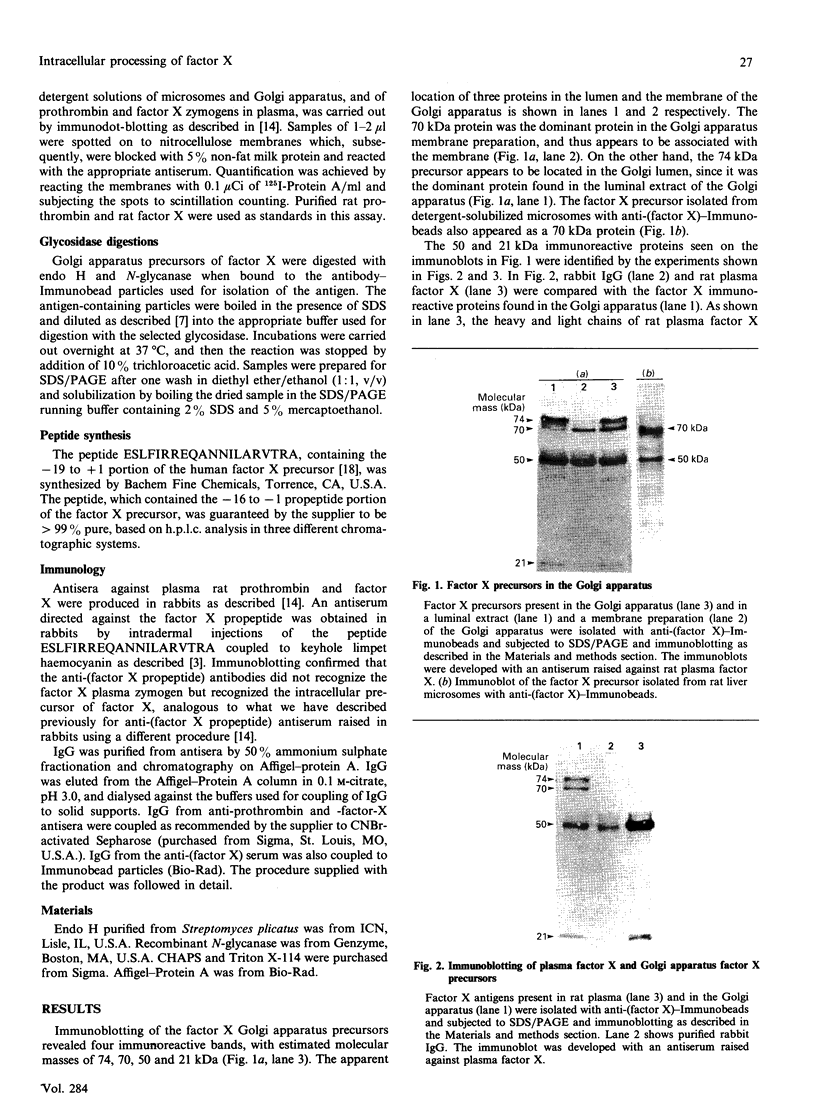
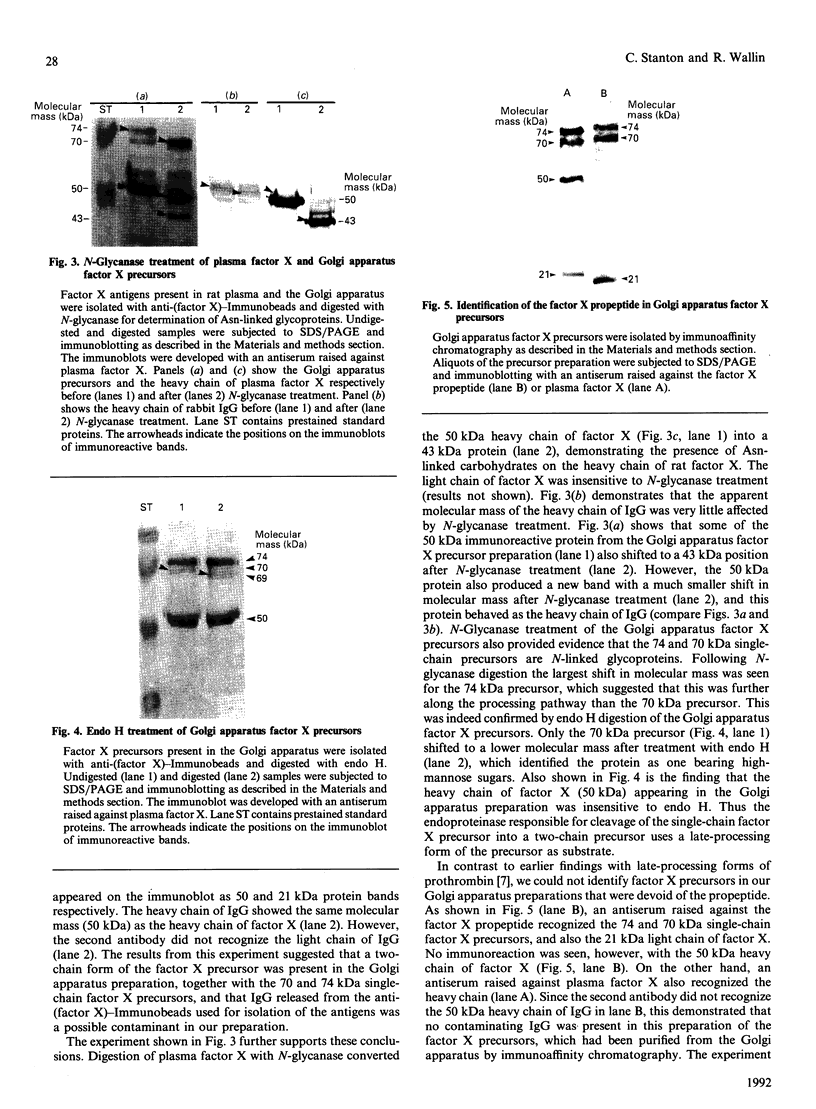
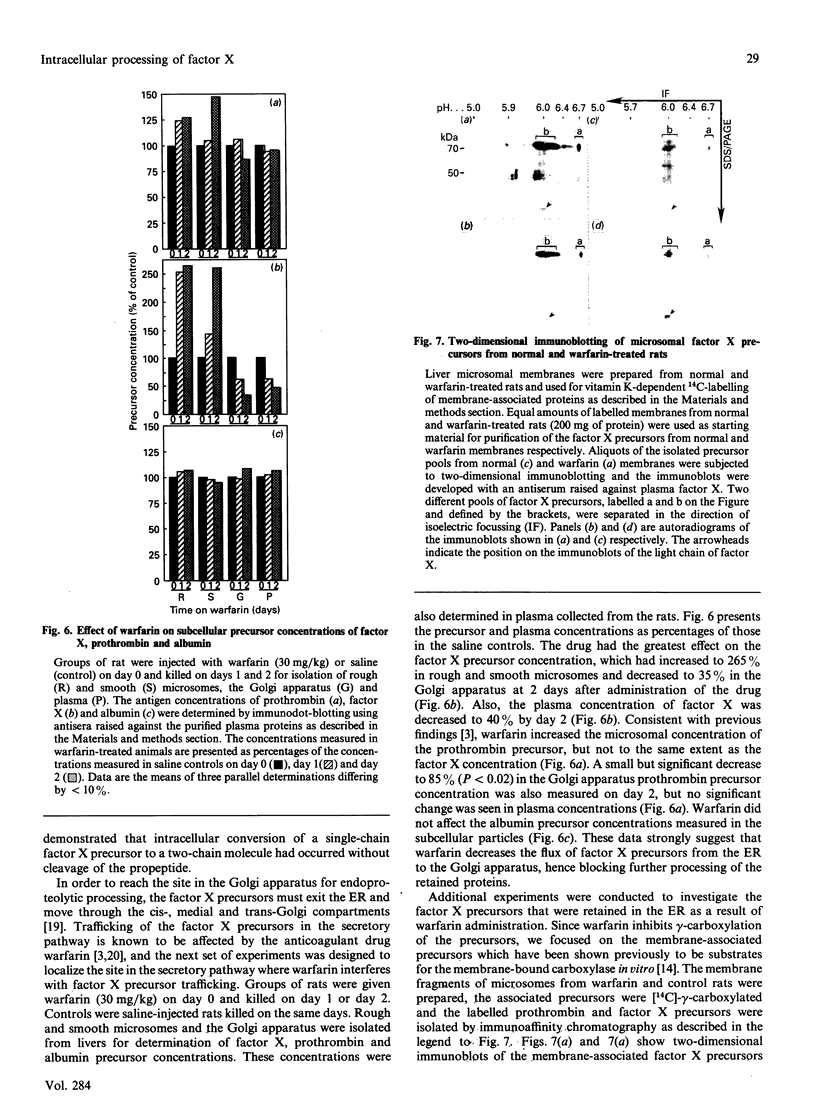
Abstract
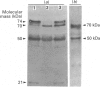
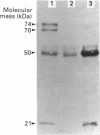
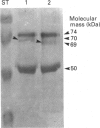
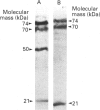
Clotting factor X undergoes several post-translational processing steps in the liver before the protein appears in blood as the mature two-chain zymogen. In this study we have followed the factor X precursor through the secretory pathway in rat liver in order to identify the site for proteolytic processing of the precursor into a two-chain form and the site for warfarin inhibition of precursor trafficking within the pathway. Isolated rat liver Golgi apparatus was shown to harbour two single-chains of factor X precursors of 70 and 74 kDa and the heavy (50 kDa) and light chains of factor X. It was demonstrated that the two-chain factor X form was produced from a late processing form of the factor X precursor, which indicated that the site for proteolytic conversion to a two-chain form was in the trans-Golgi compartment. The 70 and 74 kDa single-chain precursors and also the light chain of the two-chain form were shown to contain the factor X propeptide which is normally removed before the coagulation factor appears in blood. The data demonstrate that intra-chain cleavage of a single chain factor X precursor in the trans-Golgi compartment can precede release of the propeptide. Warfarin was shown to affect trafficking of the factor X precursor between the endoplasmic reticulum (ER) and the Golgi apparatus. The data suggest a link between vitamin K-dependent gamma-carboxylation of the precursor and its exit from the ER. Warfarin administration resulted in accumulation of factor X precursors associated with the ER membrane. These precursors appear to be stabilized from intracellular degradation while in the ER. In contrast to the large increase in the factor X precursor concentration in the ER membrane, there was no change in the prothrombin precursor concentration as a result of warfarin action on the liver. However, intracellular turnover of the microsomal prothrombin precursor pool in warfarin-treated rats resulted in a pool of less negatively charged proteins, indicating ongoing protein synthesis but inhibition of gamma-carboxylation. The data are consistent with previous findings [Wallin & Martin (1988) J. Biol. Chem. 263, 9994-10001] suggesting that prothrombin and factor X are processed differently by the vitamin K-dependent carboxylase in the ER membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E. Biochemistry of interorganelle transport. A new frontier in enzymology emerges from versatile in vitro model systems. J Biol Chem. 1989 Oct 15;264(29):16965–16968. [PubMed] [Google Scholar]
- Bathurst I. C., Brennan S. O., Carrell R. W., Cousens L. S., Brake A. J., Barr P. J. Yeast KEX2 protease has the properties of a human proalbumin converting enzyme. Science. 1987 Jan 16;235(4786):348–350. doi: 10.1126/science.3541206. [DOI] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. C., Holly R. D., Sprecher C. A., Walker K. M., Kumar A. A. Endoproteolytic processing of the human protein C precursor by the yeast Kex2 endopeptidase coexpressed in mammalian cells. Biochemistry. 1991 Jan 15;30(2):367–372. doi: 10.1021/bi00216a009. [DOI] [PubMed] [Google Scholar]
- Foster D. C., Sprecher C. A., Holly R. D., Gambee J. E., Walker K. M., Kumar A. A. Endoproteolytic processing of the dibasic cleavage site in the human protein C precursor in transfected mammalian cells: effects of sequence alterations on efficiency of cleavage. Biochemistry. 1990 Jan 16;29(2):347–354. doi: 10.1021/bi00454a007. [DOI] [PubMed] [Google Scholar]
- Furie B., Furie B. C. Molecular basis of vitamin K-dependent gamma-carboxylation. Blood. 1990 May 1;75(9):1753–1762. [PubMed] [Google Scholar]
- Furie B., Furie B. C. The molecular basis of blood coagulation. Cell. 1988 May 20;53(4):505–518. doi: 10.1016/0092-8674(88)90567-3. [DOI] [PubMed] [Google Scholar]
- Hosaka M., Nagahama M., Kim W. S., Watanabe T., Hatsuzawa K., Ikemizu J., Murakami K., Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991 Jul 5;266(19):12127–12130. [PubMed] [Google Scholar]
- Jorgensen M. J., Cantor A. B., Furie B. C., Furie B. Expression of completely gamma-carboxylated recombinant human prothrombin. J Biol Chem. 1987 May 15;262(14):6729–6734. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messier T. L., Pittman D. D., Long G. L., Kaufman R. J., Church W. R. Cloning and expression in COS-1 cells of a full-length cDNA encoding human coagulation factor X. Gene. 1991 Mar 15;99(2):291–294. doi: 10.1016/0378-1119(91)90141-w. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. The selectivity of secretion: protein sorting in the endoplasmic reticulum. Biochem Soc Trans. 1989 Oct;17(5):795–802. doi: 10.1042/bst0170795. [DOI] [PubMed] [Google Scholar]
- Shah D. V., Suttie J. W. The effect of vitamin K and warfarin on rat liver prothrombin concentrations. Arch Biochem Biophys. 1972 May;150(1):91–95. doi: 10.1016/0003-9861(72)90014-8. [DOI] [PubMed] [Google Scholar]
- Stanton C., Taylor R., Wallin R. Processing of prothrombin in the secretory pathway. Biochem J. 1991 Jul 1;277(Pt 1):59–65. doi: 10.1042/bj2770059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J. W., Jackson C. M. Prothrombin structure, activation, and biosynthesis. Physiol Rev. 1977 Jan;57(1):1–70. doi: 10.1152/physrev.1977.57.1.1. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Vitamin K-dependent carboxylase. Annu Rev Biochem. 1985;54:459–477. doi: 10.1146/annurev.bi.54.070185.002331. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Tollersrud O. K., Kvalvaag A. H., Helgeland L. Biosynthesis and clearance of prothrombin in warfarin-treated rats. Biochim Biophys Acta. 1989 Jan 17;1010(1):35–40. doi: 10.1016/0167-4889(89)90181-x. [DOI] [PubMed] [Google Scholar]
- Wallin R., Culp E. N., Coleman D. B., Goodman S. R. A structural model of human erythrocyte band 2.1: alignment of chemical and functional domains. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4095–4099. doi: 10.1073/pnas.81.13.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Early processing of prothrombin and factor X by the vitamin K-dependent carboxylase. J Biol Chem. 1988 Jul 15;263(20):9994–10001. [PubMed] [Google Scholar]
- Wallin R., Martin L. F. Warfarin poisoning and vitamin K antagonism in rat and human liver. Design of a system in vitro that mimics the situation in vivo. Biochem J. 1987 Jan 15;241(2):389–396. doi: 10.1042/bj2410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Turner R. Propeptide recognition by the vitamin K-dependent carboxylase in early processing of prothrombin and factor X. Biochem J. 1990 Dec 1;272(2):473–478. doi: 10.1042/bj2720473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. P., VanDusen W. J., Petroski C. J., Garsky V. M., Stern A. M., Friedman P. A. Bovine liver aspartyl beta-hydroxylase. Purification and characterization. J Biol Chem. 1991 Jul 25;266(21):14004–14010. [PubMed] [Google Scholar]
- de Metz M., Vermeer C., Soute B. A., van Scharrenburg G. J., Slotboom A. J., Hemker H. C. Partial purification of bovine liver vitamin K-dependent carboxylase by immunospecific adsorption onto antifactor X. FEBS Lett. 1981 Jan 26;123(2):215–218. doi: 10.1016/0014-5793(81)80290-6. [DOI] [PubMed] [Google Scholar]