Abstract
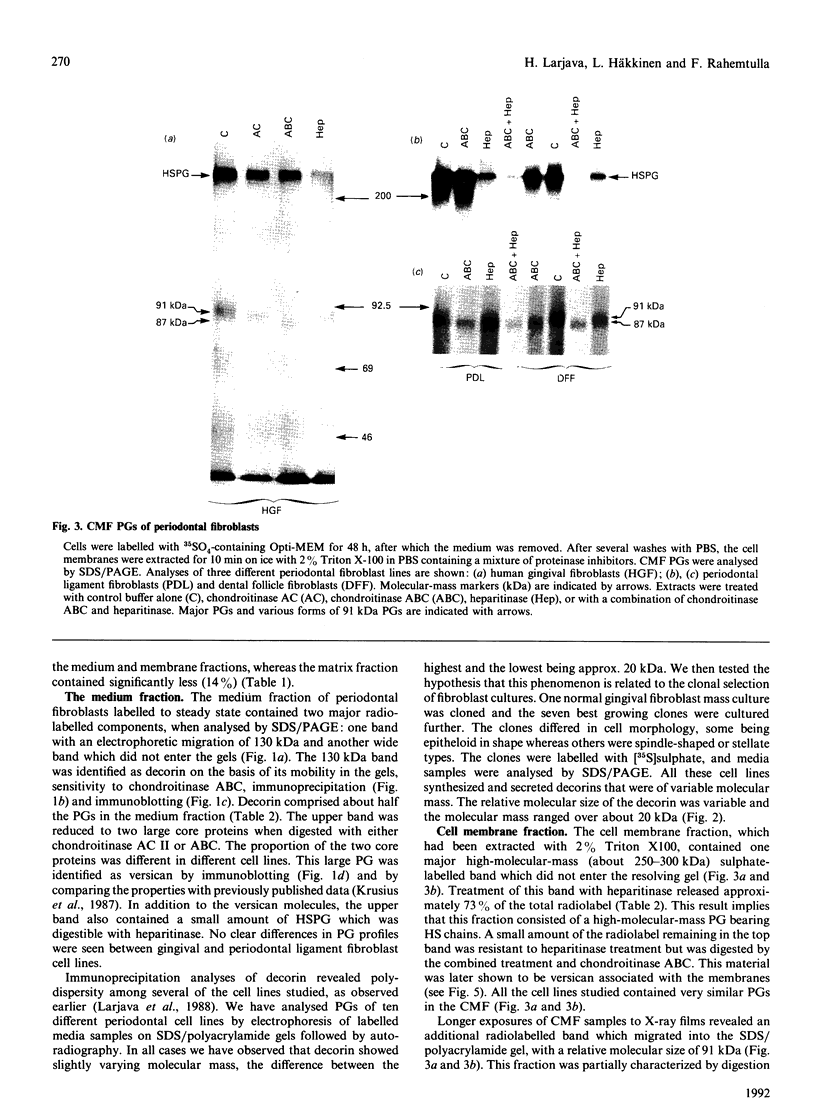
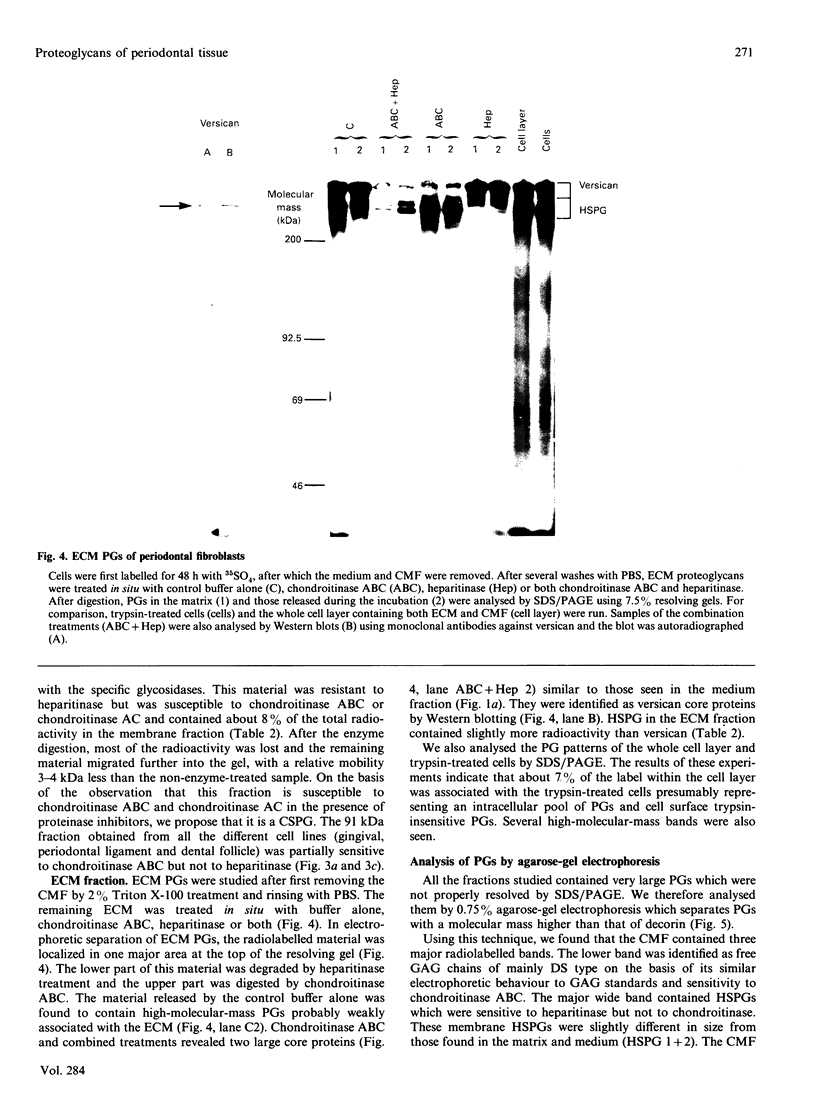
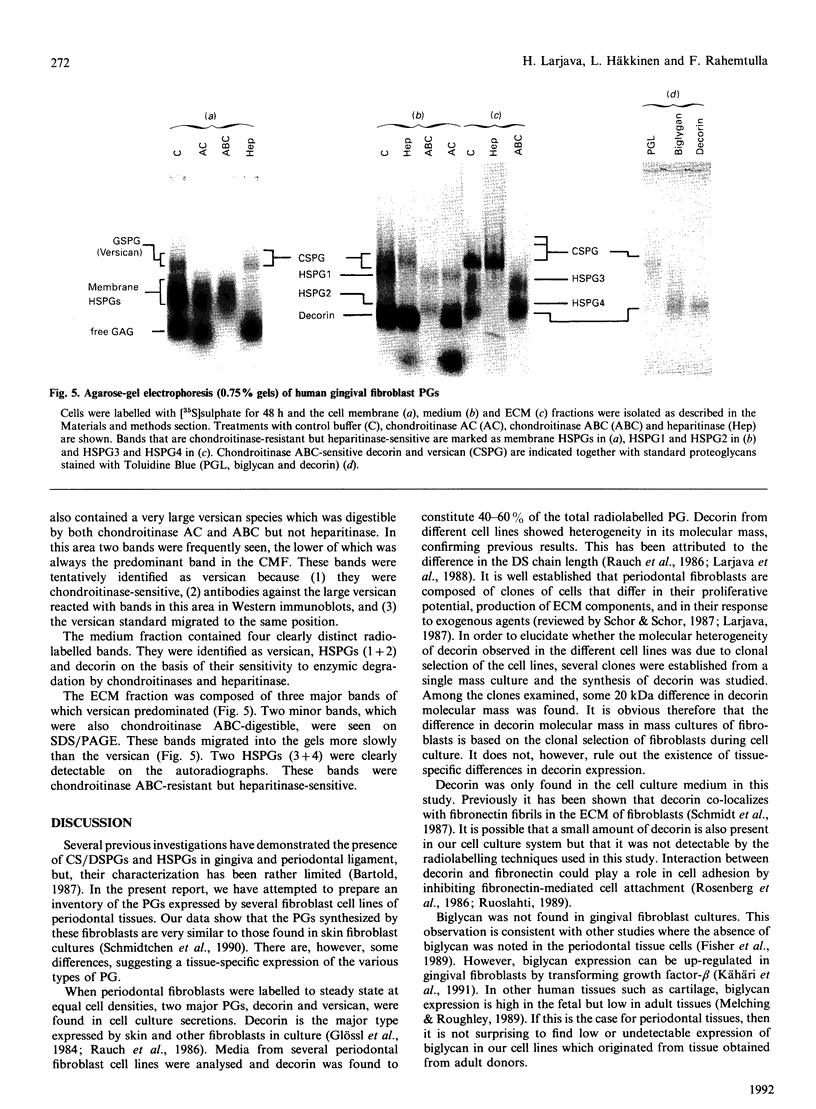
Proteoglycans synthesized by periodontal (gingival, periodontal ligament, dental follicle) fibroblasts were analysed by SDS/polyacrylamide and agarose gel electrophoresis after being labelled with radioactive sulphate. Medium, cell membrane and extracellular matrix fractions were analysed separately. Samples were treated with chondroitinase AC, chondroitinase ABC, heparitinase or a combination of chondroitinase ABC and heparitinase before electrophoretic separation of proteoglycans. Antibodies to versican and decorin were used to identify these molecules by Western immunoblots. For steady-state metabolic radiolabelling of fibroblasts, medium and cell membrane fractions contained about equal proportions of radiolabelled proteoglycans (about 43%), whereas less radioactivity (about 14%) was found in proteoglycans of the matrix fraction. Periodontal fibroblasts produce six major proteoglycans: versican, a high-molecular-mass chondroitin sulphate proteoglycan (CSPG); decorin, a dermatan sulphate proteoglycan (DSPG); a membrane-associated heparan sulphate proteoglycan (HSPG); two medium- or matrix-associated HSPGs; and a 91 kDa membrane-associated CSPG. Variation in decorin molecular size was observed in mass cultures of fibroblasts. Similar polydispersity in molecular size of decorin was seen in several clones established from one mass culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bartold P. M. Proteoglycans of the periodontium: structure, role and function. J Periodontal Res. 1987 Nov;22(6):431–444. doi: 10.1111/j.1600-0765.1987.tb02052.x. [DOI] [PubMed] [Google Scholar]
- David G., Lories V., Decock B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 2):3165–3176. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Lories V., Heremans A., Van der Schueren B., Cassiman J. J., Van den Berghe H. Membrane-associated chondroitin sulfate proteoglycans of human lung fibroblasts. J Cell Biol. 1989 Mar;108(3):1165–1173. doi: 10.1083/jcb.108.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Wayner E. A., Hoffman P. A., St John T., Butcher E. C., Carter W. G. Structural homology between lymphocyte receptors for high endothelium and class III extracellular matrix receptor. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4654–4658. doi: 10.1073/pnas.86.12.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Hassell J. R., Kimura J. H., Hascall V. C. Proteoglycan core protein families. Annu Rev Biochem. 1986;55:539–567. doi: 10.1146/annurev.bi.55.070186.002543. [DOI] [PubMed] [Google Scholar]
- Heremans A., De Cock B., Cassiman J. J., Van den Berghe H., David G. The core protein of the matrix-associated heparan sulfate proteoglycan binds to fibronectin. J Biol Chem. 1990 May 25;265(15):8716–8724. [PubMed] [Google Scholar]
- Heremans A., van der Schueren B., de Cock B., Paulsson M., Cassiman J. J., van den Berghe H., David G. Matrix-associated heparan sulfate proteoglycan: core protein-specific monoclonal antibodies decorate the pericellular matrix of connective tissue cells and the stromal side of basement membranes. J Cell Biol. 1989 Dec;109(6 Pt 1):3199–3211. doi: 10.1083/jcb.109.6.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Krusius T., Gehlsen K. R., Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem. 1987 Sep 25;262(27):13120–13125. [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larjava H. Fibroblasts-bacteria interactions. Proc Finn Dent Soc. 1987;83(3):85–93. [PubMed] [Google Scholar]
- Larjava H., Heino J., Krusius T., Vuorio E., Tammi M. The small dermatan sulphate proteoglycans synthesized by fibroblasts derived from skin, synovium and gingiva show tissue-related heterogeneity. Biochem J. 1988 Nov 15;256(1):35–40. doi: 10.1042/bj2560035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Multiple distinct membrane heparan sulfate proteoglycans in human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7009–7016. [PubMed] [Google Scholar]
- Mali M., Jaakkola P., Arvilommi A. M., Jalkanen M. Sequence of human syndecan indicates a novel gene family of integral membrane proteoglycans. J Biol Chem. 1990 Apr 25;265(12):6884–6889. [PubMed] [Google Scholar]
- Marynen P., Zhang J., Cassiman J. J., Van den Berghe H., David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989 Apr 25;264(12):7017–7024. [PubMed] [Google Scholar]
- Melching L. I., Roughley P. J. The synthesis of dermatan sulphate proteoglycans by fetal and adult human articular cartilage. Biochem J. 1989 Jul 15;261(2):501–508. doi: 10.1042/bj2610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Nakache M., Butcher E. C. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989 Aug;109(2):927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahemtulla F. Proteoglycans of oral tissues. Crit Rev Oral Biol Med. 1992;3(1-2):135–162. doi: 10.1177/10454411920030010301. [DOI] [PubMed] [Google Scholar]
- Rauch U., Glössl J., Kresse H. Comparison of small proteoglycans from skin fibroblasts and vascular smooth-muscle cells. Biochem J. 1986 Sep 1;238(2):465–474. doi: 10.1042/bj2380465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. G., Yamasaki A., Pinero G. J., Mahan C. J. Human periodontal ligament cells in vitro. J Periodontal Res. 1987 Jan;22(1):20–28. doi: 10.1111/j.1600-0765.1987.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Poole A. R., Lewandowska K., Culp L. A. Biological roles of dermatan sulphate proteoglycans. Ciba Found Symp. 1986;124:47–68. doi: 10.1002/9780470513385.ch4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtchen A., Carlstedt I., Malmström A., Fransson L. A. Inventory of human skin fibroblast proteoglycans. Identification of multiple heparan and chondroitin/dermatan sulphate proteoglycans. Biochem J. 1990 Jan 1;265(1):289–300. doi: 10.1042/bj2650289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor S. L., Schor A. M. Clonal heterogeneity in fibroblast phenotype: implications for the control of epithelial-mesenchymal interactions. Bioessays. 1987 Nov;7(5):200–204. doi: 10.1002/bies.950070503. [DOI] [PubMed] [Google Scholar]
- Silbert J. E., Palmer M. E., Humphries D. E., Silbert C. K. Formation of dermatan sulfate by cultured human skin fibroblasts. Effects of sulfate concentration on proportions of dermatan/chondroitin. J Biol Chem. 1986 Oct 15;261(29):13397–13400. [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Kiviranta I., Jurvelin J., Helminen H. J. Levels of chondroitin-6-sulfate and nonaggregating proteoglycans at articular cartilage contact sites in the knees of young dogs subjected to moderate running exercise. Arthritis Rheum. 1989 Oct;32(10):1282–1292. doi: 10.1002/anr.1780321014. [DOI] [PubMed] [Google Scholar]
- Takagi M., Hishikawa H., Hosokawa Y., Kagami A., Rahemtulla F. Immunohistochemical localization of glycosaminoglycans and proteoglycans in predentin and dentin of rat incisors. J Histochem Cytochem. 1990 Mar;38(3):319–324. doi: 10.1177/38.3.1689334. [DOI] [PubMed] [Google Scholar]
- Wight T. N. Cell biology of arterial proteoglycans. Arteriosclerosis. 1989 Jan-Feb;9(1):1–20. doi: 10.1161/01.atv.9.1.1. [DOI] [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]