Abstract
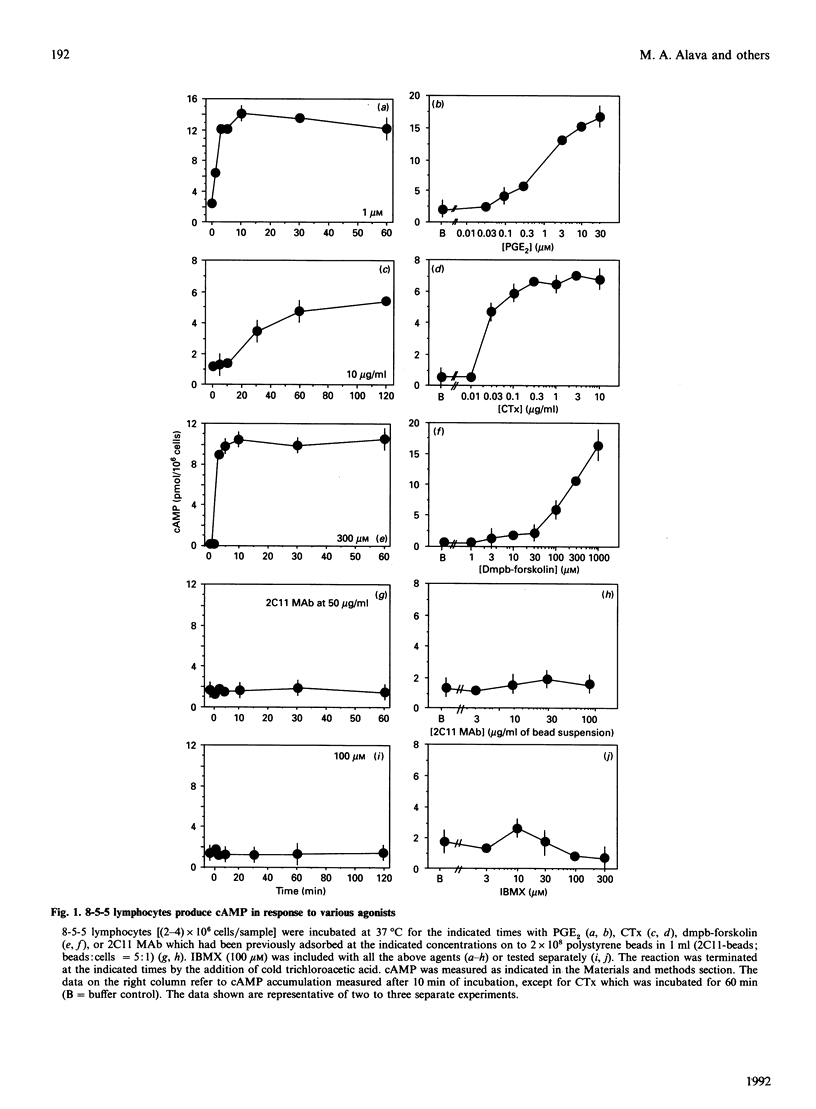
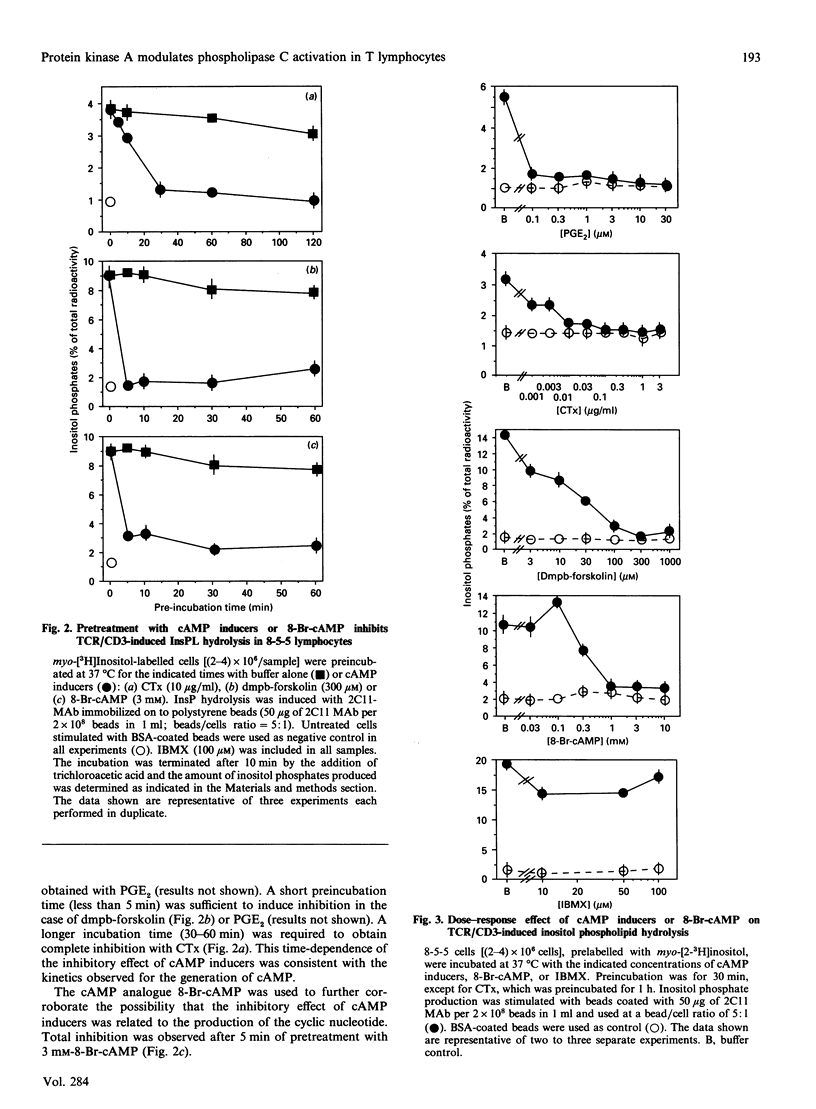
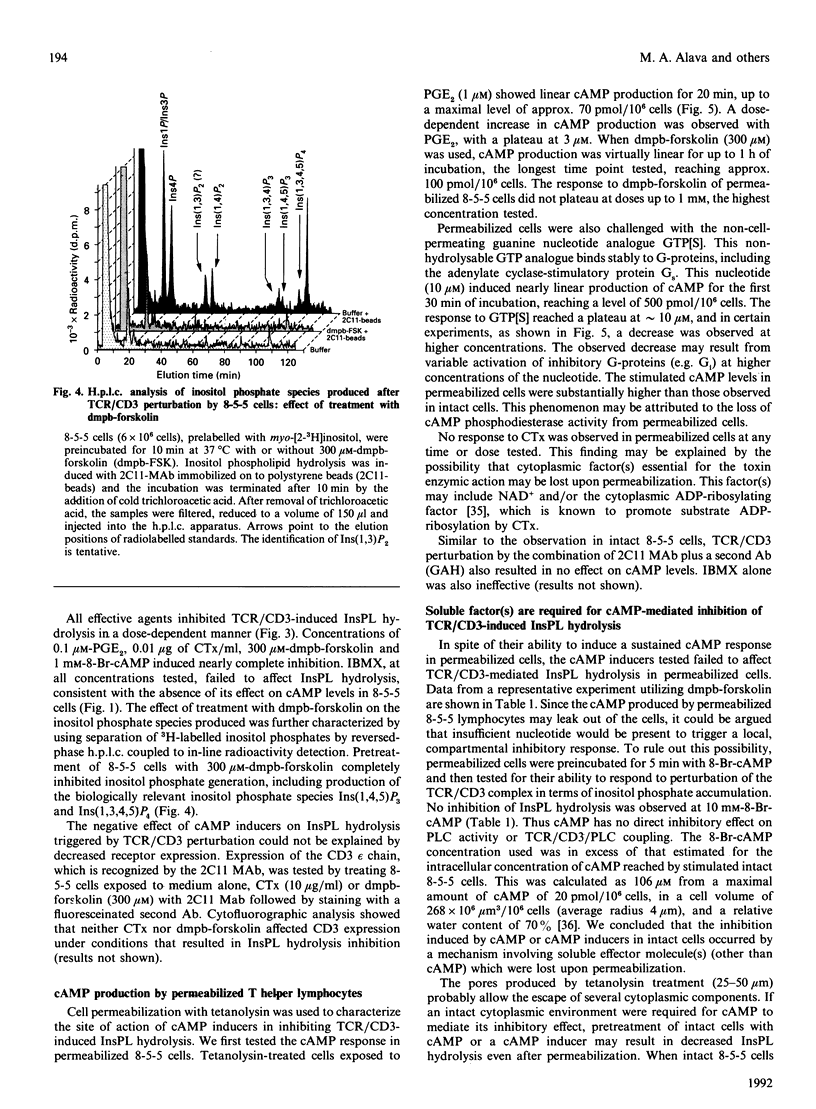
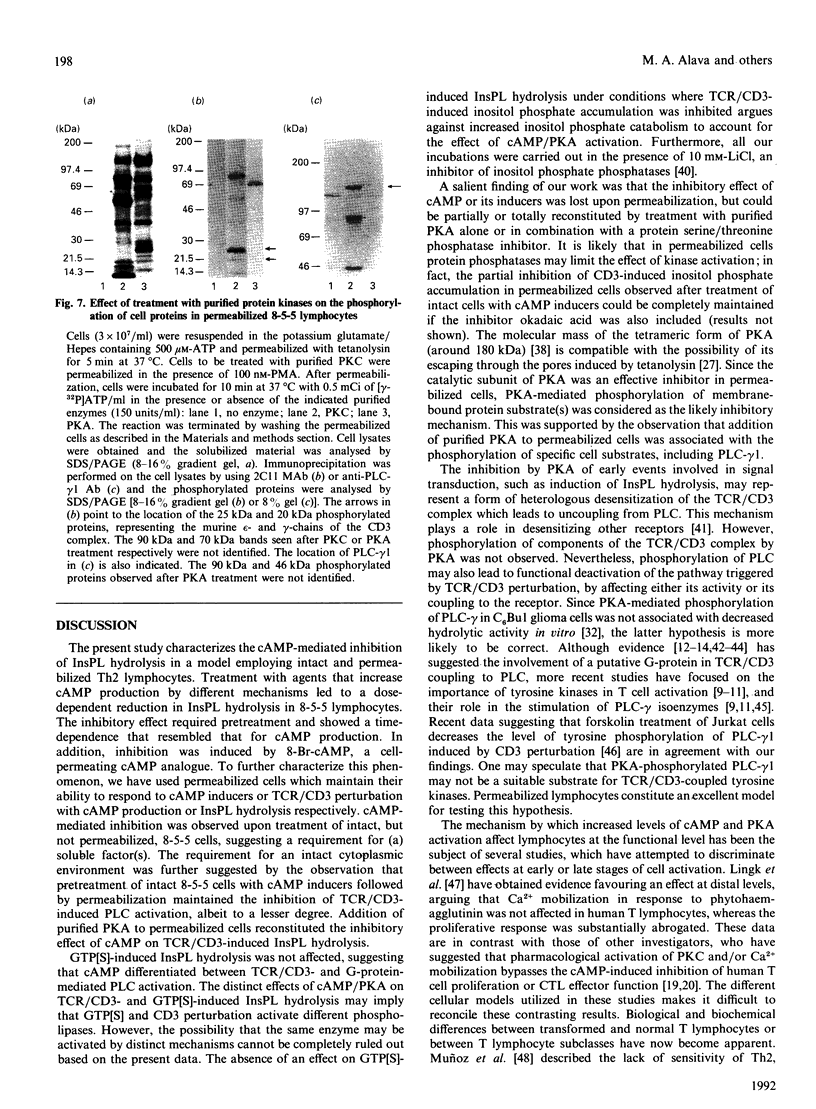
Modulation of inositol phospholipid (InsPL) hydrolysis in response to increasing intracellular concentrations of cyclic AMP (cAMP) was studied in a murine T helper type II (Th2) lymphocyte clone, 8-5-5. Intact 8-5-5 cells produced maximal amounts of cAMP in response to prostaglandin E2 (PGE2), cholera toxin (CTx) or 7 beta-deacetyl-7 beta-(gamma-N-methylpiperazino)butyryl forskolin (dmpb-forskolin). cAMP generation reached a plateau after 5 min of treatment with dmpb-forskolin (300 microM) or PGE2 (1 microM), but required 60 min of treatment with CTx (1 microgram/ml). Preincubation of 8-5-5 cells with 1 microM-PGE2 or 300 microM-dmpb-forskolin (10 min at 37 degrees C) or with 1 microgram of CTx/ml (60 min at 37 degrees C) completely inhibited InsPL hydrolysis induced by perturbation of the T cell receptor (TCR)/CD3 complex with the monoclonal antibody 145.2C11. Preincubation with the cAMP analogue 8-bromo-cyclic AMP (8-Br-cAMP) also inhibited InsPL hydrolysis. Tetanolysin-permeabilized 8-5-5 cells produced cAMP in response to PGE2, dmpb-forskolin and guanosine 5'-[gamma-thio]triphosphate (GTP[S]), a non-cell-permeating, non-hydrolysable analogue of GTP that directly activates G-proteins. No inhibition of TCR/CD3-induced InsPL hydrolysis was observed under these conditions. InsPL hydrolysis was also unaffected when permeabilized cells were incubated with up to 10 mM-8-Br-cAMP, suggesting that permeabilized cells lost (a) soluble effector molecule(s) involved in mediating the inhibitory effect observed in intact cells. Treatment of 8-5-5 cells with dmpb-forskolin or CTx prior to permeabilization resulted in inhibition of TCR/CD3-induced InsPL hydrolysis, but did not affect InsPL hydrolysis induced via G-protein stimulation with GTP[S]. Treatment of permeabilized 8-5-5 cells with purified cAMP-dependent protein kinase (PKA) resulted in inhibition of TCR/CD3- but not GTP[S]-induced InsPL hydrolysis. This effect was associated with phosphorylation of phospholipase (PLC)-gamma 1 in the absence of phosphorylation of components of the TCR/CD3 complex. These results suggest that PKA-mediated phosphorylation of PLC may regulate TCR/CD3-induced InsPL hydrolysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano Y., Hodes R. J. T cell regulation of b cell activation. Cloned Lyt-1+2-T suppressor cells inhibit the major histocompatibility complex-restricted interaction of T helper cells with B cells and/or accessory cells. J Exp Med. 1983 Oct 1;158(4):1178–1190. doi: 10.1084/jem.158.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvini E., DeBell K. E., Kolber M. A., Hoffman T., Hodes R. J., Taplits M. S. Hydrolysis of inositol phospholipids induced by stimulation of the T cell antigen receptor complex in antigen-specific, murine helper T cell clones. Requirement for exogenous calcium. J Immunol. 1989 Jul 15;143(2):587–595. [PubMed] [Google Scholar]
- Bonvini E., Debell K. E., Taplits M. S., Brando C., Laurenza A., Seamon K., Hoffman T. A role for guanine-nucleotide-binding proteins in mediating T-cell-receptor coupling to inositol phospholipid hydrolysis in a murine T-helper (type II) lymphocyte clone. Biochem J. 1991 May 1;275(Pt 3):689–696. doi: 10.1042/bj2750689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brando C., Hoffman T., Bonvini E. High-performance liquid chromatographic separation of inositol phosphate isomers employing a reversed-phase column and a micellar mobile phase. J Chromatogr. 1990 Jul 13;529(1):65–80. doi: 10.1016/s0378-4347(00)83808-6. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Robb R. J., Welte K., Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987 Aug;80(2):333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Joels L. A., Hughes P. J. The interaction of lithium ions with inositol lipid signalling systems. Biochem Soc Trans. 1987 Feb;15(1):32–35. doi: 10.1042/bst0150032. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja C., Lin L. L., Yunis E. J., Relias V., Dasgupta J. D. PLC gamma 1, a possible mediator of T cell receptor function. J Biol Chem. 1991 Sep 5;266(25):16277–16280. [PubMed] [Google Scholar]
- Graves J. D., Lucas S. C., Alexander D. R., Cantrell D. A. Guanine nucleotide regulation of inositol phospholipid hydrolysis and CD3-antigen phosphorylation in permeabilized T lymphocytes. Biochem J. 1990 Jan 15;265(2):407–413. doi: 10.1042/bj2650407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa-Sasaki H., Lutz F., Sasaki T. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of phosphatidylinositol 4,5-bisphosphate in a human T cell line. Microbiol Immunol. 1988;32(3):293–304. doi: 10.1111/j.1348-0421.1988.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Shoback D. M., Pattison G., Stobo J. D. Cholera toxin inhibits the T-cell antigen receptor-mediated increases in inositol trisphosphate and cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5673–5677. doi: 10.1073/pnas.83.15.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Protein kinase C and calcium ion in mitogenic response of macrophage-depleted human peripheral lymphocytes. J Biol Chem. 1985 Feb 10;260(3):1366–1369. [PubMed] [Google Scholar]
- Kammer G. M., Boehm C. A., Rudolph S. A., Schultz L. A. Mobility of the human T lymphocyte surface molecules CD3, CD4, and CD8: regulation by a cAMP-dependent pathway. Proc Natl Acad Sci U S A. 1988 Feb;85(3):792–796. doi: 10.1073/pnas.85.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kim U. H., Kim J. W., Rhee S. G. Phosphorylation of phospholipase C-gamma by cAMP-dependent protein kinase. J Biol Chem. 1989 Dec 5;264(34):20167–20170. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurenza A., Khandelwal Y., De Souza N. J., Rupp R. H., Metzger H., Seamon K. B. Stimulation of adenylate cyclase by water-soluble analogues of forskolin. Mol Pharmacol. 1987 Jul;32(1):133–139. [PubMed] [Google Scholar]
- Ledbetter J. A., Parsons M., Martin P. J., Hansen J. A., Rabinovitch P. S., June C. H. Antibody binding to CD5 (Tp67) and Tp44 T cell surface molecules: effects on cyclic nucleotides, cytoplasmic free calcium, and cAMP-mediated suppression. J Immunol. 1986 Nov 15;137(10):3299–3305. [PubMed] [Google Scholar]
- Lerner A., Jacobson B., Miller R. A. Cyclic AMP concentrations modulate both calcium flux and hydrolysis of phosphatidylinositol phosphates in mouse T lymphocytes. J Immunol. 1988 Feb 1;140(3):936–940. [PubMed] [Google Scholar]
- Lingk D. S., Chan M. A., Gelfand E. W. Increased cyclic adenosine monophosphate levels block progression but not initiation of human T cell proliferation. J Immunol. 1990 Jul 15;145(2):449–455. [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Mustelin T. GTP dependence of the transduction of mitogenic signals through the T3 complex in T lymphocytes indicates the involvement of a G-protein. FEBS Lett. 1987 Mar 9;213(1):199–203. doi: 10.1016/0014-5793(87)81491-6. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Zubiaga A. M., Merrow M., Sauter N. P., Huber B. T. Cholera toxin discriminates between T helper 1 and 2 cells in T cell receptor-mediated activation: role of cAMP in T cell proliferation. J Exp Med. 1990 Jul 1;172(1):95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Shea J. J., Urdahl K. B., Luong H. T., Chused T. M., Samelson L. E., Klausner R. D. Aluminum fluoride induces phosphatidylinositol turnover, elevation of cytoplasmic free calcium, and phosphorylation of the T cell antigen receptor in murine T cells. J Immunol. 1987 Nov 15;139(10):3463–3469. [PubMed] [Google Scholar]
- Papadogiannakis N., Nordström T. E., Andersson L. C., Wolff C. H. cAMP inhibits the OKT3-induced increase in cytoplasmic free calcium in the Jurkat T cell line: the degree of inhibition correlates inversely with the amount of CD3 binding ligand used. Eur J Immunol. 1989 Oct;19(10):1953–1956. doi: 10.1002/eji.1830191029. [DOI] [PubMed] [Google Scholar]
- Park D. J., Rho H. W., Rhee S. G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., Thistle L. Reduced prostaglandin E2 (PGE2) receptors on atopic T lymphocytes. Cell Immunol. 1986 Apr 15;99(1):294–299. doi: 10.1016/0008-8749(86)90237-6. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., O'Shea J. J., Luong H., Ross P., Urdahl K. B., Klausner R. D., Bluestone J. T cell antigen receptor phosphorylation induced by an anti-receptor antibody. J Immunol. 1987 Oct 15;139(8):2708–2714. [PubMed] [Google Scholar]
- Schrezenmeier H., Ahnert-Hilger G., Fleischer B. A T cell receptor-associated GTP-binding protein triggers T cell receptor-mediated granule exocytosis in cytotoxic T lymphocytes. J Immunol. 1988 Dec 1;141(11):3785–3790. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Secrist J. P., Karnitz L., Abraham R. T. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem. 1991 Jul 5;266(19):12135–12139. [PubMed] [Google Scholar]
- Suh P. G., Ryu S. H., Choi W. C., Lee K. Y., Rhee S. G. Monoclonal antibodies to three phospholipase C isozymes from bovine brain. J Biol Chem. 1988 Oct 5;263(28):14497–14504. [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Wehling M., Kuhls S., Armanini D. Volume regulation of human lymphocytes by aldosterone in isotonic media. Am J Physiol. 1989 Aug;257(2 Pt 1):E170–E174. doi: 10.1152/ajpendo.1989.257.2.E170. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Bonifacino J. S., Klausner R. D., Samelson L. E., O'Shea J. J. T cell antigen receptor: structure, assembly and function. Year Immunol. 1989;4:74–93. [PubMed] [Google Scholar]



