Abstract
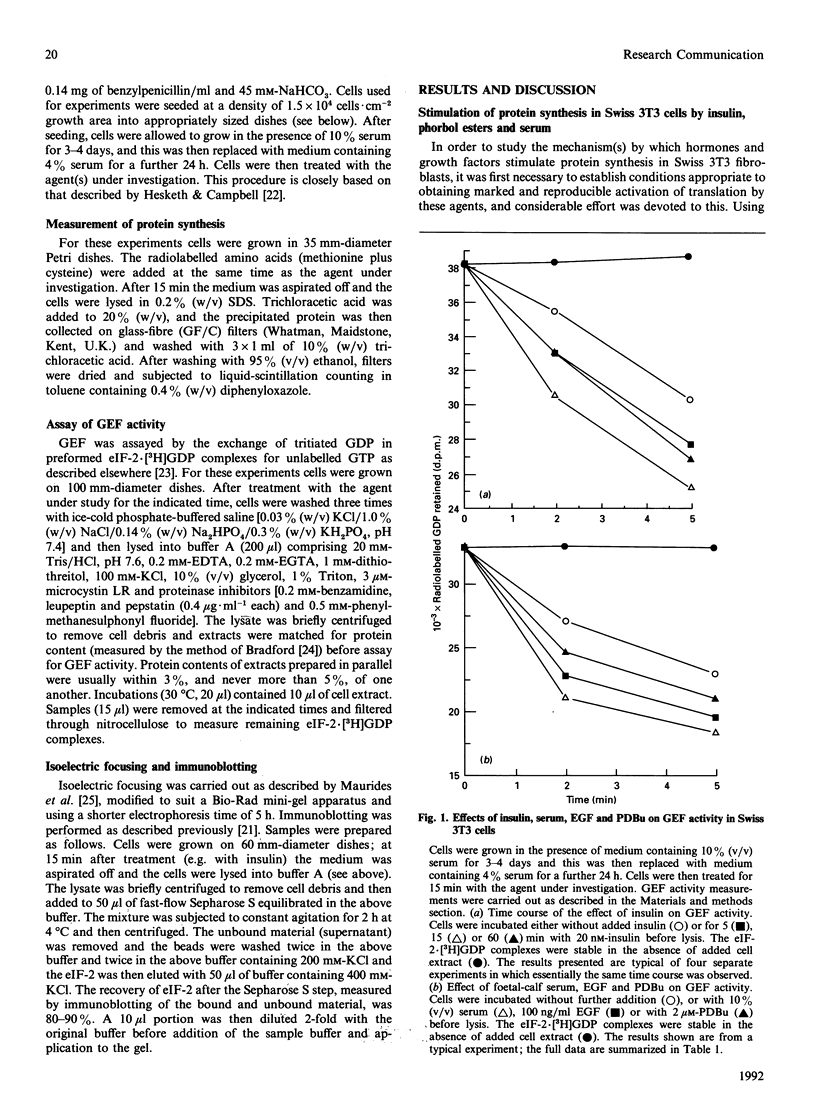
Insulin, whole serum, phorbol esters and epidermal growth factor each rapidly stimulate protein synthesis in serum-depleted Swiss 3T3 fibroblasts. The activation of protein synthesis by each of these agents is associated with stimulation of the activity of the guanine-nucleotide-exchange factor (GEF). This protein recycles the initiation factor eIF-2 by promoting exchange of GDP bound to eIF-2 for GTP. Activation of GEF is rapid, becoming maximal within 15 min. The degree of activation of GEF by these stimuli (to greater than 170% of control for insulin, serum or epidermal growth factor; 120% for phorbol dibutyrate) is more than enough to account for their effects on the overall rate of translation. Stimulation of protein synthesis and GEF activity occurs at low nanomolar insulin concentrations, indicating they are mediated through the insulin receptor. The best-characterized mechanism for regulating GEF activity is through changes in the phosphorylation of the smallest subunit of eIF-2 (eIF-2 alpha); however, none of the stimuli studied altered the level of phosphorylation of eIF-2 alpha in Swiss fibroblasts. It seems that direct regulation of GEF activity may be occurring here, and possible mechanisms for this are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990 Jan;87(2):821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Clemens M. J., Galpine A., Austin S. A., Panniers R., Henshaw E. C., Duncan R., Hershey J. W., Pollard J. W. Regulation of polypeptide chain initiation in Chinese hamster ovary cells with a temperature-sensitive leucyl-tRNA synthetase. Changes in phosphorylation of initiation factor eIF-2 and in the activity of the guanine nucleotide exchange factor GEF. J Biol Chem. 1987 Jan 15;262(2):767–771. [PubMed] [Google Scholar]
- Corps A. N., Brown K. D. Ligand-receptor interactions involved in the stimulation of Swiss 3T3 fibroblasts by insulin-like growth factors and insulin. Biochem J. 1988 May 15;252(1):119–125. doi: 10.1042/bj2520119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Activation of hemin-regulated initiation factor-2 kinase in heat-shocked HeLa cells. J Biol Chem. 1986 Jan 5;261(1):338–342. [PubMed] [Google Scholar]
- Dholakia J. N., Mueser T. C., Woodley C. L., Parkhurst L. J., Wahba A. J. The association of NADPH with the guanine nucleotide exchange factor from rabbit reticulocytes: a role of pyridine dinucleotides in eukaryotic polypeptide chain initiation. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6746–6750. doi: 10.1073/pnas.83.18.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dholakia J. N., Wahba A. J. Phosphorylation of the guanine nucleotide exchange factor from rabbit reticulocytes regulates its activity in polypeptide chain initiation. Proc Natl Acad Sci U S A. 1988 Jan;85(1):51–54. doi: 10.1073/pnas.85.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Rapid alterations in initiation rate and recruitment of inactive RNA are temporally correlated with S6 phosphorylation. Eur J Biochem. 1982 Apr;123(3):539–544. doi: 10.1111/j.1432-1033.1982.tb06565.x. [DOI] [PubMed] [Google Scholar]
- Everson W. V., Flaim K. E., Susco D. M., Kimball S. R., Jefferson L. S. Effect of amino acid deprivation on initiation of protein synthesis in rat hepatocytes. Am J Physiol. 1989 Jan;256(1 Pt 1):C18–C27. doi: 10.1152/ajpcell.1989.256.1.C18. [DOI] [PubMed] [Google Scholar]
- Gross M., Rubino M. S., Starn T. K. Regulation of protein synthesis in rabbit reticulocyte lysate. Glucose 6-phosphate is required to maintain the activity of eukaryotic initiation factor (eIF)-2B by a mechanism that is independent of the phosphorylation of eIF-2 alpha. J Biol Chem. 1988 Sep 5;263(25):12486–12492. [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Hesketh J. E., Campbell G. P. Effects of insulin, pertussis toxin and cholera toxin on protein synthesis and diacylglycerol production in 3T3 fibroblasts: evidence for a G-protein mediated activation of phospholipase C in the insulin signal mechanism. Biosci Rep. 1987 Jul;7(7):533–541. doi: 10.1007/BF01119769. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Jeffrey I. W., Kelly F. J., Duncan R., Hershey J. W., Pain V. M. Effect of starvation and diabetes on the activity of the eukaryotic initiation factor eIF-2 in rat skeletal muscle. Biochimie. 1990 Oct;72(10):751–757. doi: 10.1016/0300-9084(90)90160-i. [DOI] [PubMed] [Google Scholar]
- Kelly F. J., Jefferson L. S. Control of peptide-chain initiation in rat skeletal muscle. Development of methods for preparation of native ribosomal subunits and analysis of the effect of insulin on formation of 40 S initiation complexes. J Biol Chem. 1985 Jun 10;260(11):6677–6683. [PubMed] [Google Scholar]
- Kimball S. R., Antonetti D. A., Brawley R. M., Jefferson L. S. Mechanism of inhibition of peptide chain initiation by amino acid deprivation in perfused rat liver. Regulation involving inhibition of eukaryotic initiation factor 2 alpha phosphatase activity. J Biol Chem. 1991 Jan 25;266(3):1969–1976. [PubMed] [Google Scholar]
- Kimball S. R., Everson W. V., Flaim K. E., Jefferson L. S. Initiation of protein synthesis in a cell-free system prepared from rat hepatocytes. Am J Physiol. 1989 Jan;256(1 Pt 1):C28–C34. doi: 10.1152/ajpcell.1989.256.1.C28. [DOI] [PubMed] [Google Scholar]
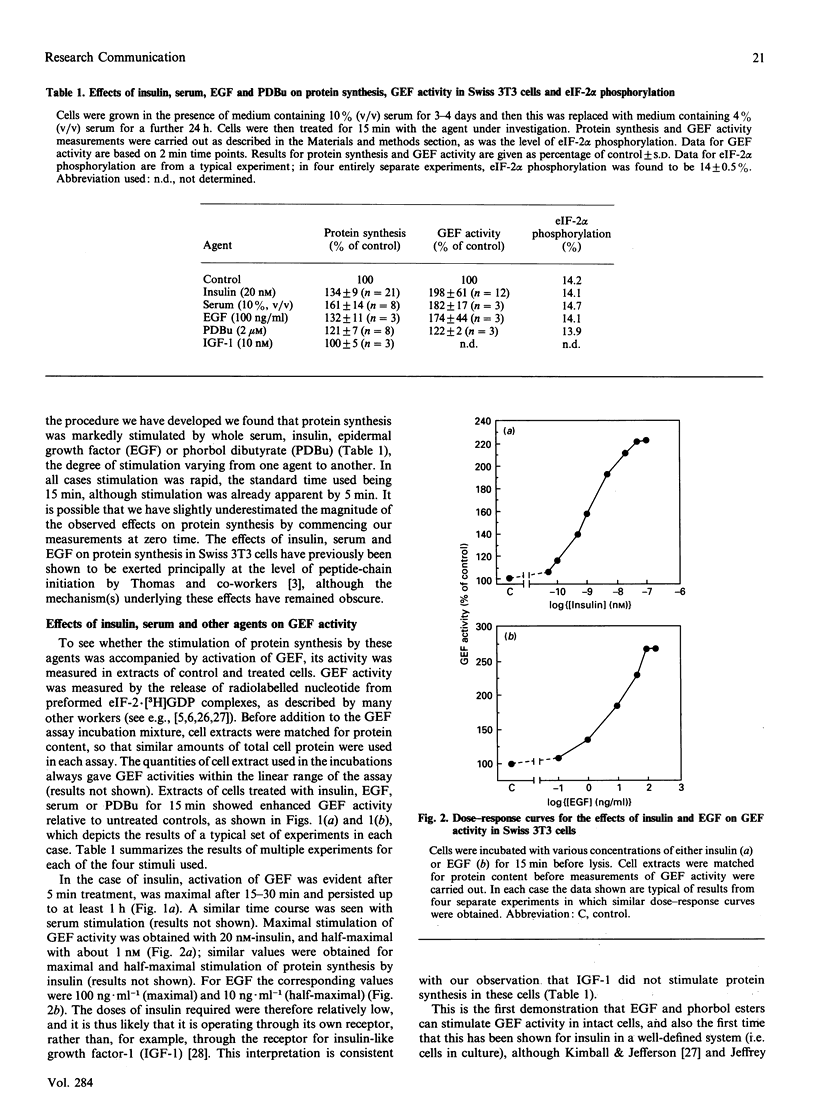
- Kimball S. R., Jefferson L. S. Effect of diabetes on guanine nucleotide exchange factor activity in skeletal muscle and heart. Biochem Biophys Res Commun. 1988 Oct 31;156(2):706–711. doi: 10.1016/s0006-291x(88)80900-8. [DOI] [PubMed] [Google Scholar]
- Lyons R. T., Nordeen S. K., Young D. A. Effects of fasting and insulin administration on polyribosome formation in rat epididymal fat cells. J Biol Chem. 1980 Jul 10;255(13):6330–6334. [PubMed] [Google Scholar]
- Maurides P. A., Akkaraju G. R., Jagus R. Evaluation of protein phosphorylation state by a combination of vertical slab gel isoelectric focusing and immunoblotting. Anal Biochem. 1989 Nov 15;183(1):144–151. doi: 10.1016/0003-2697(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Mehta H. B., Woodley C. L., Wahba A. J. Protein synthesis in brine shrimp embryos and rabbit reticulocytes. The effect of Mg2+ on binary (eukaryotic initiation factor 2 X GDP) and ternary (eukaryotic initiation factor 2 X GTP X met-tRNAf) complex formation. J Biol Chem. 1983 Mar 25;258(6):3438–3441. [PubMed] [Google Scholar]
- Montine K. S., Henshaw E. C. Serum growth factors cause rapid stimulation of protein synthesis and dephosphorylation of eIF-2 in serum deprived Ehrlich cells. Biochim Biophys Acta. 1989 Dec 14;1014(3):282–288. doi: 10.1016/0167-4889(89)90224-3. [DOI] [PubMed] [Google Scholar]
- Price N. T., Nakielny S. F., Clark S. J., Proud C. G. The two forms of the beta-subunit of initiation factor-2 from reticulocyte lysates arise from proteolytic degradation. Biochim Biophys Acta. 1989 Jul 7;1008(2):177–182. doi: 10.1016/0167-4781(80)90005-6. [DOI] [PubMed] [Google Scholar]
- Price N. T., Welsh G. I., Proud C. G. Phosphorylation of only serine-51 in protein synthesis initiation factor-2 is associated with inhibition of peptide-chain initiation in reticulocyte lysates. Biochem Biophys Res Commun. 1991 May 15;176(3):993–999. doi: 10.1016/0006-291x(91)90380-p. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. Differing effects of the protein phosphatase inhibitors okadaic acid and microcystin on translation in reticulocyte lysates. Biochim Biophys Acta. 1991 Jun 7;1093(1):36–41. doi: 10.1016/0167-4889(91)90135-k. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rowlands A. G., Montine K. S., Henshaw E. C., Panniers R. Physiological stresses inhibit guanine-nucleotide-exchange factor in Ehrlich cells. Eur J Biochem. 1988 Jul 15;175(1):93–99. doi: 10.1111/j.1432-1033.1988.tb14170.x. [DOI] [PubMed] [Google Scholar]
- Rowlands A. G., Panniers R., Henshaw E. C. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation factor 2. J Biol Chem. 1988 Apr 25;263(12):5526–5533. [PubMed] [Google Scholar]
- Salimans M. M., van Heugten H. A., van Steeg H., Voorma H. O. The effect of serum deprivation on the initiation of protein synthesis in mouse neuroblastoma cells. Biochim Biophys Acta. 1985 Jan 29;824(1):16–26. doi: 10.1016/0167-4781(85)90024-7. [DOI] [PubMed] [Google Scholar]
- Sarre T. F. The phosphorylation of eukaryotic initiation factor 2: a principle of translational control in mammalian cells. Biosystems. 1989;22(4):311–325. doi: 10.1016/0303-2647(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Scorsone K. A., Panniers R., Rowlands A. G., Henshaw E. C. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J Biol Chem. 1987 Oct 25;262(30):14538–14543. [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Gordon J. Multiple phosphorylation of ribosomal protein S6 during transition of quiescent 3T3 cells into early G1, and cellular compartmentalization of the phosphate donor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3952–3956. doi: 10.1073/pnas.76.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle C. A., Mankin H. J., Avruch J., Treadwell B. V. Insulin promoted decrease in the phosphorylation of protein synthesis initiation factor eIF-2. Biochem Biophys Res Commun. 1984 May 31;121(1):134–140. doi: 10.1016/0006-291x(84)90697-1. [DOI] [PubMed] [Google Scholar]


