophenolate mofetil | 20 (71.4%) | 3 | 16 (57.1%) | 0 | 11 (47.8%) | 3 |
| Mycophenolic acid | 8 (28.6%) | 3 | 12 (42.9%) | 0 | 12 (52.2%) | 3 |
| Steroid | 31 (100.0%) | 0 | 28 (100.0%) | 0 | 26 (100.0%) | 0 |
Abbreviations: CSII, continuous subcutaneous insulin infusion; BMI, body mass index; CMV, cytomegalovirus; PRA, panel reactive antibodies.
n (%), mean ± SD, median (Q1–Q3).
HbA1c Levels at Month 3 and During Follow-up
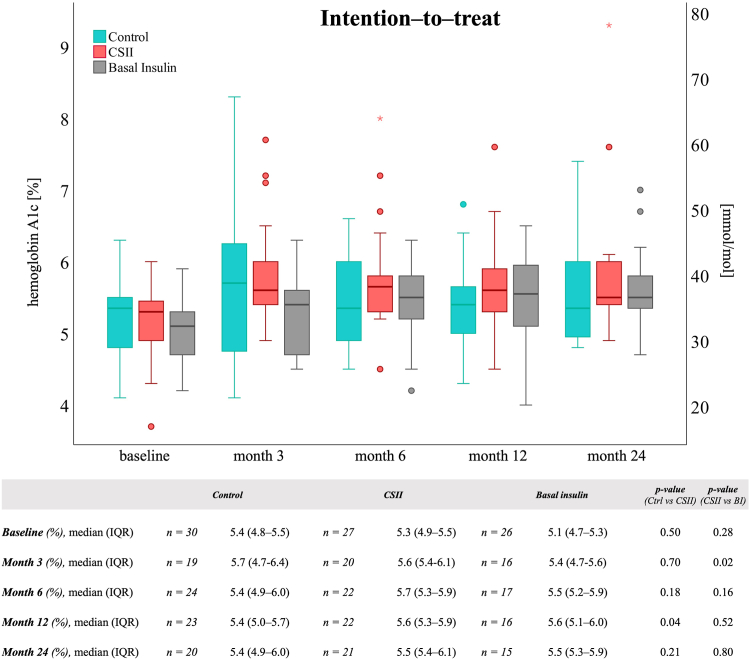
At month 3, most participants had adequate glucose control, regardless of the group with HbA1c values ≥ 6.5% (48 mmol/mol) noted in 4 (21%), 4 (20%), and 0 (0%) in the control group, CSII group and BI group, respectively (Table S1). In the ITT population, median (IQR) HbA1c levels at month 3 were 5.7% (4.7-6.4) in the control group, 5.6% (5.4-6.1) in the CSII group (P = 0.70, primary endpoint) and 5.4% (4.7-5.6) in the BI group (P = 0.02), respectively (Fig 2 and Fig S2 for the PP population). The corresponding changes from baseline and during follow-up were similar between groups (Table S2). HbA1c level increases of ≥0.5% from baseline are provided in Table S3.
Figure 2.
Glycated hemoglobin A1c. Hemoglobin A1c (HbA1c) levels in kidney transplant recipients of the intention-to-treat population Boxplots show median and interquartile ranges (IQR) of HbA1c. ∗ = extreme outliers. Variables were tested using the Mann–Whitney U test. Abbreviations: Ctrl, control; CSII, continuous subcutaneous insulin infusion; BI, basal insulin.
Hyperglycemia and Glucose-lowering Medication
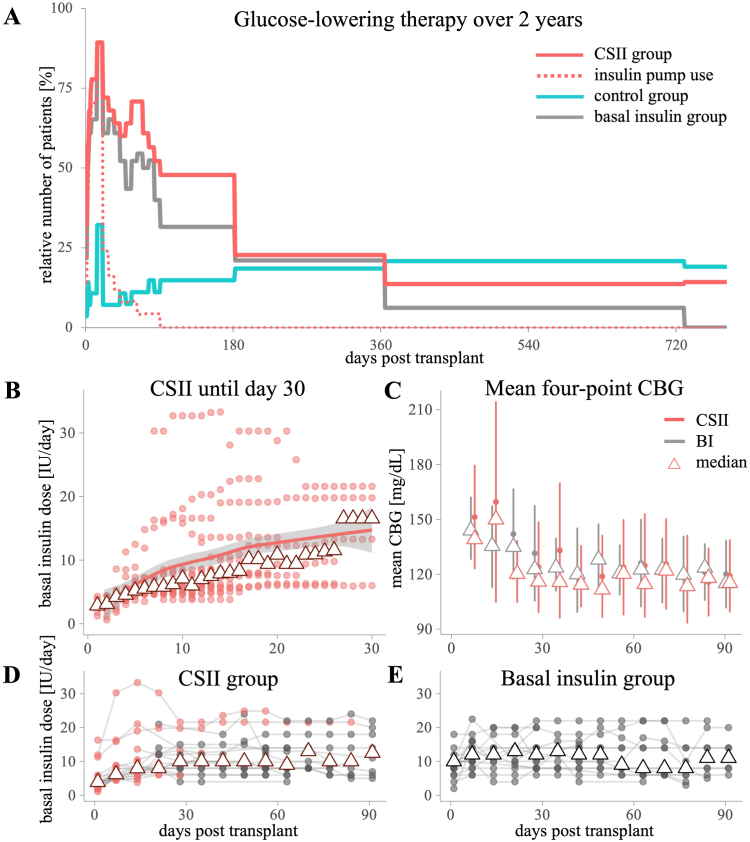
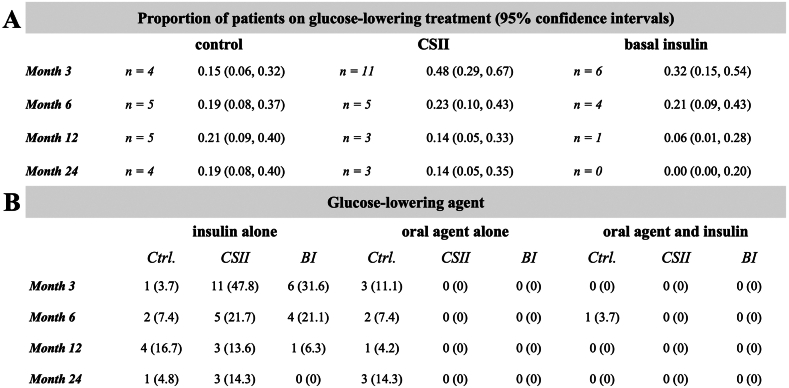
Glucose-lowering therapy and BI doses are provided in Figure 3. A detailed description of the insulin doses in the CSII group was previously provided by Werzowa et al.19 CSII therapy lasted until median (IQR) postoperative day 18 (14-28). Four participants continued CSII therapy after hospital discharge. CSII was mostly replaced by subcutaneous injections (19 out of 24 participants) (Panel D). Over the first 3 months, the median (IQR) cumulative basal insulin dose was 632 (336-963) IU in the BI group versus 571 (258-711) in the CSII group. The median BI dose per treatment day was 11.2 (8.2-14.3) versus 8.2 (5.7-13.4) IU, respectively. The insulin doses administered by CSII only were 85 (52-151) IU and 6.0 (5.2-8.7) IU per CSII treatment day. Although daily basal insulin doses tended to be lower in the CSII group, mean (SD) CBG values were similar compared with the BI group (Panel C). In months 12 and 24, 5 (21%) and 4 (19%) participants in the control group versus 3 (14%) and 3 (14%) participants in the CSII group still received (any) glucose-lowering treatment, respectively (Fig 4). The four-point CBG measurements of the CSII and the BI groups are provided in Figure S4.
Figure 3.
Glucose-lowering medication: Part 1. Glucose-lowering therapy in the CSII versus control group and basal insulin group. (A) Relative numbers of participants with glucose-lowering medication (insulin and/or oral glucose-lowering agents). Proportion of patients receiving treatment within 14 days (representing the immediate post-transplant period) are displayed as a peak in the figure. Note: CSII group participants received insulin beyond the insulin pump maximum until day 88 (red dotted line). After discontinuation of the insulin pumps, most participants changed to injectable isophane insulin (any treatment=solid red line). (B) Basal insulin rates administered through insulin pump in the CSII group. Color gradient (from light to dark) indicates overlapping values. Low doses on the first and the last day of CSII were not displayed. Lines and gray ribbons: loess smoothing line indicates the trend over time. Triangles indicate medians. (C) Mean of 4-point CBG (capillary blood glucose) measurements presented as means ± standard deviations and medians (triangles). Daily documentations in week 1 were averaged and presented on day 7. (D, E) Basal insulin doses at study visits until month 3 in the CSII group (D) and basal insulin group (E). Minimum and maximum doses in week 1 are displayed at days 1 and 7. Triangles indicate medians. In the CSII group, basal insulin administration through injections is displayed as gray dots, and administration through CSII is displayed as red dots. Abbreviations: Ctrl, control; CSII, continuous subcutaneous insulin infusion; BI, basal insulin.
Figure 4.
Glucose-lowering medication: Part 2. Glucose-lowering therapy in the CSII versus control group and basal insulin group. (F) Proportions and their 95% confidence intervals for glucose-lowering medication at months 3, 6, 12, and 24 post-transplantation. (G) Type of glucose-lowering medication. Abbreviations: Ctrl, control; CSII, continuous subcutaneous insulin infusion; BI, basal insulin.
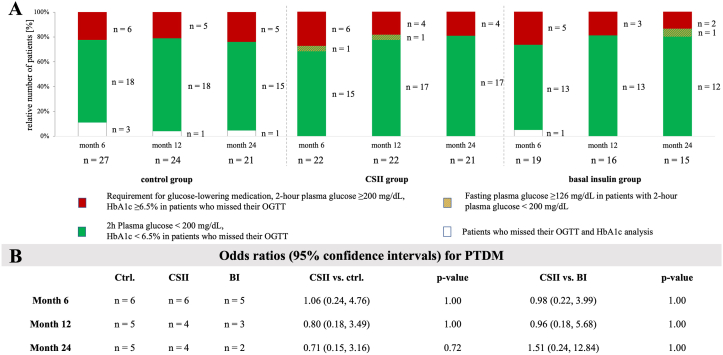
PTDM at 12 and 24 Months
Proportion of patients with PTDM at months 6, 12 and 24 is provided in Figure 5. Detailed OGTT outcome is provided in Figure S5. The odds for PTDM in months 12 and 24 in the CSII versus control group were not significantly different (odds ratio [95% confidence interval], 0.80 [0.18-3.49] and 0.71 [0.15-3.16], respectively; ITT analysis). Likewise, the odds for PTDM in months 12 and 24 in the CSII versus BI group were not significantly different (odds ratio [95% confidence interval], 0.96 [0.18-5.68] and 1.51 [0.24-12.84]; ITT analysis).
Figure 5.
PTDM development. Oral glucose tolerance test (OGTT) outcomes at 6, 12, and 24 months post-transplantation. (A) Post-transplantation diabetes mellitus (PTDM) (red) was defined by requirement for glucose-lowering medication, 2-hour plasma glucose ≥ 200 mg/dL, or HbA1c level ≥ 6.5% in participants who missed their OGTT. Absence of diabetes (green) was defined by 2-hour plasma glucose (2hPG) < 200 mg/dL or HbA1c level < 6.5% in participants who missed their OGTT. Fasting plasma glucose ≥126 mg/dL in participants with 2-hour plasma glucose < 200 mg/dL is shown above the green part of the bar chart. Missing data for oral glucose tolerance test or HbA1c (white) are presented at the bottom, and a more detailed presentation of the glucose metabolism is provided in Supplemental Figure 4. (B) Odds ratios and their 95% confidence intervals for diabetes at months 6, 12, and 24 in the intention-to treat population. Diabetes definition using the additional FPG ≥ 126 mg/dL criterion is provided in Supplemental Figure 4. Abbreviations: Ctrl, control; CSII, continuous subcutaneous insulin infusion; BI, basal insulin; FPG, fasting plasma glucose.
Metabolic Parameters and Laboratory Values
Laboratory values pretransplantation and over the 2-year follow-up post-transplant are provided in Table 2 (ITT) and Table S4 (PP). At month 3 post-transplant, estimated glomerular filtration rate (eGFR) measurements were similar between groups, whereas at month 12, eGFR in the CSII group was higher than in the BI group (P = 0.02). The number of patients with a protein-to-creatinine ratio ≥ 500 mg/g or protein excretion rate ≥ 500 mg/day at month 12 was 6 (33.3%) in the control group versus 1 (5%) in the CSII (P = 0.04) and 2 (14.3%) in the BI group (P = 0.41).
Table 2.
Metabolic Parameters, Electrolytes, and Parameters of Kidney Function: Intention-to-Treat
|
Characteristic |
Baseline, N = 85 |
Month 3, N = 69 |
Month 6, N = 68 |
Month 12, N = 62 |
Month 24, N = 57 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control, N = 31a |
Pump, N = 28a |
Basal insulin, N = 26a |
Control, N = 27a |
Pump, N = 23a |
Basal Insulin, N = 19a |
Control, N = 27a |
Pump, N = 22a |
Basal Insulin, N = 19a |
Control, N = 24a |
Pump, N = 22a |
Basal Insulin, N = 16a |
Control, N = 21a |
Pump, N = 21a |
Basal Insulin, N = 15a |
|
| Weight (kg) | 75.5 (68.8-85.1) | 76.8 (70.0-88.3) | 76.7 (70.5-85.7) | 71.0 (56.3-75.8) | 70.1 (66.8-80.8) | 72.0 (69.0-80.0) | 75.5 (56.8-83.0) | 73.5 (67.4-82.6) | 72.0 (63.5-79.5) | 72.5 (59.5-82.5) | 75.0 (68.0-86.5) | 74.0 (67.6-82.0) | 74.8 (59.6-81.5) | 74.8 (70.5-86.0) | 75.0 (69.0-79.0) |
| BMI (kg/m2) | 26.1 (22.5-28.5) | 25.2 (22.6-29.7) | 25.6 (23.4-30.1) | 24.4 (19.7-25.4) | 23.8 (21.8-24.4) | 23.3 (20.8-25.2) | 25.3 (20.5-28.1) | 23.7 (22.2-25.1) | 23.5 (20.9-25.9) | 24.4 (21.9-28.1) | 23.8 (22.0-25.8) | 23.9 (21.8-27.2) | 24.5 (21.7-28.2) | 24.3 (22.1-26.3) | 24.9 (22.8-26.5) |
| Creatinine (mg/dL) | 7.9 (5.4-10.1) | 6.5 (5.2-8.7) | 6.9 (6.2-9.5) | 1.6 (1.2-1.9) | 1.7 (1.3-2.0) | 1.9 (1.3-2.4) | 1.4 (1.1-1.7) | 1.6 (1.2-1.8) | 1.8 (1.3-1.9) | 1.3 (1.0-1.6) | 1.3 (1.1-1.6) | 1.7 (1.5-1.9) | 1.3 (1.1-1.8) | 1.3 (1.1-1.8) | 1.5 (1.4-1.9) |
| eGFR (mL/min/1.73m2) | 6.5 (5.1-9.3) | 7.9 (6.2-11.2) | 7.0 (4.9-9.3) | 50.2 (37.2-60.1) | 42.9 (34.6-58.5) | 41.2 (29.4-60.2) | 55.2 (37.6-63.8) | 52.0 (36.4-70.0) | 44.4 (38.8-55.3) | 58.8 (40.3-66.4) | 55.8 (49.1-78.3) | 46.1 (38.0-50.1) | 59.0 (40.2-73.3) | 55.7 (43.5-74.4) | 44.3 (40.3-57.8) |
| Hemoglobin (g/dL) | 11.2 (10.4-12.1) | 11.4 (10.0-12.2) | 11.2 (10.3-12.4) | 11.6 (9.6-12.9) | 12.4 (11.6-13.0) | 10.9 (9.5-11.8) | 12.5 (12.0-13.7) | 13.0 (12.6-14.4) | 12.3 (11.7-13.5) | 13.0 (10.6-14.0) | 14.2 (12.4-15.4) | 12.8 (11.5-14.9) | 13.2 (11.8-14.3) | 13.9 (13.3-14.6) | 14.3 (12.6-15.0) |
| Potassium (mmol/L) | 4.9 (4.4-5.6) | 4.6 (4.4-5.1) | 4.9 (4.4-5.2) | 4.4 (4.0-4.9) | 4.6 (4.4-4.8) | 4.8 (4.6-5.0) | 4.4 (4.2-4.8) | 4.6 (4.3-5.0) | 4.3 (4.1-4.9) | 4.4 (4.3-4.8) | 4.5 (4.2-4.8) | 4.6 (4.5-4.7) | 4.7 (4.3-4.8) | 4.4 (4.1-4.6) | 4.5 (4.4-4.8) |
| Sodium (mmol/L) | 137.0 (135.8-139.0) | 138.0 (137.0-139.0) | 138.0 (137.0-140.0) | 139.0 (138.0-140.5) | 142.0 (139.5-143.0) | 141.0 (139.0-142.0) | 140.0 (139.0-140.5) | 141.0 (138.0-141.5) | 141.0 (139.0-142.8) | 140.0 (138.5-142.0) | 141.0 (137.3-142.0) | 139.0 (137.0-143.0) | 141.0 (138.0-142.3) | 140.0 (138.0-142.5) | 141.0 (139.0-143.0) |
| Uric acid (mg/dL) | 5.4 (4.3-7.1) | 5.0 (3.8-6.1) | 5.4 (4.2-6.0) | 6.4 (5.6-7.2) | 7.0 (6.1-7.9) | 7.0 (6.0-8.0) | 6.7 (5.8-9.5) | 6.5 (5.6-7.2) | 7.1 (6.0-8.4) | 6.4 (5.5-8.6) | 6.7 (5.8-7.5) | 7.2 (6.7-8.1) | 6.5 (5.9-7.9) | 6.7 (5.6-8.1) | 7.9 (7.1-8.1) |
| Triglycerides (mg/dL) | 122.5 (92.0-194.0) | 142.5 (99.3-217.5) | 146.5 (100.3-231.0) | 148.0 (126.5-185.5) | 212.0 (150.0-247.0) | 159.0 (118.0-185.0) | 117.0 (91.0-171.5) | 128.5 (101.8-176.8) | 144.5 (127.0-194.5) | 153.0 (102.0-216.0) | 166.5 (131.5-222.0) | 128.5 (116.0-204.5) | 157.5 (107.3-173.3) | 146.0 (120.0-168.0) | 131.5 (100.8-188.5) |
| HDL (mg/dL) | 44.0 (35.3-61.8) | 43.0 (34.0-51.0) | 47.0 (40.8-65.3) | 48.0 (44.5-59.0) | 41.0 (35.0-52.5) | 52.0 (44.0-60.0) | 63.0 (53.0-74.0) | 54.5 (41.0-61.8) | 53.5 (43.0-65.5) | 58.0 (47.0-67.0) | 56.0 (50.0-89.0) | 60.5 (52.3-67.3) | 55.5 (45.8-67.3) | 59.0 (47.0-74.0) | 62.0 (56.8-69.5) |
| LDL (mg/dL) | 98.3 (69.9-112.9) | 93.5 (78.3-123.8) | 87.7 (77.9-109.8) | 125.4 (57.9-146.7) | 126.4 (112.4-189.6) | 96.0 (90.0-141.2) | 121.0 (96.0-138.6) | 132.8 (118.5-154.0) | 110.7 (87.5-140.4) | 111.6 (98.2-149.5) | 120.0 (102.6-137.4) | 113.7 (98.6-148.9) | 116.5 (101.5-126.7) | 116.5 (99.0-138.9) | 117.7 (103.5-142.7) |
| C–reactive protein (mg/dL) | 0.6 (0.2-1.5) | 0.4 (0.2-1.6) | 0.4 (0.2-0.8) | 0.3 (0.1-1.7) | 0.2 (0.1-0.4) | 0.3 (0.1-0.8) | 0.2 (0.1-0.6) | 0.2 (0.1-0.3) | 0.2 (0.1-0.6) | 0.2 (0.1-0.9) | 0.2 (0.1-0.4) | 0.2 (0.1-0.3) | 0.3 (0.1-0.3) | 0.3 (0.2-0.7) | 0.2 (0.1-0.6) |
| PCR (mg/g) | NA | NA | NA | NA | NA | NA | 153.0 (106.0-289.0) | 112.0 (84.8-208.0) | 141.0 (96.0-286.0) | 182.5 (93.3-703.8) | 169.0 (99.5-263.0) | 250.0 (99.3-418.3) | 191.0 (69.0-608.5) | 166.0 (98.0-323.0) | 95.0 (74.0-405.5) |
| Urine albumin (mg/L) | NA | NA | NA | NA | NA | NA | 28.6 (8.7-87.0) | 11.5 (6.7-35.2) | 21.3 (10.6-61.7) | 43.6 (6.0-464.9) | 30.0 (10.9-55.6) | 26.3 (7.8-78.8) | 25.3 (7.2-236.2) | 35.2 (11.1-110.9) | 13.7 (7.7-21.2) |
Abbreviations: eGFR, estimated glomerular filtration rate using the 2021 CKD-EPI formula; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PCR, urine protein-to-creatinine ratio; NA, not applicable because data were not measured per the study’s protocol.
Median (Q1–Q3).
CSII did not influence weight change between baseline and month 3 and between month 3 and month 12 compared with the control group (P > 0.05). Hemoglobin levels in the CSII group were not significantly different from the control group at baseline and month 3 (P = 1.0 and P = 0.18, respectively). In the total cohort, hemoglobin levels at baseline and month 3 as well as the hemoglobin change did not correlate with HbA1c levels at baseline and month 3 as well as the HbA1c change (all P > 0.05).
Safety of CSII Therapy
Adverse events during the first 3 weeks have previously been published.19 No severe hypoglycemia (blood glucose measurements ≤ 40 mg/dL [2.2 mmol/L]) was observed (Table 3). Mild hypoglycemia events occurred in the CSII (N = 2) and the BI (N = 2) groups (blood glucose 40-60 mg/dL [2.3-3.3 mmol/L]), both of which occurred before month 3. One hypoglycemia event in the CSII group was reported to be symptomatic.
Table 3.
Hypoglycemia Events and Other Safety Endpoints
| Baseline-Month 3 |
Months 3-6 |
Months 6-12 |
Months 12-24 |
Total |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control, N = 31a |
CSII, N = 28a |
Basal Insulin, N = 26a |
Control, N = 27a |
CSII, N = 23a |
Basal Insulin, N = 19a |
Control, N = 27a |
CSII, N = 22a |
Basal Insulin, N = 19a |
Control, N = 24a |
CSII, N = 22a |
Basal Insulin, N = 16a |
Control, N = 31a |
CSII, N = 28a |
Basal Insulin, N = 26a |
|
| Hypoglycemia events (total) | 0 (0.0%) | 2 (7.1%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.1%) | 2 (7.7%) |
| Symptomatic | 0 (0.0%) | 1 (3.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.6%) | 0 (0.0%) |
| BG 41-60 mg/dL | 0 (0.0%) | 2 (7.1%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (7.1%) | 2 (7.7%) |
| BG < 40 mg/dL | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Death | 0 (0.0%) | 0 (0.0%) | 2 (7.7%) | 0 (0.0%) | 1 (4.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (4.2%) | 1 (4.5%) | 0 (0.0%) | 1 (3.2%) | 2 (7.1%) | 2 (7.7%) |
| Rejection episode | 2 (6.5%) | 1(3.6%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) | 1 (5.3%) | 2 (7.4%) | 0 (0.0%) | 0 (0.0%) | 2 (8.3%) | 0 (0.0%) | 1 (6.3%) | 4 (12.9%) | 1 (3.6%) | 4 (15.4%) |
| Graft loss | 2 (6.5%) | 3 (10.7%) | 2 (7.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 0 (0.0%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 4 (12.9%) | 3 (10.7%) | 2 (7.7%) |
| Hospitalization | 4 (12.9%) | 4 (14.3%) | 6 (23.1%) | 5 (18.5%) | 3 (13.0%) | 6 (31.6%) | 6 (22.2%) | 3 (13.6%) | 3 (15.8%) | 5 (20.8%) | 6 (27.3%) | 4 (25.0%) | 10 (32.3%) | 12 (42.9%) | 12 (46.2%) |
Notes: Total refers to number of patients within the study population from baseline to 24 months. Number of hypoglycemia events is equivalent to number of patients with hypoglycemia events.
Abbreviations: CSII, continuous subcutaneous insulin infusion group; BG, blood glucose.
n (%).
Post Hoc Analyses of the Study Outcomes by Risk Group and Sex
In randomized participants, 20 (64.5%), 17 (60.7%), and 19 (73%) patients were identified as being at increased risk for PTDM in the control group, CSII group, and BI group, respectively. Over the 2-year follow-up time, HbA1c values tended to be higher in the high-risk group (Fig S6). Treatment group sizes stratified by risk group were very small. Among individuals with high baseline risk, BI treatment group participants seemed to have slightly better HbA1c control and OGTT results (Figs S6 and S7). HbA1c measurements and the number of patients with PTDM were similar between men and women (Figs S8 and S9).
Discussion
In the present trial, we investigated whether early postoperative insulin intervention using CSII therapy provided superior glycemic control in kidney transplant recipients (KTRs) without previous history of diabetes. By month 3, most participants in the 3 groups had adequate glucose control. CSII therapy was, however, not superior at reducing HbA1c levels at month 3 or PTDM prevalence at months 12 and 24 compared with the control or BI group.
Our negative trial results may be interpreted in the context of a relatively normoglycemic control group, evaluated using HbA1c levels at month 3 and the HbA1c change from baseline (median ≤0.4% during follow-up). In comparison, in our previous proof-of-concept trial evaluating BI, there had been higher steroid doses and consequently a higher mean change in HbA1c levels (+0.9 at month 3) and more patients on glucose-lowering treatment at month 12 (32%).15 We observed only 2 mild and quickly resolving episodes of hypoglycemia in both the CSII and BI groups, respectively.
We hypothesized CSII would benefit KTRs because it is effective in nontransplanted individuals.24,25 Beta-cell function decline in type 2 diabetes responds to early insulin or lifestyle interventions, and CSII achieved this effect in some studies.26, 27, 28 Given that beta-cell exhaustion plays a pivotal role in the development of PTDM, early intensive insulin therapy was seen as a plausible prevention strategy in KTRs.17,18,29, 30, 31, 32 Here, the CSII group had lower insulin doses and adequate glucose control, but showed no apparent PTDM prevention.19
An earlier trial in KTRs showed that intensive insulin therapy targeting 70-110 mg/dL glucose versus a basal bolus regimen targeting 70-180 mg/dL increased the risk for a composite of rejection or graft loss.33 In nontransplanted patients in the intensive care unit, the multicentric NICE-SUGAR study showed that maintaining 81-108 mg/dL over 24 hours increased the 90-day mortality in comparison to a control group targeting <180 mg/dL. KTRs without intensive care may need different glucose targets, but evidence is scarce.34,35 Here, rejection and graft loss rates were comparable between groups, as observed in our previous trial.16 The CSII group had less severe proteinuria than control at 12 months, and the BI group had slightly less. These differences are likely because of group differences in recurrent or de novo disease rather than a treatment effect.
Although this study demonstrates feasibility (mostly during hospital admission), the insulin pumps were very demanding to handle. Only 18 of 28 randomized patients received CSII therapy at least until day 14 post-transplant, and the majority of those who discontinued CSII were placed on BI afterward. Only 4 patients were willing to use CSII after discharge. Study staff reported that the application of CSII equipment was clearly more sophisticated than the single BI injection in the morning. Regarding user-friendliness, the availability of closed-loop systems may change the prerequisites of future studies for post-transplant hyperglycemia.36 Also, CGM sensors had to be omitted because of technical difficulties, but this could be a single-center observation that may not apply to transplanted or hospitalized patients in general. In fact, CGM can reduce mean glucose levels and the time out of target range in hospitalized patients.37,38 Some participants reported general discomfort or premature removal. Infusion set and site difficulties are common.39 Therefore, first-time use after major surgery in a population that requires insulin for few weeks to months only might not be practical. Few studies evaluated first-time in-hospital CSII therapy.40, 41, 42, 43 One study reported recruiting difficulties because patients were overwhelmed by acute illness and that CSII and CGM required a lot of attention during the study.43 These observations may reflect our experiences.
It does not appear sensible to disregard insulin pump therapy altogether. Our previous trial showed a reduction of PTDM using BI, and insulin remains the preferred treatment for post-transplant hyperglycemia.9,15 This is the first study of CSII in KTRs without a history of diabetes showing that CSII may not be beneficial for de novo hyperglycemia and moderate insulin demand. However, some findings indicated that CSII might be an effective therapy for patients with prior diabetes and poor glycemic control post-transplantation.44,45 CSII and CGM technologies have dramatically improved glucose monitoring and insulin delivery for patients with type 1 and 2 diabetes. Despite some notable challenges, these devices can also improve users’ confidence and ability to manage their disease.46 Although CSII may offer some advantages for certain patients, standard insulin delivery should remain the first-line treatment post kidney transplant. Insulin is effective and other drugs for in-hospital hyperglycemia in KTRs are understudied.9 BI may still be the preferred option, as it matches steroid-induced glucose peaks.15,16 Oral or noninsulin injectable agents and their combinations may be sufficient for moderate outpatient hyperglycemia.9
Our study has several limitations. Although we managed to recruit an adequate number of patients, there were missing HbA1c values at the time of our primary endpoint evaluation. Still, HbA1c values remained similarly low in all 3 groups. Statistical power was, as expected, too low to evaluate a reduction of PTDM. Furthermore, the fact that the use of glucose sensors was not implemented, as originally planned, is an additional study limitation. At the time when this study was carried out (earlier publication hindered by the need to publish the primary study first), sensor-augmented insulin pump therapy was the most advanced insulin delivering technology, and this system has by now been surpassed by the more up-to-date closed-loop systems.16 We also acknowledge the change of the analysis from PP to ITT for improved evaluation of the efficacy of CSII therapy in these groups because of maintained statistical power. Another study limitation is that use of sulfonylureas for persistent hyperglycemia may not constitute a standard-of-care group by today’s practices and is grounded in the timespan since study planning.47,48 Also, the parallel enrollment but consecutive analysis with the ITP-NODAT study publication in 2021 caused a delay in the publication of the present work.16
Choosing HbA1c levels as our primary endpoint may not have been ideal to start with because measurements may be influenced by post-transplant anemia, among other factors in KTRs.49,50 Here, we did not observe a systematic relationship between hemoglobin and HbA1c levels. Recent studies indicated that HbA1c levels used for diagnostic purposes showed less sensitivity compared with the OGTT and a lack of association with mortality as early as 10 weeks after transplantation.6,51 Moreover, intraindividual variability in KTRs may be higher than in nontransplanted individuals.52 Only few years before this study was conceptualized, HbA1c measurements had been shown to be associated with cardiovascular events and were recognized as a diagnostic test in the general population.53,54 HbA1c levels as a marker of hyperglycemia as early as 3 months may not necessarily indicate any benefit for these patients long-term because many patients with early PTDM revert during follow-up.55 As our previous trial showed reduced HbA1c levels at month 3 and PTDM prevalence over the first year post transplant, we hypothesized that an intensified and individualized insulin administration using CSII could lead to an even clearer benefit versus the control group.15 Overall, the use of HbA1c level as the primary endpoint (in view of its shortcomings as marker of hyperglycemia in kidney disease and anemia) limits the interpretation of our results.
Our study is further limited because we did not performed diabetes screening before inclusion. In a study by Bergrem et al,56 OGTT screening revealed 8% undiagnosed diabetes mellitus in transplant candidates. Finally, we acknowledge that our study center does not routinely collect self-reported ethnicity. Study investigators, however, reported that the large majority of our study population were European White individuals. In view of the association between ethnicity and altered HbA1c measurements, our results might not be applicable to other ethnic groups.57
In conclusion, first-time CSII use after kidney transplantation in patients without previous diabetes diagnosis did not improve HbA1c levels at month 3. Even though CSII was feasible during hospital stay, it was labor-intensive for patients and staff. This therapy may be offered safely to patients with excellent therapy adherence who are aware of the advantages and disadvantages of insulin pump therapy. Individuals who are familiar with insulin pump therapy or those with very poor glucose control after transplantation might, in the future, opt for CSII therapy, and we suggest it may be important to study their experiences further.
Article Information
Authors’ Full Names and Academic Degrees
Amelie Kurnikowski, MD, Johannes Werzowa, MD, Sebastian Hödlmoser, MSc, Simon Krenn, MD, Christopher Paschen, MD, Sebastian Mussnig, MD, Andrea Tura, PhD, Jürgen Harreiter, MD, PhD, Michael Krebs, MD, PhD, Peter X.K. Song, PhD, Kathrin Eller, MD, Julio Pascual, MD, Klemens Budde, MD, Manfred Hecking, MD, PhD, Elisabeth Schwaiger, MD, PhD
Authors’ Contributions
MH and JW designed the trial, wrote and submitted the study protocol. MH and JW enrolled participants into the trial and actively treated trial participants. JW retrieved the data, actively converting paper case report forms into electronic database. AK, ES, MH, and JW analyzed the data. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The investigator-initiated ITP-NODAT trial was supported by University of Michigan subcontract 3002300292 to the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK092475 and by Astellas Pharma and Eli Lilly in the form of contracts with the Medical University of Vienna that did not contain intellectual restrictions on publication. The investigator-initiated SAPT-NODAT trial was supported by the Austrian Diabetes Association (ÖDG) in the form of a competitive research grant won in the year 2012 by M. Hecking. The SAPT-NODAT trial was also supported by Astellas Pharma and Eli Lilly in the form of contracts with the Medical University of Vienna that did not contain intellectual restrictions on publication. Medtronic Europe, Germany, provided support in the form of insulin pumps and consumables for these pumps.
Funding
University of Michigan subcontract 3002300292 to the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK092475, Astellas Pharma, Eli Lilly, Austrian Diabetes Association (ÖDG), Medtronic Europe (please refer to the Support section for more detail).
Financial Disclosures
M. Hecking reports having received speaker honoraria from Astellas Pharma, which did not influence the submitted work. M. Hecking reports having received research funding from Astellas Pharma, Boehringer Ingelheim, Eli Lilly, and Siemens Healthcare. M. Hecking reports having received speaker honoraria from Fresenius Medical Care.
Prior Presentations
European Society for Organ Transplantation (ESOT) Congress, Milan, Italy, August 29 to September 1, 2021.
Peer Review
Received November 22, 2023, as a submission to the expedited consideration track with 2 external peer reviews. Direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form February 14, 2024.
Footnotes
Complete author and article information provided before references.
Figure S1: Days hospitalized post-transplant.
Figure S2: Glycated hemoglobin A1c levels in the per-protocol population.
Figure S3: Glucose-lowering medication in the per-protocol population.
Figure S4: Capillary blood glucose measurements.
Figure S5: Detailed PTDM development.
Figure S6: Glycated hemoglobin A1c levels: high-risk versus low-risk group.
Figure S7: PTDM development: high-risk versus low-risk group.
Figure S8: Glycated hemoglobin A1c levels: female versus male participants.
Figure S9: PTDM development: female versus male participants.
Item S1: Supplemental methods.
Table S1: HbA1c Diagnostic Categories.
Table S2: Glycated Hemoglobin A1c Development.
Table S3: Patients with Increased HbA1c Levels.
Table S4: Metabolic Parameters, Electrolytes, and Parameters of Kidney Function: Per-Protocol.
Supplementary Materials
Figures S1-S9; Item S1; Tables S1-S4.
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L., et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Luan F.L., Stuckey L.J., Ojo A.O. Abnormal glucose metabolism and metabolic syndrome in non-diabetic kidney transplant recipients early after transplantation. Transplantation. 2010;89(8):1034–1039. doi: 10.1097/TP.0b013e3181d05a90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porrini E., Diaz J.M., Moreso F., et al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int. 2019;96(6):1374–1380. doi: 10.1016/j.kint.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Wauters R.P., Cosio F.G., Suarez Fernandez M.L., Kudva Y., Shah P., Torres V.E. Cardiovascular consequences of new-onset hyperglycemia after kidney transplantation. Transplantation. 2012;94(4):377–382. doi: 10.1097/TP.0b013e3182584831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosio F.G., Kudva Y., van der Velde M., et al. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67(6):2415–2421. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 6.Eide I.A., Halden T.A., Hartmann A., et al. Mortality risk in post-transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl Int. 2016;29(5):568–578. doi: 10.1111/tri.12757. [DOI] [PubMed] [Google Scholar]
- 7.Valderhaug T.G., Hjelmesæth J., Hartmann A., et al. The association of early post-transplant glucose levels with long-term mortality. Diabetologia. 2011;54(6):1341–1349. doi: 10.1007/s00125-011-2105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakkera H.A., Weil E.J., Castro J., et al. Hyperglycemia during the immediate period after kidney transplantation. Clin J Am Soc Nephrol. 2009;4(4):853–859. doi: 10.2215/cjn.05471008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharif A., Chakkera H., de Vries A.P.J., et al. International consensus on post-transplantation diabetes mellitus. Nephrol Dial Transplant. 2024;39(3):531–549. doi: 10.1093/ndt/gfad258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amiel S.A. The consequences of hypoglycaemia. Diabetologia. 2021;64(5):963–970. doi: 10.1007/s00125-020-05366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pozzilli P., Battelino T., Danne T., Hovorka R., Jarosz-Chobot P., Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32(1):21–39. doi: 10.1002/dmrr.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connell M.A., Donath S., O'Neal D.N., et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250–1257. doi: 10.1007/s00125-009-1365-0. [DOI] [PubMed] [Google Scholar]
- 13.Coronel-Restrepo N., Blanco V.M., Palacio A., et al. Real-world effectiveness and safety of sensor-augmented insulin pump therapy in adults with type 1 diabetes: Long-term follow-up. Endocrinol Diabetes Nutr (Engl Ed) 2020 doi: 10.1016/j.endinu.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Luo P., Cheng Q., Chen B., et al. Hypoglycemia and blood glucose fluctuations in the application of a sensor-augmented insulin pump. Diabetes Technol Ther. 2013;15(12):984–989. doi: 10.1089/dia.2013.0078. [DOI] [PubMed] [Google Scholar]
- 15.Hecking M., Haidinger M., Döller D., et al. Early basal insulin therapy decreases new-onset diabetes after renal transplantation. J Am Soc Nephrol. 2012;23(4):739–749. doi: 10.1681/ASN.2011080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwaiger E., Krenn S., Kurnikowski A., et al. Early postoperative basal insulin therapy versus standard of care for the prevention of diabetes mellitus after kidney transplantation: a multicenter randomized trial. J Am Soc Nephrol. 2021;32(8):2083–2098. doi: 10.1681/asn.2021010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakkera H.A., Weil E.J., Pham P.T., Pomeroy J., Knowler W.C. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care. May 2013;36(5):1406–1412. doi: 10.2337/dc12-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecking M., Sharif A., Port F.K., Saemann M.D. Comment on: Chakkera et al. Can new-onset diabetes after kidney transplant be prevented? Diabetes Care. 2013;36:1406–1412. doi: 10.2337/dc13-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Werzowa J.M., Saemann M.D., Mohl A., et al. A randomized controlled trial-based algorithm for insulin-pump therapy in hyperglycemic patients early after kidney transplantation. PLOS ONE. 2018;13(3) doi: 10.1371/journal.pone.0193569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cosio F.G., Pesavento T.E., Kim S., Osei K., Henry M., Ferguson R.M. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62(4):1440–1446. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 21.Romagnoli J., Citterio F., Violi P., Cadeddu F., Nanni G., Castagneto M. Post-transplant diabetes mellitus: a case-control analysis of the risk factors. Transpl Int. 2005;18(3):309–312. doi: 10.1111/j.1432-2277.2004.00043.x. [DOI] [PubMed] [Google Scholar]
- 22.Torres A., Hernández D., Moreso F., et al. Randomized controlled trial assessing the impact of tacrolimus versus cyclosporine on the incidence of posttransplant diabetes mellitus. Kidney Int Rep. 2018;3(6):1304–1315. doi: 10.1016/j.ekir.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah T., Kasravi A., Huang E., et al. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82(12):1673–1676. doi: 10.1097/01.tp.0000250756.66348.9a. [DOI] [PubMed] [Google Scholar]
- 24.Reznik Y., Cohen O., Aronson R., et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet. 2014;384(9950):1265–1272. doi: 10.1016/s0140-6736(14)61037-0. [DOI] [PubMed] [Google Scholar]
- 25.Grunberger G., Bhargava A., Ly T., et al. Human regular U-500 insulin via continuous subcutaneous insulin infusion versus multiple daily injections in adults with type 2 diabetes: The VIVID study. Diabetes Obes Metab. 2020;22(3):434–441. doi: 10.1111/dom.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer C.K., Zinman B., Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1(1):28–34. doi: 10.1016/s2213-8587(13)70006-8. [DOI] [PubMed] [Google Scholar]
- 27.Taylor R., Al-Mrabeh A., Zhyzhneuskaya S., et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but Is dependent upon capacity for β cell recovery. Cell Metab. 2018;28(4):547–556.e3. doi: 10.1016/j.cmet.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Retnakaran R., Pu J., Emery A., et al. Determinants of sustained stabilization of beta-cell function following short-term insulin therapy in type 2 diabetes. Nat Commun. 2023;14(1):4514. doi: 10.1038/s41467-023-40287-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam J.H., Mun J.I., Kim S.I., et al. Beta-cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation. 2001;71(10):1417–1423. doi: 10.1097/00007890-200105270-00011. [DOI] [PubMed] [Google Scholar]
- 30.Zelle D.M., Corpeleijn E., Deinum J., et al. Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care. 2013;36(7):1926–1932. doi: 10.2337/dc12-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hecking M., Kainz A., Werzowa J., et al. Glucose metabolism after renal transplantation. Diabetes Care. 2013;36(9):2763–2771. doi: 10.2337/dc12-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halden T.A., Egeland E.J., Åsberg A., et al. GLP-1 restores altered insulin and glucagon secretion in posttransplantation diabetes. Diabetes Care. 2016;39(4):617–624. doi: 10.2337/dc15-2383. [DOI] [PubMed] [Google Scholar]
- 33.Hermayer K.L., Egidi M.F., Finch N.J., et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. J Clin Endocrinol Metab. 2012;97(12):4399–4406. doi: 10.1210/jc.2012-1979. [DOI] [PubMed] [Google Scholar]
- 34.NICE-SUGAR Study Investigators. Finfer S., Chittock D.R., et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 35.Lo C., Jun M., Badve S.V., et al. Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients. Cochrane Database Syst Rev. 2017;2(2) doi: 10.1002/14651858.CD009966.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haidar A., Legault L., Raffray M., et al. Comparison between closed-loop insulin delivery system (the artificial pancreas) and sensor-augmented pump therapy: a randomized-controlled crossover trial. Diabetes Technol Ther. 2021;23(3):168–174. doi: 10.1089/dia.2020.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortmann A.L., Spierling Bagsic S.R., Talavera L., et al. Glucose as the fifth vital sign: a randomized controlled trial of continuous glucose monitoring in a non-ICU hospital setting. Diabetes Care. 2020;43(11):2873–2877. doi: 10.2337/dc20-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buschur E.O., Faulds E., Dungan K. CGM in the hospital: is it ready for prime time? Curr Diab Rep. 2022;22(9):451–460. doi: 10.1007/s11892-022-01484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickup J.C., Yemane N., Brackenridge A., Pender S. Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol Ther. 2014;16(3):145–149. doi: 10.1089/dia.2013.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodur H.A., Saygili F., Saygili S., Doganay L.H., Yesil S. Continuous infusion of subcutaneous compared to intravenous insulin for tight glycaemic control in medical intensive care unit patients. Anaesth Intensive Care. 2008;36(4):520–527. doi: 10.1177/0310057x0803600421. [DOI] [PubMed] [Google Scholar]
- 41.Lee I.T., Liau Y.J., Lee W.J., Huang C.N., Sheu W.H. Continuous subcutaneous insulin infusion providing better glycemic control and quality of life in Type 2 diabetic subjects hospitalized for marked hyperglycemia. J Eval Clin Pract. 2010;16(1):202–205. doi: 10.1111/j.1365-2753.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 42.Kannampilly J.J. Role of continuous subcutaneous insulin infusion (insulin pump) in reducing blood glucose in four patients with type 2 diabetes and cirrhosis: a case series. Diabetes Technol Ther. 2010;12(7):543–545. doi: 10.1089/dia.2009.0166. [DOI] [PubMed] [Google Scholar]
- 43.Levitt D.L., Spanakis E.K., Ryan K.A., Silver K.D. Insulin pump and continuous glucose monitor initiation in hospitalized patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2018;20(1):32–38. doi: 10.1089/dia.2017.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitz O., Sorensen S.S., Alberti K.G., Orskov H., Hansen H.E. Metabolic control in newly kidney transplanted insulin-dependent diabetics: improvement by insulin pump treatment (CSII) J Diabet Complications. 1987;1(3):81–86. doi: 10.1016/s0891-6632(87)80061-2. [DOI] [PubMed] [Google Scholar]
- 45.Kaneyama N., Toyoda M., Kato E., et al. Glycemic control after kidney transplantation using sensor-augmented insulin pump therapy in a patient with slowly progressive type 1 diabetes mellitus. Tokai J Exp Clin Med. 2020;45(1):49–52. [PubMed] [Google Scholar]
- 46.Natale P., Chen S., Chow C.K., et al. Patient experiences of continuous glucose monitoring and sensor-augmented insulin pump therapy for diabetes: A systematic review of qualitative studies. J Diabetes. 2023;15(12):1048–1069. doi: 10.1111/1753-0407.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azoulay L., Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40(5):706–714. doi: 10.2337/dc16-1943. [DOI] [PubMed] [Google Scholar]
- 48.Douros A., Yin H., Yu O.H.Y., Filion K.B., Azoulay L., Suissa S. Pharmacologic differences of sulfonylureas and the risk of adverse cardiovascular and hypoglycemic events. Diabetes Care. 2017;40(11):1506–1513. doi: 10.2337/dc17-0595. [DOI] [PubMed] [Google Scholar]
- 49.Sharif A., Baboolal K. Diagnostic application of the A(1c) assay in renal disease. J Am Soc Nephrol. 2010;21(3):383–385. doi: 10.1681/ASN.2010010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkelmayer W.C., Chandraker A. Pottransplantation anemia: management and rationale. Clin J Am Soc Nephrol. 2008;3 Suppl 2(Suppl 2):S49–S55. doi: 10.2215/cjn.03290807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eide I.A., Halden T.A., Hartmann A., et al. Limitations of hemoglobin A1c for the diagnosis of posttransplant diabetes mellitus. Transplantation. 2015;99(3):629–635. doi: 10.1097/tp.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 52.Pimentel A.L., Camargo J.L. Variability of glycated hemoglobin levels in the first year post renal transplantation in patients without diabetes. Clin Biochem. 2017;50(18):997–1001. doi: 10.1016/j.clinbiochem.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Selvin E., Steffes M.W., Zhu H., et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Porrini E.L., Diaz J.M., Moreso F., et al. Clinical evolution of post-transplant diabetes mellitus. Nephrol Dial Transplant. 2016;31(3):495–505. doi: 10.1093/ndt/gfv368. [DOI] [PubMed] [Google Scholar]
- 56.Bergrem H.A., Valderhaug T.G., Hartmann A., et al. Undiagnosed diabetes in kidney transplant candidates: a case-finding strategy. Clin J Am Soc Nephrol. 2010;5(4):616–622. doi: 10.2215/cjn.07501009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selvin E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care. 2016;39(8):1462–1467. doi: 10.2337/dc16-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S9; Item S1; Tables S1-S4.