Highlights
-
•
GABA+ and Glx values were not disparate between patients with PD and healthy controls in the medial frontal cortex and thalamus.
-
•
GABA+ levels increased in the medial frontal cortex in DPD, which may be more closely related to depressive pathology.
-
•
Patients with PD had lower N-acetyl aspartate and choline levels in the left thalamus.
Keywords: γ-Aminobutyric acid, Depression, Medial frontal cortex, MEGA-PRESS, Parkinson’s disease
Abstract
Objective
The pathogenesis of depression in patients with Parkinson’s disease (PD) is poorly understood. Therefore, this study aimed to explore the changes in γ-aminobutyric acid (GABA) and glutamate plus glutamine (Glx) levels in patients with PD with or without depression determined using MEscher-GArwood Point Resolved Spectroscopy (MEGA-PRESS).
Materials and methods
A total of 83 patients with primary PD and 24 healthy controls were included. Patients with PD were categorized into depressed PD (DPD, n = 19) and nondepressed PD (NDPD, n = 64) based on the 17-item Hamilton Depression Rating Scale. All participants underwent T1-weighted imaging and MEGA-PRESS sequence to acquire GABA+ and Glx values. The MEGA-PRESS sequence was conducted using 18.48 mL voxels in the left thalamus and medial frontal cortex. The GABA+, Glx, and creatine values were quantified using Gannet 3.1 software.
Results
The GABA+ and Glx values were not significantly disparate between patients with PD and controls in the thalamus and medial frontal cortex. However, the levels of N-acetyl aspartate/creatine and choline/creatine in the left thalamus were significantly lower in patients with PD than in controls (P = .031, P = .009). The GABA+/Water and GABA+/Creatine in the medial frontal cortex were higher in DPD than in NDPD (P = .001, P = .004). The effects of depression on Glx or other metabolite levels were not evident, and no significant difference in metabolite values was noted in the left thalamus among all groups (P > .05).
Conclusions
GABA+ levels increased in the medial frontal cortex in DPD, which may be more closely related to depressive pathology. Thus, alterations in GABAergic function in special brain structures may be related to the clinical manifestations of PD symptoms, and hence mediating this function might help in treating depression in PD.
1. Introduction
Depression is a common non-motor symptom in patients with Parkinson's disease (PD), which is characterized by low mood, cognitive slowing, and reduced volitional activity (Lachner et al., 2017). The depression can be manifest in any stage of PD, particularly as the disease progresses (Reijnders et al., 2008).
The pathophysiology of depression in PD (DPD) involves complex alterations in dopamine, noradrenaline, and serotonin that are thought to contribute to PD (Schapira et al., 2017). Patients with DPD often have less dopamine transporter availability in specific brain regions than patients with nondepressed PD (NDPD) (Remy et al., 2005, Vriend et al., 2014). Imaging findings show dysfunction of dopaminergic and noradrenergic innervation in the locus coeruleus, thalamus, and limbic regions of the brain (Brown et al., 2011, Burn et al., 2012, Frisina et al., 2009). These changes are associated with multiple neurotransmitters, including γ-aminobutyric acid (GABA) and glutamate. GABA, as a major transmitter in the interneurons of the cerebral cortex, plays a critical role in regulating local neurotransmitter systems, particularly the glutamatergic system. GABA is the brain's most abundant inhibitory neurotransmitter, while glutamate is the most abundant excitatory neurotransmitter (Zeng et al., 2023).
Neuroanatomical and functional changes in limbic structures, particularly the prefrontal cortex, are characteristic of depression. These alterations have been linked to mood regulation and cognitive functions affected in depression. These changes may exacerbate depressive symptoms due to the added burden of neurodegenerative processes in PD (Ballanger et al., 2012, Kostic et al., 2012). Depression is associated with a lack of inhibitory synaptic transmission to key glutamatergic neurons, resulting in an imbalance of excitation and inhibition in the prefrontal cortex (Fogaça and Duman, 2019). It has been reported that the pathophysiology of depression involves dysfunction of GABAergic and glutamatergic neurons (Kantrowitz et al., 2021). These alterations are associated with reduced GAD67 and GABA levels, changes in GABA receptor expression (Fogaça and Duman, 2019), and diminished GABA concentrations in the cerebrospinal fluid (Gerner and Hare, 1981) and plasma (Petty, 1994, Petty et al., 1995) in patients with depression. A depression model using rats has been demonstrated to reduce GABA-B receptor binding in the prefrontal cortex (Dennis et al., 1993). Also, clinical trials of the potential of GABA agonists to alleviate depressive symptoms have displayed promise for GABAergic modulation as a therapeutic avenue (Gunduz-Bruce et al., 2019). However, magnetic resonance spectroscopy (MRS) studies have yielded mixed results regarding GABA levels in depression, which can be attributed to methodologic differences, variations in patient populations, and heterogeneity of depression itself. Some studies reported reduced GABA levels in patients with depression (Romeo et al., 2018, Sanacora et al., 2004), contrasting with others that observed no such change (Schür et al., 2016). These disparities highlight the complexity of neurochemical changes in depression and the need for further research. These disparities underscore the complex relationship between depression and GABAergic function. Furthermore, neuroimaging studies consistently reveal structural deficits across diverse brain regions and disruptions in connectivity between neural networks in depression, especially within the limbic–cortical–striatal–pallidal–thalamic circuit (Sheline, 2003), with the thalamus emerging as a key node in the pathology of depression (Zhang et al., 2022). The thalamus is crucial in regulating mood and cognitive functions. In the context of PD, its involvement becomes more vital due to its connections with the motor and limbic systems. Cardoso et al. (2009) demonstrated decreased activation in the thalamus and an increased volume in mediodorsal thalamic nuclei bilaterally in PD patients with depression, suggesting thalamic key role in the pathology of depressive symptoms in PD. Although studies have reported both increases and decreases in GABA levels in the brains of patients with PD (Terkelsen et al., 2022), these differences may depend on clinical symptom profiles.
No previous studies comprehensively assessed GABA and glutamate levels in patients with DPD. Thus, this study employed MRS to examine GABA+ and glutamate plus glutamine (Glx) levels in the medial frontal cortex (MFC) and thalamus of participants with DPD and NDPD, as well as age-matched healthy participants. We anticipated finding reduced GABA+ levels in patients with DPD, like depression, and we aimed to establish correlations between GABA+ reductions and the severity of depressive symptoms. This study aims to offer new insights into the pathogenesis of DPD, as well as potential treatment strategies.
2. Material and methods
2.1. Participants
The study had local ethics committee approval and all participants had informed consent. We prospectively recruited 83 patients with mild-to-moderate idiopathic PD (age, 63.9 ± 8.04 years; 44 male participants; and Hoehn and Yahr scale, 1–3) and 24 age- and sex-matched healthy controls (age, 60.8 ± 8.37 years; 12 male participants). Table 1 lists the demographic and clinical information for all participants. The diagnosis of PD was made using the Movement Disorder Society Clinical Diagnostic Criteria. Using the 17-item Hamilton Depression Rating Scale (HAMD-17), PD patients were classified into DPD group (HAMD-17 ≥ 17 scores, n = 19) and NDPD group (HAMD-17 < 17 scores, n = 64). The clinical diagnoses have been confirmed by an experienced and independent psychiatrist.
Table 1.
Demographics and clinical assessment of the participants.
| Variable | HC | NDPD | DPD | Sig. |
|---|---|---|---|---|
| N | 24 | 64 | 19 | |
| Age, year | 60.8 (8.4) | 64.2 (7.9) | 62.8 (8.7) | 0.225 |
| Male, n (%) | 12 (50.0) | 31 (48.4) | 7 (36.8) | 0.630 |
| Duration of PD, year | – | 7.5 (4.8) | 8.9 (5.5) | 0.286 |
| UPDRS-III scores (OFF states) | – | 35.1 (16.1) | 30.6 (9.8) | 0.280 |
| UPDRS-III scores (ON states) | – | 17.9 (10.1) | 18.5 (7.4) | 0.811 |
| HAMD-17 | 0.9 (1.1) | 9.3 (4.2) | 20.5 (6.0) | 0 < .001a, b, c |
| HAMA-14 | 1.0 (0.9) | 10.1 (5.4) | 18.3 (8.1) | 0 < .001a, b, c |
| MMSE | 27.3 (1.4) | 27.3 (1.8) | 27.1 (2.0) | 0.864 |
| PSQI | 3.8 (3.2) | 7.5 (4.6) | 10.3 (4.1) | 0 < .001a, b, c |
| UPDRS-Ⅰ | 0.5 (0.5) | 3.1 (2.3) | 4.9 (2.5) | 0 < .001a, b, c |
| UPDRS-Ⅱ | 0.0 (0.0) | 10.3 (6.7) | 9.9 (6.4) | 0 < .001a, b |
| UPDRS-Ⅳ | – | 3.4 (2.4) | 4.7 (3.2) | 0.064 |
Abbreviations: HC = healthy control; NDPD = nondepressed Parkinson's disease; DPD = depression in Parkinson's disease; UPDRS-III = Unified Parkinson’s Disease Rating Scale, motor subsection; HAMD-17 = the 17-item Hamilton Depression Rating Scale; HAMA-14 = the 14-items Hamilton Anxiety Rating Scale; MMSE = Mini-Mental State Examination; PSQI = Pittsburgh Sleep Quality Index; UPDRS-Ⅰ = Unified Parkinson’s Disease Rating Scale, mental, behavioral and emotional subsection; UPDRS-Ⅱ = Unified Parkinson’s Disease Rating Scale, daily living subsection; UPDRS-Ⅳ = Unified Parkinson’s Disease Rating Scale, treatment complications subsection.
Significant (P < 0.05) Bonferroni post hoc tests:
Control versus NDPD.
Control versus DPD.
NDPD versus DPD.
The healthy controls in the study showed no signs of neurological or psychiatric disorders, including depression. The exclusion criteria included treated with antidepressants; cognitive dysfunction, defined as Mini-Mental State Examination (MMSE) < 24; severe neuropsychiatric disorders; history of substance misuse or alcohol; history of cranial surgery, and contraindications to magnetic resonance imaging (MRI). All the participants were right-handed.
2.2. Clinical assessment
The motor symptoms in PD patients were evaluated by a trained neurologist using the Unified Parkinson's Disease Rating Scale part III (UPDRS-Ⅲ) and the Hoehn and Yahr scale. The UPDRS-III scores were evaluated in the OFF state, that is, patients abstained from consuming levodopa agonists for at least 72 h and levodopa for at least 12 h. After evaluation in the OFF state, the patients were administered the acute levodopa-test impact dose. They were re-evaluated the motor symptoms, and the ON-state UPDRS-III scores were recorded. All participants underwent a comprehensive evaluation for depression, anxiety, cognitive function, sleep quality, and other non-motor functions based on the HAMD-17, the 14-item Hamilton Anxiety Rating Scale (HAMA-14), MMSE, the Pittsburgh Sleep Quality Index (PSQI), and UPDRS-Ⅰ, Ⅱ. The UPDRS-Ⅳ was used to assess the treatment complications of PD.
2.3. MRI data acquisition
The scanning of participants was conducted using a 3.0 T MRI scanner (Ingenia Elition, Philips) equipped with a 32-channel coil. All PD patients were scanned 1–2 h after taking dopaminergic medication to make them in the same state.
Structural MRI used 3D T1-weighted imaging with sagittal acquisition (resolution 1.0 × 1.0 × 1.0 mm3, TR/TE = 7.6 ms/3.6 ms, matrix 256 × 256, field of view 256 × 256 mm2, and flip angle 8°) to obtain high-resolution brain images for the volume of interest (VOI) location and tissue segmentation.
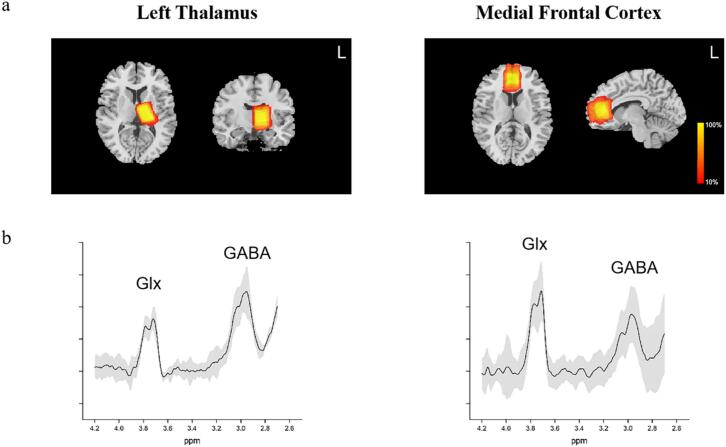
MEGA-PRESS (Mescher et al., 1998) spectra detected GABA signals from a 30 × 22 × 28 mm3 (AP×LR×FH) voxel in the left thalamus and MFC (Fig. 1a), using the following parameters: TR/TE = 2000 ms/68 ms, acquisition bandwidth = 2000 Hz, 288 averages; flip angle = 90° and scan duration 9 min 54 s. The water unsuppressed spectra were collected in the beginning and VAPOR (Variable Power and Optimized Relaxations Delays) water suppression. The shimming used the Philips MRI machine's built-in automatic shimming algorithm in the actual scan, and the shimming frame was manually adjusted to the actual head size of the patient. The placements of the MFC VOIs were parallel to the longitudinal fissure of the brain, with the sagittal lower edge aligned with the lower edge of the cingulate gyrus. The left thalamic VOIs localized the anterior edge at the posterior margin of the head of the caudate nucleus and the posterior edge at the posterior margin of the thalamus.
Fig. 1.
MEGA-PRESS spectra. Panel (a) depicts a heat plot of the MRS voxels in the left thalamus and medial prefrontal cortex. This plot was generated by normalizing all anatomical T1 scans to standard stereotactic space and calculating the overlap of regions of interest between subjects. Panel (b) depicts the standard deviations (shaded area) and the mean spectra of PD group. Abbreviations: HC = healthy control; NDPD = nondepressed Parkinson’s disease; DPD = depression in Parkinson’s disease; GABA = γ-aminobutyric acid; Glx = glutamate plus glutamine.
MEGA-PRESS is a spectral editing technique for separating and quantifying GABA. By applying 14 ms editing pulses at 1.89 ppm (often referred to as “ON”) and 7.46 ppm (often referred to as “OFF”) during the acquisition of odd and even numbers, respectively. Subtracting the OFF spectrum from the ON spectrum preserves the peaks affected by the editing pulse, resulting in the GABA signal at 3.00 ppm and the Glx signal at 3.75 ppm. Because the signal detected around 3.00 ppm includes contributions from co-edited macromolecules and homocarnosine, the total signal in this area is labelled GABA+.
MEGA-PRESS data were analyzed with the Gannet 3.1 toolkit (Edden et al., 2014, Myers et al., 2014), a MATLAB-based (The Mathworks, MA, USA) open-source software with 5 modules: (1) GannetLoad for loading and preprocessing MEGA-PRESS data; (2) GannetFit for modeling metabolite signals and providing basic quantification results and spectral quantitative information of GABA, Glx, and creatine levels. The spectrum findings were reported as the ratios of metabolites to Water and Cr. The fit error and full width at half maximum were the primary quality control measures for the spectra. Average fit error, full width at half maximum values and signal to noise ratio by subgroup are shown in Table 2. A previous study used a fitting error of 15 % as a critical value for quality control of spectral data (Wenneberg et al., 2020). Therefore, according to the references, this study used a threshold of 15 % to exclude poor quality spectra. Spectral data were excluded from the final statistical analysis if they exhibited a fit error greater than 15 % or if they were identified as outliers. The outliers were defined as values that differed from the mean by more than three standard deviations. This was done in order to ensure the reliability of the results; (3) GannetCoRegister for producing a figure showing the localization of the voxel on the structural image in three orthogonal slices; (4) GannetSegment for calling SPM12, a toolkit of MATLAB, to segment 3D T1WI for calculating tissue composition and producing cerebrospinal fluid-corrected metabolite estimates (Fig. 1b); and (5) GannetQuantify for partial volume tissue correction using the segmentation information.
Table 2.
The quality control of spectra between groups.
| Medial frontal cortex |
Left thalamus |
|||||
|---|---|---|---|---|---|---|
| HC | PD | Sig. | HC | PD | Sig. | |
| FWHM(Hz) | ||||||
| Water | 10.57(2.13) | 10.53(1.66) | 0.911 | 11.24(1.34) | 10.93(1.20) | 0.295 |
| Cr | 9.26(1.72) | 9.71(1.47) | 0.222 | 10.12(0.94) | 9.88(0.85) | 0.262 |
| Fit Error(%) | ||||||
| GABA+, Water | 9.53(2.54) | 9.91(2.59) | 0.547 | 8.67(2.13) | 7.76(2.18) | 0.084 |
| GABA+, Cr | 9.66(2.49) | 10.06(2.55) | 0.518 | 9.01(2.35) | 7.99(2.15) | 0.057 |
| Glx, Water | 7.14(1.94) | 7.70(2.39) | 0.309 | 11.62(2.25) | 11.37(2.26) | 0.644 |
| Glx, Cr | 7.31(1.90) | 7.89(2.35) | 0.284 | 11.72(2.19) | 11.52(2.25) | 0.710 |
| SNR GABA | 15.93(0.85) | 16.44(2.78) | 0.730 | 18.86(1.63) | 17.94(2.42) | 0.554 |
Abbreviations: FWHM = full width at half maximum; HC = healthy control; PD = Parkinson's disease; Cr = creatine; GABA = γ-aminobutyric acid; Glx = glutamate plus glutamine; SNR = signal to noise ratio.
The GABA+/Water and Glx/Water contents were obtained for subsequent analysis using the Gasparovic et al. (2006) method, with measurements that correct for partial volume effects that attenuate the observed water and metabolite signals. Additionally, Glx, NAA and choline use the default Gannet method for fit the quantization (Evans et al., 2013, Maria et al., 2021). Representative MR spectra of the two voxels in a Parkinson's disease patient is presented in Fig. 1c.
2.4. Dopaminergic intervention
All patients with PD were subjected to MRI scanning after dopaminergic medication. Because differences in the reports of the response to levodopa treatment on GABA content have been described (Seger et al., 2021, van Nuland et al., 2020), some PD patients in this study were subjected to MRI scanning on two separate occasions: (1) 1–2 h after taking dopaminergic medication (ON state); (2) no levodopa agonists for at least 72 h or no levodopa analogs for at least 12 h (OFF state). The MRI scanning sequences were repeated as described earlier. The time interval between scans was two days, and the VOIs were manually placed by the same investigator so that the two positions were as consistent as possible.
2.5. Statistical analysis
The Statistical Package for Social Sciences software, version 25, was used to analyze MRI data and clinical variables. P < 0.05 was used as the threshold for statistical significance. For clinical variables, independent sample t tests or analysis of variance were used for group comparisons of continuous variables, and χ2 tests were used for comparing categorical variables.
For the MRI data, the analysis was divided into 4 main sections: (1) finding differences in GABA+, Glx, creatine, NAA, and choline levels between patients with PD and healthy controls using analysis of covariance, with percentage of gray matter used as a covariate for the comparison; (2) testing whether GABA+, Glx, and other metabolites levels were influenced by depression in patients with PD using analysis of covariance and Bonferroni post hoc tests; and (3) investigating whether GABA+ and Glx levels were correlated with the severity of depression. Linear correlations between GABA+ and Glx levels and HAMD-17 scores were evaluated using Pearson correlation coefficients; and (4) the paired samples t test was used to test the influence of dopaminergic drugs on GABA+ levels.
3. Results
3.1. Demographics and clinical assessment
No significant differences in age, gender, or MMSE scores between DPD, NDPD, and controls (see Table 1). No differences in the course of the disease, UPDRS-Ⅲ (OFF and ON states), and UPDRS-Ⅱ and UPDRS-Ⅳ scales were observed between the DPD and NDPD groups. However, patients in the DPD group had higher UPDRS Ⅰ scores (P < 0.001), were more likely to be anxious according to the HAMA-14 sores (DPD > NDPD > controls) (P < 0.001), and had inferior sleep quality according to the PSQI sores (DPD > NDPD > controls) (P < 0.001). As expected, the HAMD-17 scores were significantly higher in the DPD group than in the NDPD group.
3.2. Effects of PD on metabolite levels
Few participants failed to meet the MRS quality-assurance criteria, as mentioned earlier; therefore, 92 participants (16 DPD, 53 NDPD, 23 controls) for the MFC and 95 participants (17 DPD, 55 NDPD, and 23 controls) for the left thalamus were included in the MRS analysis. There were no significant differences between excluded and non-excluded participants in terms of age, gender, or other clinical variables.
Table 3 depicts the proportions of gray matter and white matter within the MFC and the thalamus for each of the groups. The proportion of gray matter in the MFC voxel was significantly lower in PD patients than in healthy controls (P = .012). No significant differences in the proportion of gray or white matter within the left thalamus were observed between PD and healthy controls and between DPD and NDPD groups. The observed reduction in GM volume of MFC may be linked to atrophy in the frontal lobe (Burton et al., 2004) and uncertainties inherent in the manual localization of the VOI.
Table 3.
Proportion of gray and white matter within the voxel.
| HC | NDPD | DPD | Sig. | |
|---|---|---|---|---|
| Medial frontal cortex | ||||
| GM in voxel | 0.55 (0.05) | 0.50 (0.07) | 0.52 (0.06) | 0.012a |
| WM in voxel | 0.20 (0.04) | 0.20 (0.05) | 0.20 (0.05) | 0.946 |
| CSF in voxel | 0.25 (0.06) | 0.30 (0.07) | 0.28 (0.08) | 0.023a |
| Left thalamus | ||||
| GM in voxel | 0.24 (0.03) | 0.23 (0.03) | 0.22 (0.03) | 0.335 |
| WM in voxel | 0.58 (0.05) | 0.58 (0.05) | 0.60 (0.04) | 0.421 |
| CSF in voxel | 0.18 (0.05) | 0.19 (0.06) | 0.18 (0.03) | 0.432 |
Abbreviations: HC = healthy control; NDPD = nondepressed Parkinson's disease; DPD = depression in Parkinson's disease; GM = gray matter; WM = white matter; CSF = cerebrospinal fluid.
Significant (P < 0.05) Bonferroni post hoc tests:
Control versus NDPD.
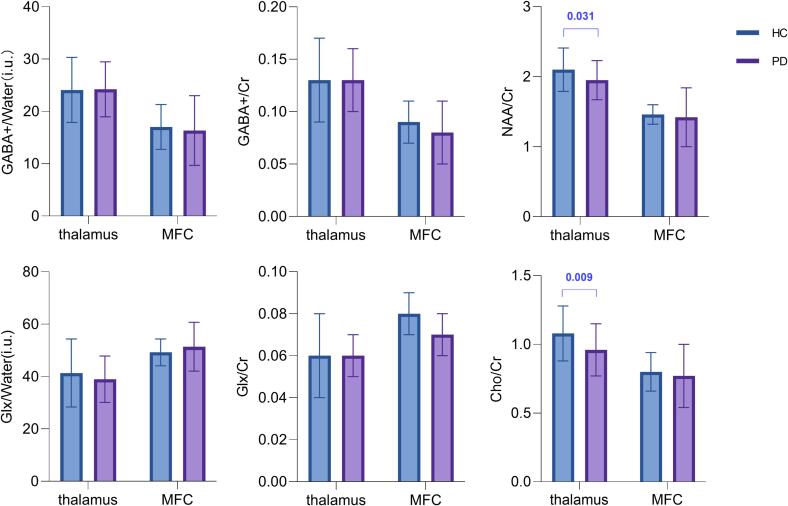
In order to exclude the effect caused by the percentage of gray matter, an analysis of covariance was performed, in which the percentage of gray matter was used as a covariate for the comparison between the two groups. The levels of NAA/creatine and choline/creatine in the left thalamus were considerably lower in the PD group than in the control group (P = .031, P = .009), see Fig. 2. However, no considerable differences in GABA+, Glx, and creatine values in the left thalamus were observed between the PD and control groups. No substantial differences in the concentrations of metabolites, including GABA+, in the MFC were observed between patients and controls (Fig. 2), and that the effect of the covariate, the percentage of gray matter in the MFC, was also not significant (P > 0.05).
Fig. 2.
Metabolites, including GABA+, change in Parkinson’s disease (PD), demonstrating the GABA+, Glx, NAA, and Cho levels in patients with PD and healthy controls. Only the levels of NAA/Cr and Cho/Cr in the left thalamus were significantly lower in patients with PD than in HC (P = .031, P = .009); others had no difference. Abbreviations: GABA = γ-aminobutyric acid; Glx = glutamate plus glutamine; Cr = creatine; NAA = N-acetyl aspartate; Cho = Choline; MFC = medial prefrontal cortex; HC = healthy controls; PD = Parkinson's disease.
3.3. Depressive symptoms effects on GABAergic in PD
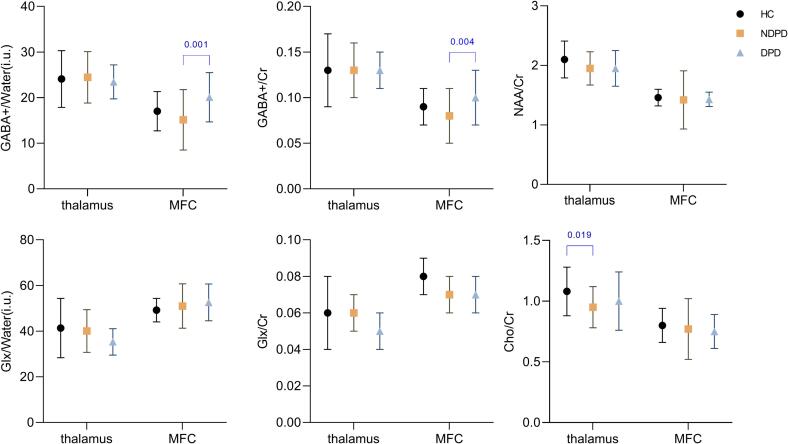
The results of metabolite comparisons among all three groups of DPD, NDPD, and healthy controls are shown in Fig. 3. Because there were differences in HAMA and PSQI scores among the three groups and to exclude potential effects due to the percentage of gray matter, an analysis of covariance was used to compare the difference of metabolite contents, with the percentage of gray matter, HAMA and PSQI scores used as covariates. The results showed that GABA+/Water and GABA+/Creatine in MFC differed among the three groups (P = .002, P = .003), and Bonferroni post hoc test showed that GABA+/Water and GABA+/Creatine content in MFC were significantly higher in the DPD group than in the NDPD group (P = .001, P = .004). Cho/creatine in the left thalamus was statistically different among the three groups (P = .022), and Bonferroni post hoc test showed that the NDPD patients have lower Cho/creatine contents in the left thalamus than that in the healthy control group (P = .019), which was mainly due to the differences between the PD and control groups. Furthermore, no impact of depressive symptoms on other metabolite levels was observed (P > .05). Metabolites referenced to water using the method of Harris et al (with alpha correction, with and without normalization) demonstrated similar outcomes, as shown in Supplementary Tables 3, 4, 6 and 7.
Fig. 3.
Comparison of metabolites between DPD, NDPD and healthy control group. The GABA+/Water and GABA+/Cr of MFC were disparate according to the analysis of covariance (P = .002, P = .003), with post hoc Bonferroni test revealing that GABA+/Water and GABA+/Cr increased in DPD relative to NDPD (P = .001, P = .004). Cho/Cr levels in the thalamus were higher in the healthy control group than in the NDPD group (P = .019). Abbreviations: GABA = γ-aminobutyric acid; Glx = glutamate plus glutamine; Cr = creatine; NAA = N-acetyl aspartate; Cho = Choline; MFC = medial prefrontal cortex; HC = healthy control; NDPD = nondepressed Parkinson’s disease; DPD = depression in Parkinson’s disease.
3.4. Dopaminergic medication effects on GABAergic in PD
Fifteen patients with PD were included in this part of MRS analysis. There were no significant differences in the proportion of gray or white matter within the voxels between the two separate occasions, and no effect of medication was noted on GABA+, Glx, creatine, NAA, or choline values. This result might indicate that the dopaminergic medication had no effect on the GABA+ and Glx levels.
3.5. Correlations between the severity of emotional disorder and metabolite levels
The Pearson correlation analysis of GABA+ or Glx and emotional disorder severity (HAMD-17 and HAMA-14 scores) in both DPD group and all patients with PD revealed no correlation between metabolite levels and HAMD-17 or HAMA-14 scores. Furthermore, no substantial correlations between GABA+ or Glx and age, disease duration, UPDRS, MMSE, and PSQI scores were observed in patients with PD.
4. Discussion
In this quest for new insights into the pathogenesis and potential treatment strategies for PD with co-occurring depression, we examined the differences of GABA+ values in the MFC and thalamus between PD patients and healthy controls. We also examined whether the GABA+ levels were affected by depressive symptoms and dopaminergic medications in PD patients. We hypothesized that PD with depression had lower GABA+ levels in the MFC than healthy controls. In addition, we also explored whether thalamus GABA+ levels were altered in DPD patients because of the limbic–cortical–striatal–pallidal–thalamic circuit, expecting that GABA+ levels would predict the severity of emotional disorder symptoms, particularly depression.
In this study, the concentrations and ratios of GABA+ and Glx obtained by us are close to the data reported in several previous studies in this area (Firbank et al., 2018, van Nuland et al., 2020), which to some extent proves the reliability of our results. We found that the levels of NAA and choline were noted to be lower in the left thalamus in patients with PD than in healthy controls. However, no difference in the GABA+ and Glx values was observed between patients with PD and healthy controls. Contrary to our predictions, we noted higher (not lower) GABA+ levels in the MFC in patients with DPD than in those with NDPD. The effects of depression on Glx and thalamus GABA+ levels or the influence of dopaminergic medication on GABA+ levels were also not evident. These results implied that GABA had a regulatory function in the development of PD symptoms that were unrelated to dopaminergic medication.
4.1. Depression in PD was associated with increased GABA+ levels in the MFC
Contrary to our initial hypothesis, we could not confirm the reduction in GABA+ levels in DPD. GABA+ levels were higher in patients with DPD than in those with NDPD, whereas Glx values revealed no difference between the 2 populations. These findings supported the hypothesis that depression stemmed from an imbalance in the overall excitation–inhibition, which might be caused by the changes in either GABA, glutamate, or both. An imbalance between excitation and inhibition, particularly in the prefrontal regions, has been linked to a change in the mental focus from external to internal environments, which is a hallmark feature of major depressive symptoms (Lemogne et al., 2010). Higher levels of GABA in the MFC of patients with DPD suggested decreased neural activity, leading to increased inhibition and, ultimately, suppressed excitatory neurotransmission. However, the levels of Glx, an excitatory neurotransmitter, were not altered correspondingly, causing a disturbance in the balance between excitation and inhibition. This disruption contributed to the depressive symptoms experienced by patients with PD.
Our findings agreed with those of previous functional MRI research, which reported widespread functional connection abnormalities in the brain of patients with DPD and decreased connections in interacting brain regions at the whole-brain level (Assogna et al., 2020, Guo et al., 2020). Increased GABA levels within the MFC led to tonic inhibition of GABA-mediated glutaminergic projections, thus promoting decreased activation and functional connections. The frontal cortex facilitates the integration and evaluation of multimodal information and decision-making (Huang et al., 2016), and damage to this region can severely affect an individual’s social behavior and emotion processing (Bechara et al., 2000).
The findings of this study were contrary to previous findings of reduced GABA+ in the frontal cortex of depressed patients (Romeo et al., 2018). It is possible that this difference was due to the differences in the participants studied. Depression was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders in previous studies. In contrast, the diagnosis of depression in this study was based on the HAMA in this study, which focused on the depressive symptoms. The depressive symptoms of patients with DPD were milder in this study compared with previous studies. The GABAergic changes may differ in various stages of depression. In addition, this difference might be attributed to the unique pathophysiology of depression in the context of PD, which could differ from general depression. Neurochemical alterations in DPD could be a compensatory mechanism or a result of the interaction between PD pathology and depressive symptoms. This finding challenges the conventional understanding and suggests the need for further research into the specific neurobiologic mechanisms of DPD and following the development of GABAergic function in DPD.
4.2. Is GABA+ level in MFC and thalamus associated with PD?
In this study, no substantial abnormalities in GABA+ and Glx levels were found in PD patients compared to healthy controls. Our findings contradicted the notion that alterations in GABA levels were linked with PD. The discrepancies between our results and those of others might be attributed to different sample sizes or methodologic or other factors. First, the validity of the results was unlikely to be compromised by a small sample size. Takashima et al. (2022) using 11C-FMZ PET imaging, reported a reduction in the GABAergic binding potential in the striatum and frontal cortical areas of patients with PD, but their study included only 13 patients (compared with 78 patients in this study). Dharmadhikari et al. (2015) demonstrated increased GABA+ levels in the thalamus of only 19 PD patients. However, our results were consistent with those of previous studies with large sample sizes. For example, Delli et al. (2020) demonstrated no difference in the GABA levels in the MFC between 42 patients with PD and healthy controls. Nuland revealed no alterations in GABA+ levels in 60 patients with PD (van Nuland et al., 2020). Second, previous researches used either a GABA-to-water ratio or a GABA-to-creatine ratio. The majority of scholars concur that creatine is a more stable metabolite in brain tissue, and the majority of studies have confirmed that creatine concentration is largely unaltered in the basal ganglia region of patients with PD (Kickler et al., 2007, Weiduschat et al., 2015). However, some studies have suggested that creatine concentrations in PD may alter (Gong et al., 2018). Therefore, we investigated both GABA+/Water and GABA+/Creatine, finding no major discrepancies in the outcomes from the two approaches. Third, two distinct methodologies were employed for the quantification of GABA+ and Glx in the Gannet 3.1 toolkit. The outcomes of the Gasparovic et al. (2006) method are presented in this paper, while the Harris et al. (2015) method is detailed in the Supplementary Material. Both methods yielded comparable results, further corroborating the reliability of the findings. Fourth, although all patients with PD were scanned after dopaminergic medication administration, some of these patients had another MRI scanning in the OFF state. No difference in the GABA levels was observed between the 2 states, which was in line with the previous study, in which the GABA+ levels were unaltered by dopaminergic medication (van Nuland et al., 2020). Therefore, the impact of dopaminergic medication on GABA levels could be partially eliminated. Fourth, our study included patients with PD in the early or middle stages of the disease. It was possible that the diffusion of alpha-synuclein pathology, a protein implicated in GABAergic function, had not reached remarkable levels in the thalamus and MFC in these stages.
In addition, we observed gray matter loss in the MFC voxel in patients with PD, which may result from frontal lobe atrophy and uncertainties associated with manual localization of the VOI. Potgieser et al.'s study found reduced gray matter in PD's bilateral anterior temporal lobes and left inferior frontal gyrus (Potgieser et al., 2014).
In conclusion, our findings contradicted the notion that alterations in GABA levels were linked to PD. Therefore, the observed changes in inhibitory neurotransmission may be more closely related to depressive symptoms. We also speculated that alterations in GABAergic function in specific structures might be related to the clinical symptoms of PD. This notion was consistent with the research demonstrating that patients with somatic symptoms, with or without PD, had higher levels of GABA+/creatine in the MFC than in PD patients without a somatic symptom disorder (Delli et al., 2020).
4.3. Decreased NAA and choline levels in the thalamus in PD
We observed lower NAA and choline levels in the left thalamus of patients with PD compared with controls, aligning with studies demonstrating reduced NAA/creatine in the substantia nigra and tegmentum of patients with PD (Gröger et al., 2011). Lower NAA level, a marker of neuronal integrity suggests thalamic neuronal loss or dysfunction in PD. Choline, one component of phospholipid metabolism in cell membranes, participates in the synthesis and disintegration of cell membranes. Thus, the choline peak reflects the renewal of cell membranes, and reduced choline may indicate slowed cell membrane renewal. These reductions reflect PD’s neurodegenerative impact on thalamic function.
4.4. Limitations
Our study's limitations included a large VOI for GABA+ detection, inevitably encompassing other structures, such as the internal capsule and caudate nucleus in the thalamus and the corpus callosum in the MFC, with resultant inability to accurately measure GABA+ levels in the desired area. This was necessary due to low GABA levels in the brain, needing a larger volume for enhanced signal-to-noise ratio of the spectrum. Additionally, the MEGA-PRESS-detected GABA+ signal included macromolecules and homocarnosine, complicating “pure” GABA detection. No evidence suggested altered macromolecule or homocarnosine levels in patients with PD, but their influence on our results could not be ruled out. All patients with PD were scanned post-dopaminergic medication. Also, although the impact of dopaminergic medication on GABAergic values is debated, no substantial differences in GABA+ levels were noted between pre- and post-dopaminergic medication scans. This aligned with larger studies (van Nuland et al., 2020), enhancing the reliability of our findings.
5. Conclusion
GABA+ levels are increased in the MFC of patients with DPD, a phenomenon that appears to be more closely related to depressive pathology. This suggests a specific role for the GABAergic system in PD-related depression. We propose that GABAergic activity alterations in certain neuroanatomical structures are related to the clinical manifestations of PD symptoms. Targeting the GABAergic system can offer a new therapeutic strategy, particularly for PD depression resistant to standard antidepressants. Future studies combining 1H-MRS and functional MRI are needed to explore how GABAergic modulation affects neural circuit function. These studies can provide comprehensive insights into the role of the GABAergic system in PD and its depression. Additionally, longitudinal studies are crucial to understand how GABA+ levels change over time and their impact on the development and progression of depressive symptoms in PD, guiding effective therapeutic interventions.
Funding statement
This work was supported by grants from National Natural Science Foundation of China (U21A6005).
CRediT authorship contribution statement
Xinzi Liu: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yuxin Li: Formal analysis, Data curation. Yixiang Mo: Formal analysis, Data curation. Baoling Chen: Data curation. Xusheng Hou: Data curation. Jianbin Zhu: Writing – review & editing. Yongzhou Xu: Software. Jingyue Xue: Data curation. Haitao Wen: Writing – review & editing, Data curation. Xianlong Wang: Data curation. Zhibo Wen: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank all volunteers and patients for their participation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103641.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- Assogna F., Pellicano C., Savini C., Macchiusi L., Pellicano G.R., Alborghetti M., Caltagirone C., Spalletta G., Pontieri F.E. Drug choices and advancements for managing depression in Parkinson's disease. Curr. Neuropharmacol. 2020;18:277–287. doi: 10.2174/1570159X17666191016094857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanger B., Klinger H., Eche J., Lerond J., Vallet A.E., Le Bars D., Tremblay L., Sgambato-Faure V., Broussolle E., Thobois S. Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson's disease. Mov. Disord. 2012;27:84–89. doi: 10.1002/mds.23895. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Brown R.G., Landau S., Hindle J.V., Playfer J., Samuel M., Wilson K.C., Hurt C.S., Anderson R.J., Carnell J., Dickinson L., Gibson G., van Schaick R., Sellwood K., Thomas B.A., Burn D.J. Depression and anxiety related subtypes in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2011;82:803–809. doi: 10.1136/jnnp.2010.213652. [DOI] [PubMed] [Google Scholar]
- Burn D.J., Landau S., Hindle J.V., Samuel M., Wilson K.C., Hurt C.S., Brown R.G. Parkinson's disease motor subtypes and mood. Mov. Disord. 2012;27:379–386. doi: 10.1002/mds.24041. [DOI] [PubMed] [Google Scholar]
- Burton E.J., McKeith I.G., Burn D.J., Williams E.D., O'Brien J.T. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Cardoso E.F., Maia F.M., Fregni F., Myczkowski M.L., Melo L.M., Sato J.R., Marcolin M.A., Rigonatti S.P., Cruz A.J., Barbosa E.R., Amaro E.J. Depression in Parkinson's disease: convergence from voxel-based morphometry and functional magnetic resonance imaging in the limbic thalamus. Neuroimage. 2009;47:467–472. doi: 10.1016/j.neuroimage.2009.04.059. [DOI] [PubMed] [Google Scholar]
- Delli P.S., Franciotti R., Ferretti A., Edden R., Zöllner H.J., Esposito R., Bubbico G., Aiello C., Calvanese F., Sensi S.L., Tartaro A., Onofrj M., Bonanni L. High γ-aminobutyric acid content within the medial prefrontal cortex is a functional signature of somatic symptoms disorder in patients with Parkinson's disease. Mov. Disord. 2020;35:2184–2192. doi: 10.1002/mds.28221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis T., Beauchemin V., Lavoie N. Differential effects of olfactory bulbectomy on GABAA and GABAB receptors in the rat brain. Pharmacol. Biochem. Behav. 1993;46:77–82. doi: 10.1016/0091-3057(93)90320-s. [DOI] [PubMed] [Google Scholar]
- Dharmadhikari S., Ma R., Yeh C.L., Stock A.K., Snyder S., Zauber S.E., Dydak U., Beste C. Striatal and thalamic GABA level concentrations play differential roles for the modulation of response selection processes by proprioceptive information. Neuroimage. 2015;120:36–42. doi: 10.1016/j.neuroimage.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden R.A., Puts N.A., Harris A.D., Barker P.B., Evans C.J. Gannet: a batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J. Magn. Reson. Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.J., Puts N.A., Robson S.E., Boy F., McGonigle D.J., Sumner P., Singh K.D., Edden R.A. Subtraction artifacts and frequency (mis-)alignment in J-difference GABA editing. J. Magn. Reson. Imaging. 2013;38:970–975. doi: 10.1002/jmri.23923. [DOI] [PubMed] [Google Scholar]
- Firbank M.J., Parikh J., Murphy N., Killen A., Allan C.L., Collerton D., Blamire A.M., Taylor J.P. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology. 2018;91:e675–e685. doi: 10.1212/WNL.0000000000006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça M.V., Duman R.S. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front. Cell. Neurosci. 2019;13:87. doi: 10.3389/fncel.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina P.G., Haroutunian V., Libow L.S. The neuropathological basis for depression in Parkinson's disease. Parkinsonism Relat. Disord. 2009;15:144–148. doi: 10.1016/j.parkreldis.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C., Song T., Devier D., Bockholt H.J., Caprihan A., Mullins P.G., Posse S., Jung R.E., Morrison L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Gerner R.H., Hare T.A. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am. J. Psychiatry. 1981;138:1098–1101. doi: 10.1176/ajp.138.8.1098. [DOI] [PubMed] [Google Scholar]
- Gong T., Xiang Y., Saleh M.G., Gao F., Chen W., Edden R., Wang G. Inhibitory motor dysfunction in parkinson's disease subtypes. J. Magn. Reson. Imaging. 2018;47:1610–1615. doi: 10.1002/jmri.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröger A., Chadzynski G., Godau J., Berg D., Klose U. Three-dimensional magnetic resonance spectroscopic imaging in the substantia nigra of healthy controls and patients with Parkinson's disease. Eur. Radiol. 2011;21:1962–1969. doi: 10.1007/s00330-011-2123-5. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H., Silber C., Kaul I., Rothschild A.J., Riesenberg R., Sankoh A.J., Li H., Lasser R., Zorumski C.F., Rubinow D.R., Paul S.M., Jonas J., Doherty J.J., Kanes S.J. Trial of SAGE-217 in patients with major depressive disorder. N. Engl. J. Med. 2019;381:903–911. doi: 10.1056/NEJMoa1815981. [DOI] [PubMed] [Google Scholar]
- Guo T., Guan X., Zhou C., Gao T., Wu J., Song Z., Xuan M., Gu Q., Huang P., Pu J., Zhang B., Cui F., Xia S., Xu X., Zhang M. Clinically relevant connectivity features define three subtypes of Parkinson's disease patients. Hum. Brain Mapp. 2020;41:4077–4092. doi: 10.1002/hbm.25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A.D., Puts N.A., Edden R.A. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging. 2015;42:1431–1440. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P., Lou Y., Xuan M., Gu Q., Guan X., Xu X., Song Z., Luo W., Zhang M. Cortical abnormalities in Parkinson's disease patients and relationship to depression: a surface-based morphometry study. Psychiatry Res.-Neuroimag. 2016;250:24–28. doi: 10.1016/j.pscychresns.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Kantrowitz J.T., Dong Z., Milak M.S., Rashid R., Kegeles L.S., Javitt D.C., Lieberman J.A., John M.J. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder. Transl. Psychiatry. 2021;11:419. doi: 10.1038/s41398-021-01541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickler N., Krack P., Fraix V., Lebas J.F., Lamalle L., Durif F., Krainik A., Rémy C., Segebarth C., Pollak P. Glutamate measurement in Parkinson's disease using MRS at 3 T field strength. NMR Biomed. 2007;20:757–762. doi: 10.1002/nbm.1141. [DOI] [PubMed] [Google Scholar]
- Kostic V.S., Agosta F., Pievani M., Stefanova E., Jecmenica-Lukic M., Scarale A., Spica V., Filippi M. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- Lachner C., Armstrong M.J., Gruber-Baldini A.L., Rezvani Z., Reich S.G., Fishman P.S., Salazar R., Shulman L.M. Discordance between physician assessment and patient-reported depressive symptoms in Parkinson disease. J. Geriatric Psychiatry Neurol. 2017;30:191–195. doi: 10.1177/0891988717710335. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Mayberg H., Bergouignan L., Volle E., Delaveau P., Lehéricy S., Allilaire J.F., Fossati P. Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J. Affect. Disord. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Maria Y.L., Price A.N., Puts N., Hughes E.J., Edden R., McAlonan G.M., Arichi T., De Vita E. Simultaneous quantification of GABA, Glx and GSH in the neonatal human brain using magnetic resonance spectroscopy. Neuroimage. 2021;233 doi: 10.1016/j.neuroimage.2021.117930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M., Merkle H., Kirsch J., Garwood M., Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Myers J.F., Evans C.J., Kalk N.J., Edden R.A., Lingford-Hughes A.R. Measurement of GABA using J-difference edited 1H-MRS following modulation of synaptic GABA concentration with tiagabine. Synapse. 2014;68:355–362. doi: 10.1002/syn.21747. [DOI] [PubMed] [Google Scholar]
- Petty F. Plasma concentrations of gamma-aminobutyric acid (GABA) and mood disorders: a blood test for manic depressive disease? Clin. Chem. 1994;40:296–302. [PubMed] [Google Scholar]
- Petty F., Kramer G.L., Fulton M., Davis L., Rush A.J. Stability of plasma GABA at four-year follow-up in patients with primary unipolar depression. Biol. Psychiatry. 1995;37:806–810. doi: 10.1016/0006-3223(94)00226-S. [DOI] [PubMed] [Google Scholar]
- Potgieser A.R., van der Hoorn A., Meppelink A.M., Teune L.K., Koerts J., de Jong B.M. Anterior temporal atrophy and posterior progression in patients with Parkinson's disease. Neurodegener. Dis. 2014;14:125–132. doi: 10.1159/000363245. [DOI] [PubMed] [Google Scholar]
- Reijnders J.S., Ehrt U., Weber W.E., Aarsland D., Leentjens A.F. A systematic review of prevalence studies of depression in Parkinson's disease. Mov. Disord. 2008;23(183–189):313. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- Remy P., Doder M., Lees A., Turjanski N., Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Romeo B., Choucha W., Fossati P., Rotge J.Y. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression. J. Psychiatry Neurosci. 2018;43:58–66. doi: 10.1503/jpn.160228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G., Gueorguieva R., Epperson C.N., Wu Y.T., Appel M., Rothman D.L., Krystal J.H., Mason G.F. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Schapira A., Chaudhuri K.R., Jenner P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- Schür R.R., Draisma L.W., Wijnen J.P., Boks M.P., Koevoets M.G., Joëls M., Klomp D.W., Kahn R.S., Vinkers C.H. Brain GABA levels across psychiatric disorders: a systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016;37:3337–3352. doi: 10.1002/hbm.23244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger A.D., Farrher E., Doppler C., Gogishvili A., Worthoff W.A., Filss C.P., Barbe M.T., Holtbernd F., Shah N.J., Fink G.R., Sommerauer M. Putaminal y-aminobutyric acid modulates motor response to dopaminergic therapy in Parkinson's disease. Mov. Disord. 2021;36:2187–2192. doi: 10.1002/mds.28674. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I. Neuroimaging studies of mood disorder effects on the brain. Biol. Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Takashima H., Terada T., Bunai T., Matsudaira T., Obi T., Ouchi Y. In vivo illustration of altered dopaminergic and GABAergic systems in early Parkinson's disease. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.880407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkelsen M.H., Hvingelby V.S., Pavese N. Molecular imaging of the GABAergic system in Parkinson's disease and atypical parkinsonisms. Curr. Neurol. Neurosci. Rep. 2022;22:867–879. doi: 10.1007/s11910-022-01245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nuland A., den Ouden H., Zach H., Dirkx M., van Asten J., Scheenen T., Toni I., Cools R., Helmich R.C. GABAergic changes in the thalamocortical circuit in Parkinson's disease. Hum. Brain Mapp. 2020;41:1017–1029. doi: 10.1002/hbm.24857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend C., Raijmakers P., Veltman D.J., van Dijk K.D., van der Werf Y.D., Foncke E.M., Smit J.H., Berendse H.W., van den Heuvel O.A. Depressive symptoms in Parkinson's disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J. Neurol. Neurosurg. Psychiatry. 2014;85:159–164. doi: 10.1136/jnnp-2012-304811. [DOI] [PubMed] [Google Scholar]
- Weiduschat N., Mao X., Beal M.F., Nirenberg M.J., Shungu D.C., Henchcliffe C. Usefulness of proton and phosphorus MR spectroscopic imaging for early diagnosis of Parkinson's disease. J. Neuroimaging. 2015;25:105–110. doi: 10.1111/jon.12074. [DOI] [PubMed] [Google Scholar]
- Wenneberg C., Nordentoft M., Rostrup E., Glenthøj L.B., Bojesen K.B., Fagerlund B., Hjorthøj C., Krakauer K., Kristensen T.D., Schwartz C., Edden R., Broberg B.V., Glenthøj B.Y. Cerebral glutamate and gamma-aminobutyric acid levels in individuals at ultra-high risk for psychosis and the association with clinical symptoms and cognition. Biol. Psychiatry-Cogn. Neurosci. Neuroimaging. 2020;5:569–579. doi: 10.1016/j.bpsc.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Zeng Z., Dong Y., Zou L., Xu D., Luo X., Chu T., Wang J., Ren Q., Liu Q., Li X. GluCEST imaging and structural alterations of the bilateral hippocampus in first-episode and early-onset major depression disorder. J. Magn. Reson. Imaging. 2023;58:1431–1440. doi: 10.1002/jmri.28651. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Ai H., Van Dam N.T., Qian L., Hou G., Xu P. Microstructural deficits of the thalamus in major depressive disorder. Brain Commun. 2022;4 doi: 10.1093/braincomms/fcac236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.