Abstract
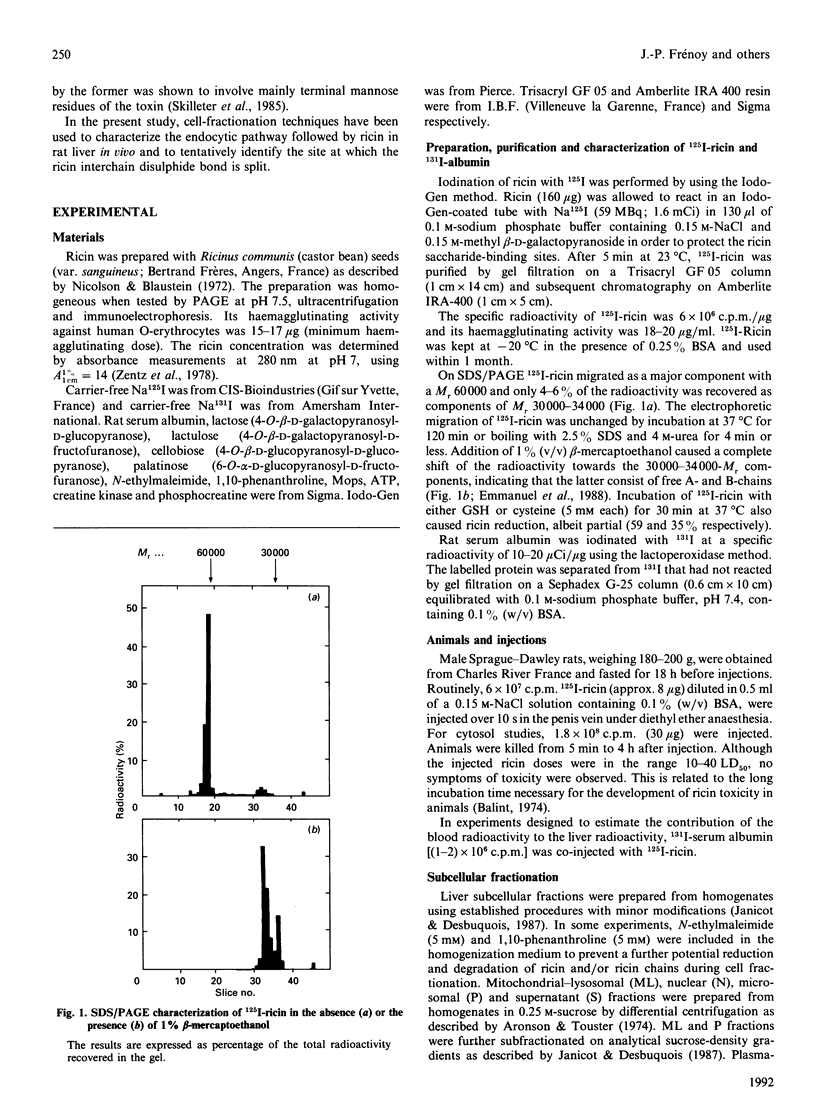
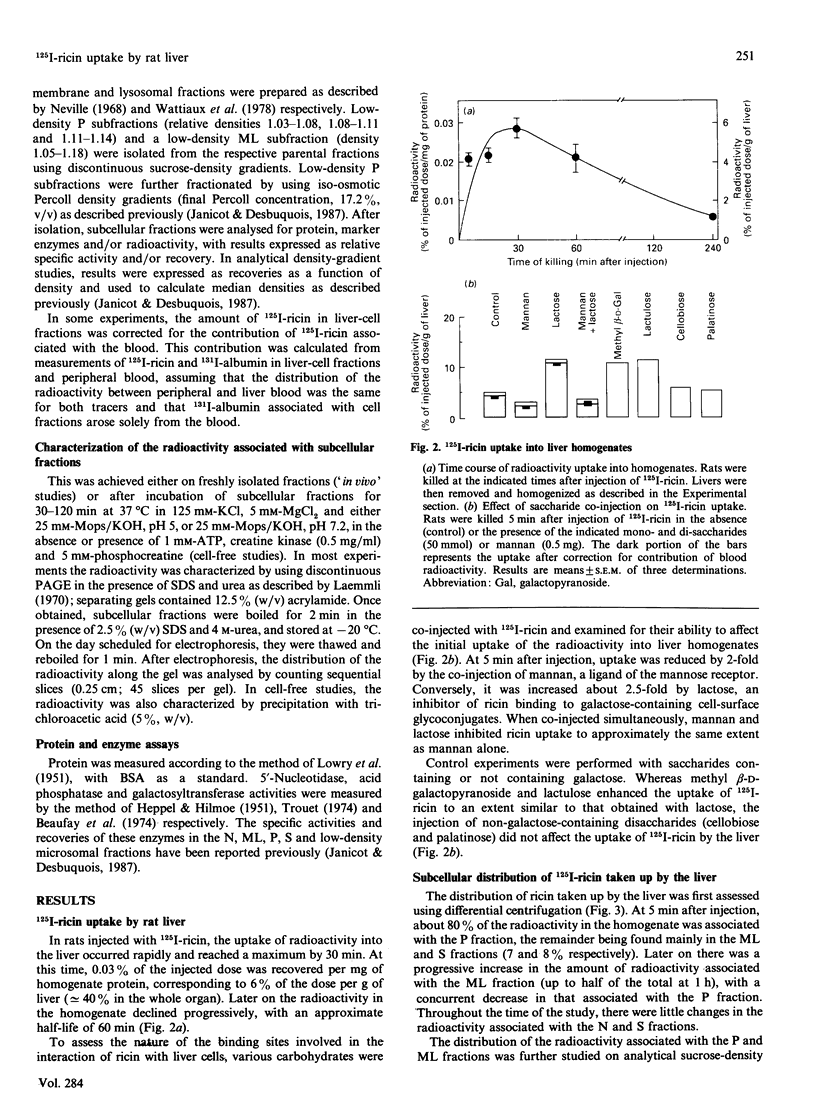
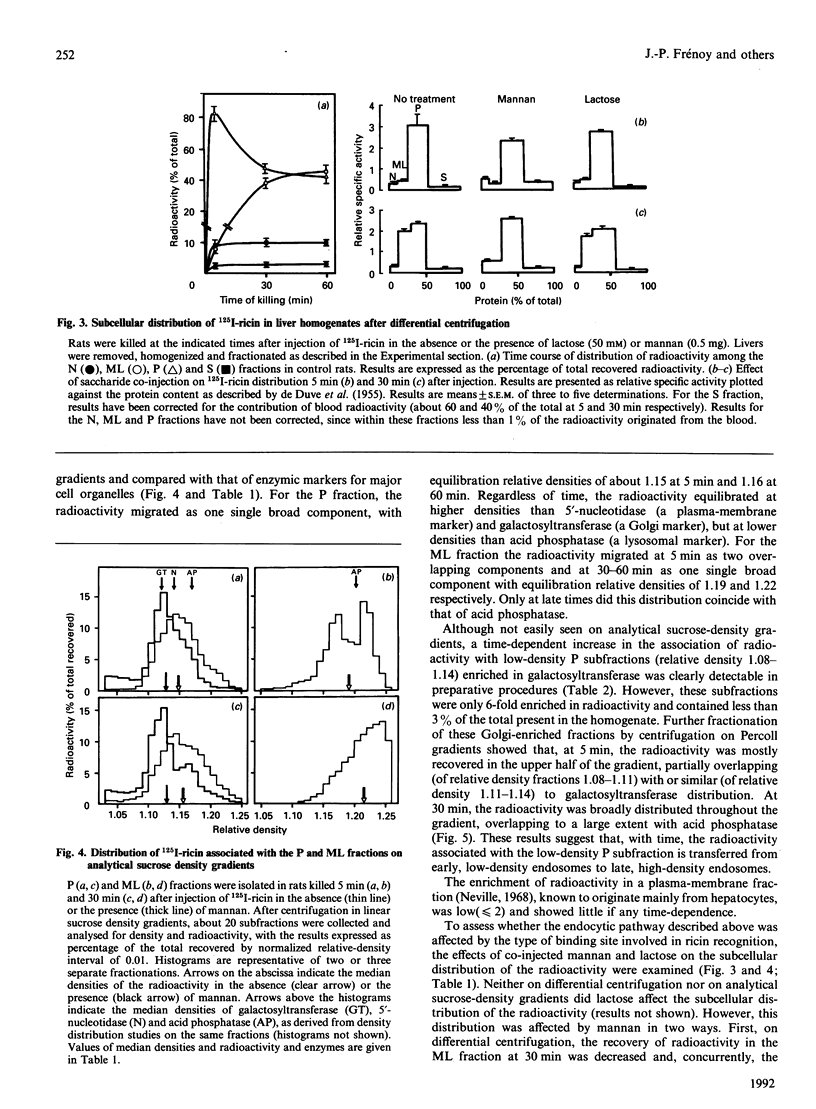
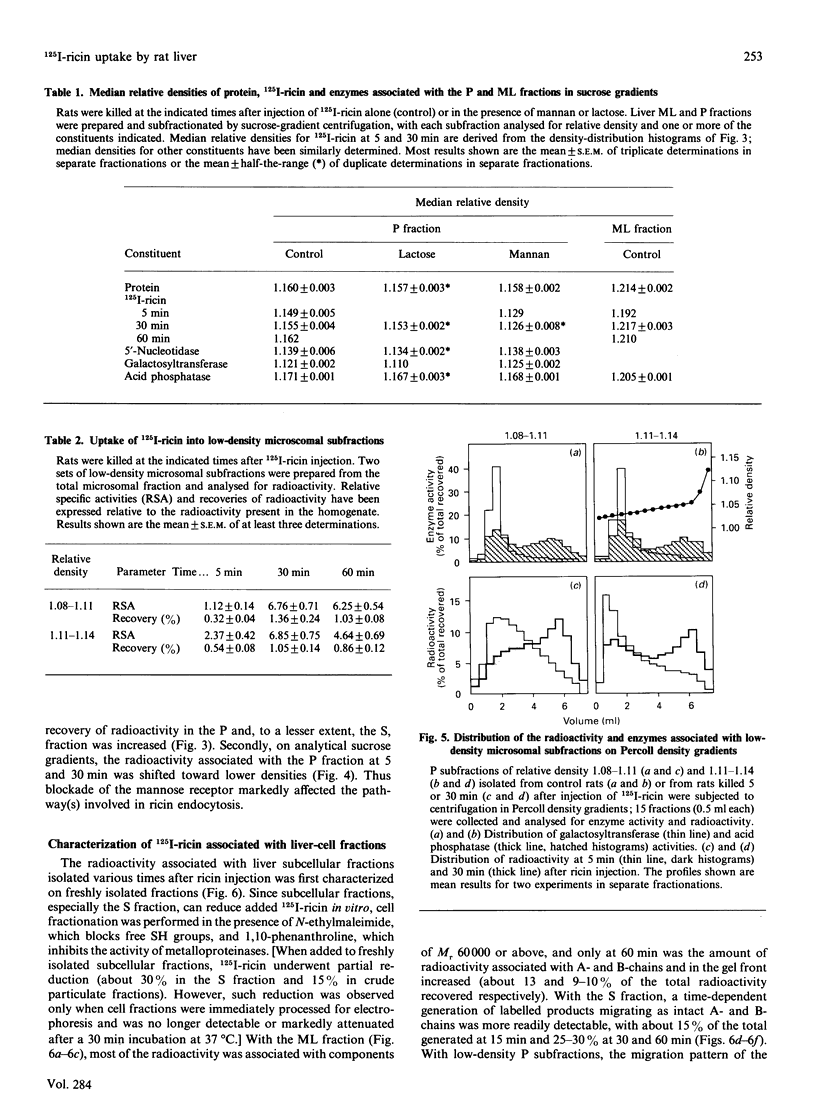
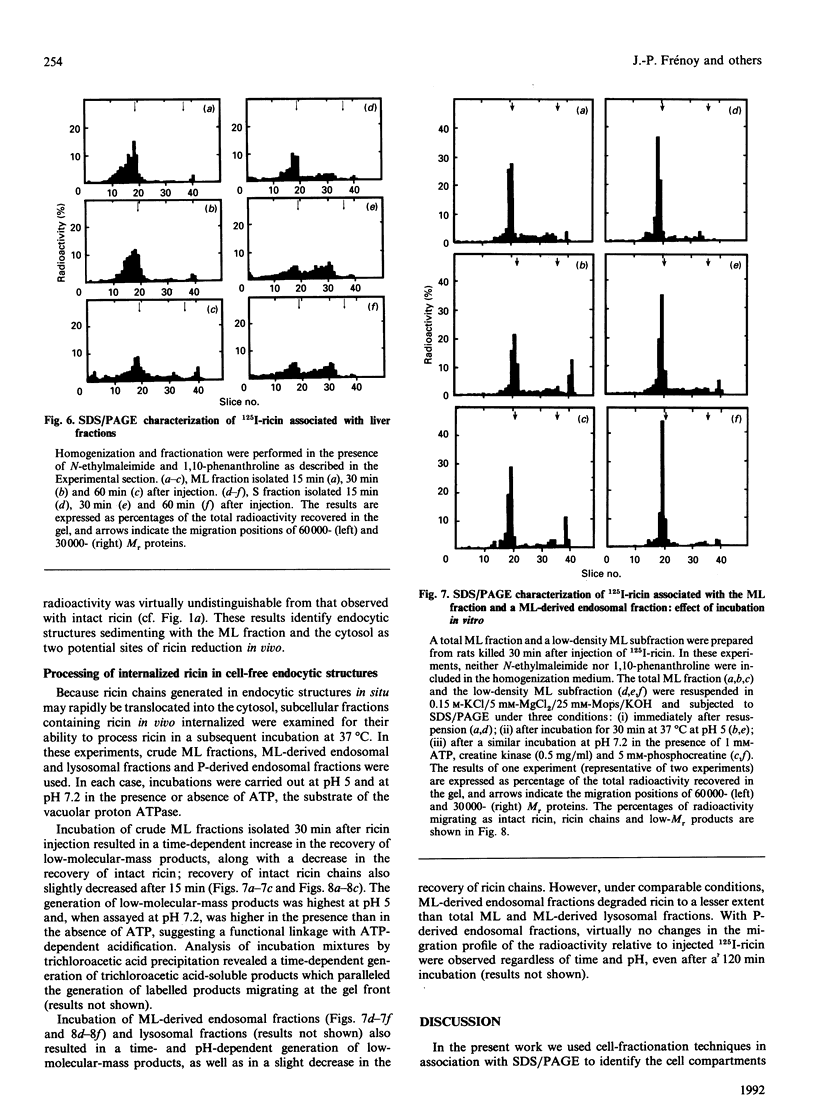
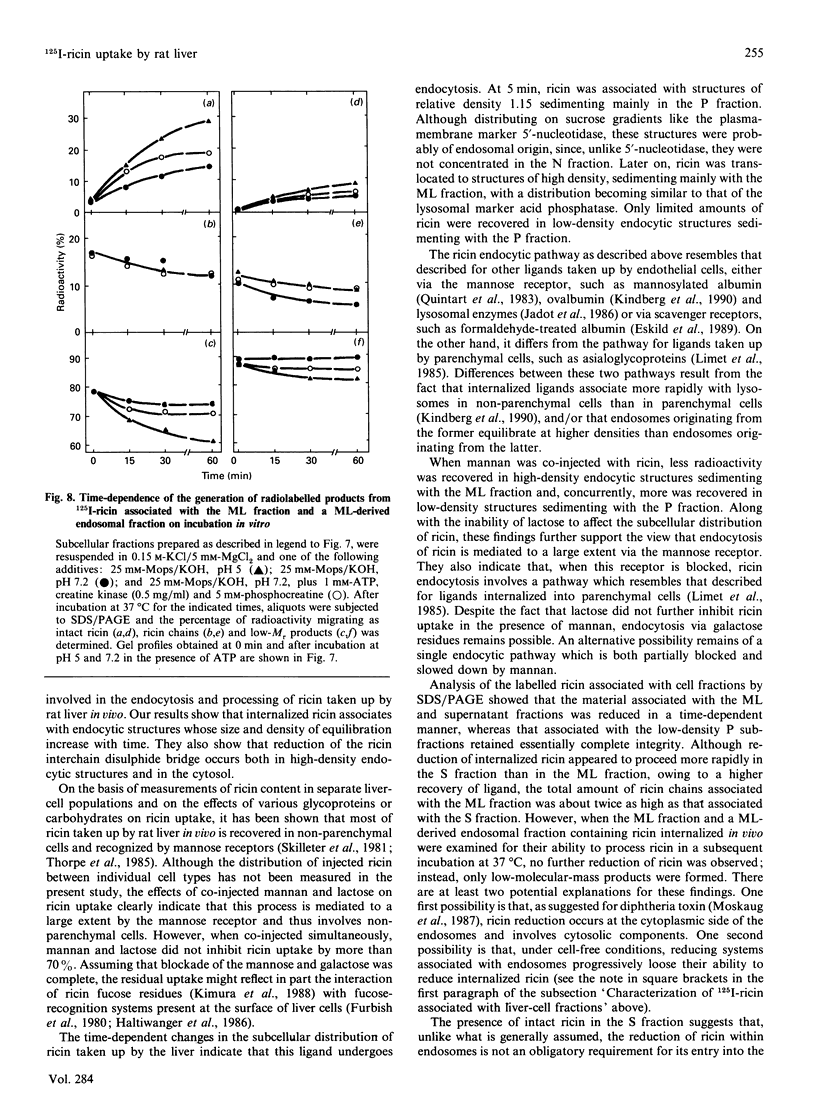
Subcellular-fractionation techniques were used to characterize the endocytic pathway followed by ricin in rat liver in vivo and tentatively identify the site(s) at which the ricin interchain disulphide bridge is split. After injection of 125I-ricin, hepatic uptake of radioactivity was maximum at 30 min (40% of injected dose). At 5 min, about 80% of the radioactivity in the homogenate was recovered in the microsomal (P) fraction, but later on the recovery of the radioactivity in the mitochondrial-lysosomal (ML) fractions progressively increased (50% at 30 min) at the expense of that in the P fraction. Subfractionation of the P and ML fractions on analytical sucrose-density gradients revealed a time-dependent translocation of the radioactivity from low- to high-density endocytic structures, with median relative densities at 5 and 60 min of about 1.15 and 1.16 (P fraction) and 1.19 and 1.22 (ML fraction) respectively. The late distribution of the radioactivity in the ML fraction was similar to that of the lysosomal marker acid phosphatase. Studies with co-injected lactose and mannan showed that ricin was internalized mainly via the mannose receptor. In the presence of mannan, the late recovery of radioactivity in the ML fraction was decreased, and the distribution of the radioactivity associated with the P fraction was shifted toward lower densities (median relative density 1.13), indicating a different pathway of endocytosis. Analysis of the radioactivity associated with the ML and S fractions by SDS/PAGE revealed a time-dependent increase in the amount of intact A- and B-chains and low-molecular-mass products. When ML fractions containing partially processed ricin were incubated at 37 degrees C at pH 5 or at pH 7.2 in the presence of ATP, only low-molecular-mass products were generated. We conclude that internalized ricin associates with endocytic structures whose size and density of equilibration increase with time, and that, although detectable in these structures, reduction of the ricin interchain disulphide bridge occurs to a large extent in the cytosol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979 Oct 10;254(19):9795–9799. [PubMed] [Google Scholar]
- Balint G. A. Ricin: the toxic protein of castor oil seeds. Toxicology. 1974 Mar;2(1):77–102. doi: 10.1016/0300-483x(74)90044-4. [DOI] [PubMed] [Google Scholar]
- Barbieri L., Battelli M. G., Stirpe F. Reduction of ricin and other plant toxins by thiol:protein disulfide oxidoreductases. Arch Biochem Biophys. 1982 Jun;216(1):380–383. doi: 10.1016/0003-9861(82)90224-7. [DOI] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat J., Molthoff C., Janssen H., Hilkens J. Endocytosis and intracellular routing of an antibody-ricin A chain conjugate. Cancer Res. 1988 Jul 1;48(13):3822–3827. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel F., Turpin E., Alfsen A., Frénoy J. P. Separation of ricin A- and B-chains after dithiothreitol reduction. Anal Biochem. 1988 Aug 15;173(1):134–141. doi: 10.1016/0003-2697(88)90170-4. [DOI] [PubMed] [Google Scholar]
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem. 1987 Apr 25;262(12):5908–5912. [PubMed] [Google Scholar]
- Endo Y., Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987 Jun 15;262(17):8128–8130. [PubMed] [Google Scholar]
- Eskild W., Kindberg G. M., Smedsrod B., Blomhoff R., Norum K. R., Berg T. Intracellular transport of formaldehyde-treated serum albumin in liver endothelial cells after uptake via scavenger receptors. Biochem J. 1989 Mar 1;258(2):511–520. doi: 10.1042/bj2580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feener E. P., Shen W. C., Ryser H. J. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J Biol Chem. 1990 Nov 5;265(31):18780–18785. [PubMed] [Google Scholar]
- Fodstad O., Olsnes S., Pihl A. Toxicity, distribution and elimination of the cancerostatic lectins abrin and ricin after parenteral injection into mice. Br J Cancer. 1976 Oct;34(4):418–425. doi: 10.1038/bjc.1976.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbish F. S., Krett N. L., Barranger J. A., Brady R. O. Fucose plays a role in the clearance and uptake of glucocerebrosidase by rat liver cells. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1768–1774. doi: 10.1016/s0006-291x(80)80103-3. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Kim S. U., Stieber A., Avrameas S. Internalization of lectins in neuronal GERL. J Cell Biol. 1977 Apr;73(1):1–13. doi: 10.1083/jcb.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HILMORE R. J. Purification and properties of 5-nucleotidase. J Biol Chem. 1951 Feb;188(2):665–676. [PubMed] [Google Scholar]
- Haltiwanger R. S., Lehrman M. A., Eckhardt A. E., Hill R. L. The distribution and localization of the fucose-binding lectin in rat tissues and the identification of a high affinity form of the mannose/N-acetylglucosamine-binding lectin in rat liver. J Biol Chem. 1986 Jun 5;261(16):7433–7439. [PubMed] [Google Scholar]
- Hansen S. H., Petersen O. W., Sandvig K., Olsnes S., van Deurs B. Internalized ricin and the plasma membrane glycoprotein MAM-6 colocalize in the trans-Golgi network of T47D human breast carcinoma cells. Exp Cell Res. 1989 Dec;185(2):373–386. doi: 10.1016/0014-4827(89)90307-8. [DOI] [PubMed] [Google Scholar]
- Hertler A. A., Frankel A. E. Immunotoxins: a clinical review of their use in the treatment of malignancies. J Clin Oncol. 1989 Dec;7(12):1932–1942. doi: 10.1200/JCO.1989.7.12.1932. [DOI] [PubMed] [Google Scholar]
- Ishida B., Cawley D. B., Reue K., Wisnieski B. J. Lipid-protein interactions during ricin toxin insertion into membranes. Evidence for A and B chain penetration. J Biol Chem. 1983 May 10;258(9):5933–5937. [PubMed] [Google Scholar]
- Jadot M., Misquith S., Dubois F., Wattiaux-De Coninck S., Wattiaux R. Intracellular pathway followed by invertase endocytosed by rat liver. Eur J Biochem. 1986 Dec 15;161(3):695–700. doi: 10.1111/j.1432-1033.1986.tb10495.x. [DOI] [PubMed] [Google Scholar]
- Janicot M., Desbuquois B. Fate of injected 125I-labeled cholera toxin taken up by rat liver in vivo. Generation of the active A1 peptide in the endosomal compartment. Eur J Biochem. 1987 Mar 2;163(2):433–442. doi: 10.1111/j.1432-1033.1987.tb10816.x. [DOI] [PubMed] [Google Scholar]
- Janicot M., Fouque F., Desbuquois B. Activation of rat liver adenylate cyclase by cholera toxin requires toxin internalization and processing in endosomes. J Biol Chem. 1991 Jul 15;266(20):12858–12865. [PubMed] [Google Scholar]
- Kimura Y., Hase S., Kobayashi Y., Kyogoku Y., Ikenaka T., Funatsu G. Structures of sugar chains of ricin D. J Biochem. 1988 Jun;103(6):944–949. doi: 10.1093/oxfordjournals.jbchem.a122391. [DOI] [PubMed] [Google Scholar]
- Kindberg G. M., Magnusson S., Berg T., Smedsrød B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem J. 1990 Aug 15;270(1):197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Youle R. J. Ricin subunit association. Thermodynamics and the role of the disulfide bond in toxicity. J Biol Chem. 1986 Sep 5;261(25):11571–11577. [PubMed] [Google Scholar]
- Limet J. N., Quintart J., Schneider Y. J., Courtoy P. J. Receptor-mediated endocytosis of polymeric IgA and galactosylated serum albumin in rat liver. Evidence for intracellular ligand sorting and identification of distinct endosomal compartments. Eur J Biochem. 1985 Feb 1;146(3):539–548. doi: 10.1111/j.1432-1033.1985.tb08685.x. [DOI] [PubMed] [Google Scholar]
- Magnusson S., Berg T., Turpin E., Frénoy J. P. Interactions of ricin with sinusoidal endothelial rat liver cells. Different involvement of two distinct carbohydrate-specific mechanisms in surface binding and internalization. Biochem J. 1991 Aug 1;277(Pt 3):855–861. doi: 10.1042/bj2770855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Mathis L. S., Stookey M., Shia S. P., Stone D. K., Draper R. K. A Chinese hamster ovary cell mutant with a heat-sensitive, conditional-lethal defect in vacuolar function. J Cell Biol. 1984 Dec;99(6):1907–1916. doi: 10.1083/jcb.99.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh D., Timar J., Davies A. J. The intracellular movement and cycling of ricin. Eur J Cell Biol. 1990 Jun;52(1):77–86. [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Strains of CHO-K1 cells resistant to Pseudomonas exotoxin A and cross-resistant to diphtheria toxin and viruses. Infect Immun. 1983 Sep;41(3):998–1009. doi: 10.1128/iai.41.3.998-1009.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano L., Cawley D., Herschman H. R. Disuccinimidyl suberate cross-linked ricin does not inhibit cell-free protein synthesis. Biochem Biophys Res Commun. 1982 Nov 16;109(1):7–13. doi: 10.1016/0006-291x(82)91558-3. [DOI] [PubMed] [Google Scholar]
- Moskaug J. O., Sandvig K., Olsnes S. Cell-mediated reduction of the interfragment disulfide in nicked diphtheria toxin. A new system to study toxin entry at low pH. J Biol Chem. 1987 Jul 25;262(21):10339–10345. [PubMed] [Google Scholar]
- Moya M., Dautry-Varsat A., Goud B., Louvard D., Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985 Aug;101(2):548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Hunter T. R. Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res. 1975 Jan;35(1):144–155. [PubMed] [Google Scholar]
- Olsnes S., Sandvig K., Petersen O. W., van Deurs B. Immunotoxins--entry into cells and mechanisms of action. Immunol Today. 1989 Sep;10(9):291–295. [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Limet J. N., Baudhuin P. Galactose-specific endocytosis in rat liver. Biochemical and morphological characterization of a low-density compartment isolated from hepatocytes. Eur J Biochem. 1983 Mar 1;131(1):105–112. doi: 10.1111/j.1432-1033.1983.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Ramsden C. S., Drayson M. T., Bell E. B. The toxicity, distribution and excretion of ricin holotoxin in rats. Toxicology. 1989 Apr;55(1-2):161–171. doi: 10.1016/0300-483x(89)90183-2. [DOI] [PubMed] [Google Scholar]
- Robbins A. R., Oliver C., Bateman J. L., Krag S. S., Galloway C. J., Mellman I. A single mutation in Chinese hamster ovary cells impairs both Golgi and endosomal functions. J Cell Biol. 1984 Oct;99(4 Pt 1):1296–1308. doi: 10.1083/jcb.99.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Inhibitory effect of ammonium chloride and chloroquine on the entry of the toxic lectin modeccin into HeLa cells. Biochem Biophys Res Commun. 1979 Sep 27;90(2):648–655. doi: 10.1016/0006-291x(79)91284-1. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Tønnessen T. I., Olsnes S. Ability of inhibitors of glycosylation and protein synthesis to sensitize cells to abrin, ricin, Shigella toxin, and Pseudomonas toxin. Cancer Res. 1986 Dec;46(12 Pt 1):6418–6422. [PubMed] [Google Scholar]
- Saxena S. K., O'Brien A. D., Ackerman E. J. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. J Biol Chem. 1989 Jan 5;264(1):596–601. [PubMed] [Google Scholar]
- Simmons B. M., Stahl P. D., Russell J. H. Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem. 1986 Jun 15;261(17):7912–7920. [PubMed] [Google Scholar]
- Skilleter D. N., Paine A. J., Stirpe F. A comparison of the accumulation of ricin by hepatic parenchymal and non-parenchymal cells and its inhibition of protein synthesis. Biochim Biophys Acta. 1981 Nov 5;677(3-4):495–500. doi: 10.1016/0304-4165(81)90264-6. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Price R. J., Thorpe P. E. Modification of the carbohydrate in ricin with metaperiodate and cyanoborohydride mixtures: effect on binding, uptake and toxicity to parenchymal and non-parenchymal cells of rat liver. Biochim Biophys Acta. 1985 Sep 27;842(1):12–21. doi: 10.1016/0304-4165(85)90287-9. [DOI] [PubMed] [Google Scholar]
- Thorpe P. E., Detre S. I., Foxwell B. M., Brown A. N., Skilleter D. N., Wilson G., Forrester J. A., Stirpe F. Modification of the carbohydrate in ricin with metaperiodate-cyanoborohydride mixtures. Effects on toxicity and in vivo distribution. Eur J Biochem. 1985 Feb 15;147(1):197–206. doi: 10.1111/j.1432-1033.1985.tb08737.x. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Turpin E., Goussault Y., Lis H., Sharon N. Nature of the receptor sites for galactosyl-specific lectins on human lymphocytes. Exp Cell Res. 1984 Jun;152(2):486–492. doi: 10.1016/0014-4827(84)90650-5. [DOI] [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-dupal M. F., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978 Aug;78(2):349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Chen C. C., Zhang M. S., Wu H. C. Disruption of the Golgi apparatus by brefeldin A inhibits the cytotoxicity of ricin, modeccin, and Pseudomonas toxin. Exp Cell Res. 1991 Feb;192(2):389–395. doi: 10.1016/0014-4827(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Chen C. H., Zhang M. S., Wu H. C. Increased cytotoxicity of ricin in a putative Golgi-defective mutant of Chinese hamster ovary cell. Exp Cell Res. 1990 Sep;190(1):11–16. doi: 10.1016/0014-4827(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Colombatti M. Hybridoma cells containing intracellular anti-ricin antibodies show ricin meets secretory antibody before entering the cytosol. J Biol Chem. 1987 Apr 5;262(10):4676–4682. [PubMed] [Google Scholar]
- Zentz C., Frénoy J. P., Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies. Biochim Biophys Acta. 1978 Sep 26;536(1):18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Pedersen L. R., Sundan A., Olsnes S., Sandvig K. Receptor-mediated endocytosis of a ricin-colloidal gold conjugate in vero cells. Intracellular routing to vacuolar and tubulo-vesicular portions of the endosomal system. Exp Cell Res. 1985 Aug;159(2):287–304. doi: 10.1016/s0014-4827(85)80003-3. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. Delivery of internalized ricin from endosomes to cisternal Golgi elements is a discontinuous, temperature-sensitive process. Exp Cell Res. 1987 Jul;171(1):137–152. doi: 10.1016/0014-4827(87)90257-6. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Sandvig K., Petersen O. W., Olsnes S., Simons K., Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988 Feb;106(2):253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]


