Abstract
Background
Molecular biological techniques are dramatically changing our view of microbial diversity in almost any environment that has so far been investigated. This study presents a systematic survey of the microbial diversity associated with a population of Acromyrmex leafcutter ants. In contrast to previous studies on social insects, which targeted specific groups of symbionts occurring in the gut (termites, Tetraponera ants) or in specialised cells (Camponotus ants) the objective of our present study was to do a total screening of all possible micro-organisms that can be found inside the bodies of these leafcutter ants.
Results
We amplified, cloned and sequenced SSU rRNA encoding gene fragments from 9 microbial groups known to have insect-associated representatives, and show that: (1) representatives of 5 out of 9 tested groups are present, (2) mostly several strains per group are present, adding up to a total of 33 different taxa. We present the microbial taxa associated with Acromymex ants in a phylogenetic context (using sequences from GenBank) to assess and illustrate to which known microorganisms they are closely related. The observed microbial diversity is discussed in the light of present knowledge on the evolutionary history of Acromyrmex leafcutter ants and their known mutualistic and parasitic symbionts.
Conclusions
The major merits of the screening approach documented here is its high sensitivity and specificity, which allowed us to identify several microorganisms that are promising candidates for further study of their interactions with Acromyrmex leafcutter ants or their gardens.
Background
Recent trends in microbial ecology reveal that in most terrestrial and aquatic ecosystems biodiversity is in large part microbial. Since the pioneering work of Carl Woese [1] and Rudolf Amann [2,3], the unraveling of microbial diversity and taxonomy no longer depends on conventional culture-dependent bacteriological methods [4]. In particular, the use of small subunit ribosomal RNA (SSU rRNA) encoding sequences has dramatically increased the understanding of microbial diversity in a variety of environments, ranging from plankton communities [5], sewage plants [6] to geothermal springs [7] and symbiotic associations with a diversity of hosts (e.g. [8]). When symbionts, microbes can either be parasites (i.e. be detrimental for the survival and reproduction of the host; [9]), or mutualists (i.e. be beneficial to the host; [10]). A clear phylogenetic identification of hosts and symbionts is essential to address detailed questions about interactions, transmission and coevolution when hosts and symbionts are known (e.g. [11]). However, the same molecular tools are also important to identify novel and hitherto unexpected associations with parasites or mutualists (e.g. [12-14]). The present study is of the latter type and focuses on a social insect that is known to have at least two microbial mutualists and one microbial parasite [15-18].
Both mutualistic and parasitic microbial associates of social insect hosts face spatially and genetically structured host populations against which their genotypes are tested by natural selection. In fact, the distribution of genetic diversity across and within colonies is likely to determine in large part the efficiency of horizontal transmission of strains between individual hosts [19-21]. In addition, maternally (vertically) transmitted symbionts may attempt to force their hosts to produce a female biased sex ratio, thereby enhancing their representation in the next generation [17,22]. Although these vertically transmitted symbionts may thus affect the expression and regulation of reproductive conflict in insect societies, rather little effort has been undertaken to estimate the total diversity of microbial associates of social insects. The studies available today suggest that surveys of this kind are rewarding, because a number of interesting specific associations between social insects and microbial symbionts have recently been discovered and analysed for their co-evolutionary interactions with molecular tools. Examples include Camponotus wood ant γ-proteobacterial mutualists [12,13,23,24], Solenopsis fire ant microsporidian parasites [25,26], diverse honey bee parasites [27-30], and endosymbiotic Wolbachia in ants [17,22,31][32][18]. However, the only social insects where a larger spectrum of taxonomically heterogeneous microbial diversity has been systematically surveyed with molecular tools are termites, whose gut communities are now known to harbour a complex and diverse community with numerous representatives of several microbial groups [33-38].
The present study aims to document the total diversity of microorganisms that can be found within the bodies of workers of the leaf-cutter ant Acromyrmex octospinosus. To do this, we have amplified, cloned and sequenced SSU rRNA encoding sequences representing eight microbial groups known to have insect-associated representatives, and one negative control group (Fibrobacter) known to contain only vertebrate associated ruminal bacteria [39].
Results
Group-specific PCR amplification
No group specific template could be amplified from the pooled sample in four out of nine tested microbial groups, including the negative control group Fibrobacter (Table 1). For these taxa either no representatives are present, or our methods failed to amplify them. The latter possibility, however, seems unlikely, since the goup-specific primer pairs were designed using all known representatives in GenBank of the respective groups, and were tested using several positive controls aimed to represent the whole diversity of the group in question [40]. For the other five taxa the group specific PCR results indicated that at least one representative from each of these groups is associated with Acromyrmex octospinosus (Table 1).
Table 1.
Results of the group specific PCR reactions and characterization of the diversity of symbiont strains in each of the microbial taxa
| Group (Kingdom) | Group specific PCR | N clones sequenced | N symbiont strains present |
| Microsporidia (EUK) | - | ||
| Parabasalidea & Diplomonadida (EUK) | - | ||
| Eumycota – Fungi (EUK) | + | 41 | 6 |
| Flavobacteria (EUB) | + | 44 | 5 |
| Fibrobacter (EUB) | - | ||
| Spirochaetes & relatives (EUB) | - | ||
| Proteobacteria (EUB) | + | 36 | 12 |
| Gram pos high GC (EUB) | + | 26 | 9 |
| Gram pos low GC (EUB) | + | 12 | 1 |
| TOTAL | 159 | 33 |
Acromyrmex octospinosus microbial associates in a phylogenetic context
Cloning and sequencing of these amplification products revealed that the Gram positive bacteria with low GC content were represented by a single sequence but that a heterogeneous template was amplified in the other group specific PCR's (Table 1). Differences smaller than 1% sequence divergence were assumed to be due to Taq polymerase reading errors and a consensus sequence was calculated for these groups of similar sequences. This procedure generated a total of 33 different bacterial strains associated with A. octospinosus (Table 1).
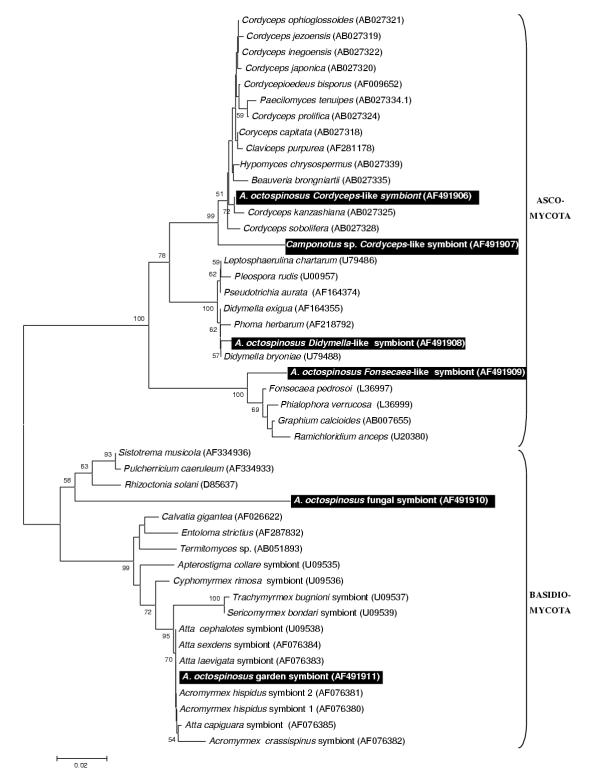
One of the six identified fungal clones (Fig 1) clustered within a monophyletic group of Basidiomycota including all known garden symbionts of fungus growing ants. This sequence was retrieved 15 times in a total of 41 sequenced clones. A second basidiomycotal sequence clustered with the plant pathogen Rhizoctonia solani but is only distantly related to it. Among the Ascomycota associated with A. octospinosus, we found a Cordyceps sp., a relative of the plant pathogen Didymella, and a more distant relative of the human pathogen Fonsecaea.
Figure 1.
Neighbour joining tree for fungal strains based on partial sequences of the 18S rRNA gene Distances were calculated using the Kimura 2-parameter in MEGA 2.0. The tree was rooted using the Basidiomycota as an outgroup for their sister group Ascomycota and vice versa. Bootstrap support values (1000 replicates) over 50% are shown above the branches. Names of strains are followed by their GenBank accession number. Sequences generated in this study are indicated on black background.
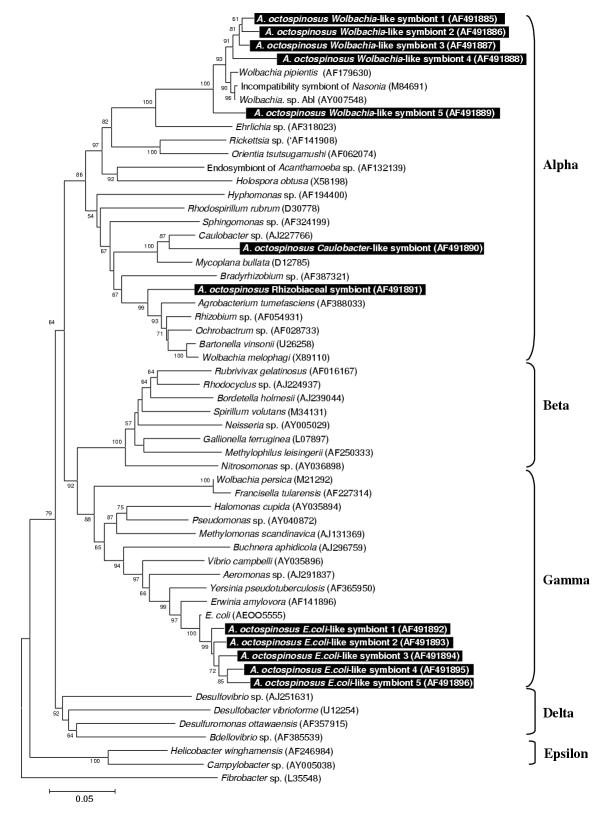
A total of 12 strains belonged to the Proteobacteria (Fig 2). Among these, 5 strains clustered within the α-proteobacterial genus Wolbachia. Two other α-Proteobacteria were present, one closely related to the freshwater bacterium Caulobacter sp., the other falling within the family Rhizobiaceae. A last group of 5 sequences is closely related to the γ-proteobacterial pathogen Escherichia coli.
Figure 2.
Neighbour joining tree for proteobacterial strains based on partial sequences of the 16S rRNA gene The tree was rooted using Fibrobacter sp. (EMBL L35548) as an outgroup sequence. See legend of Fig. 1 for further details.
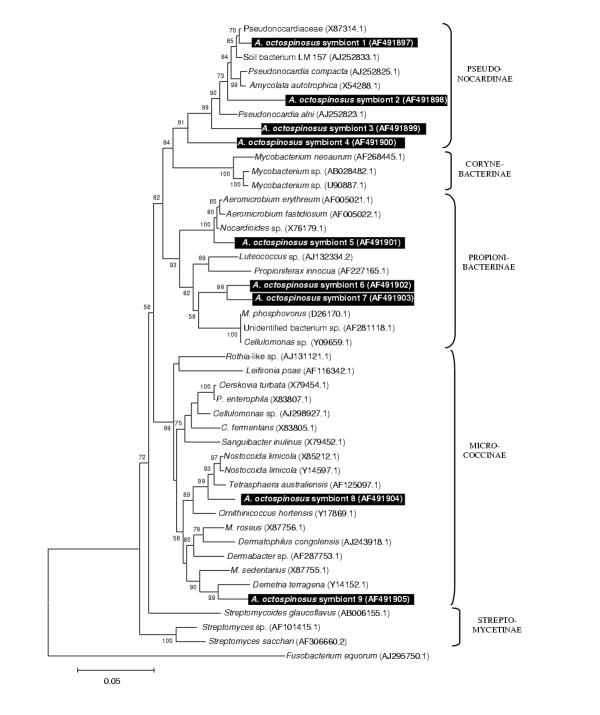
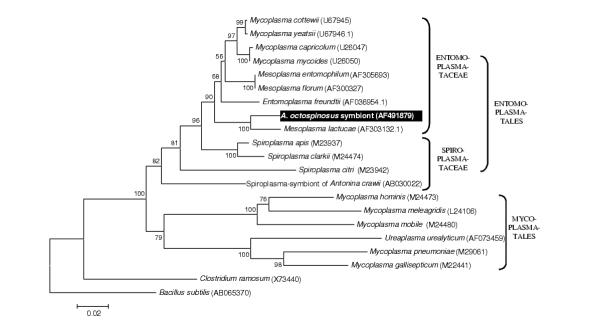
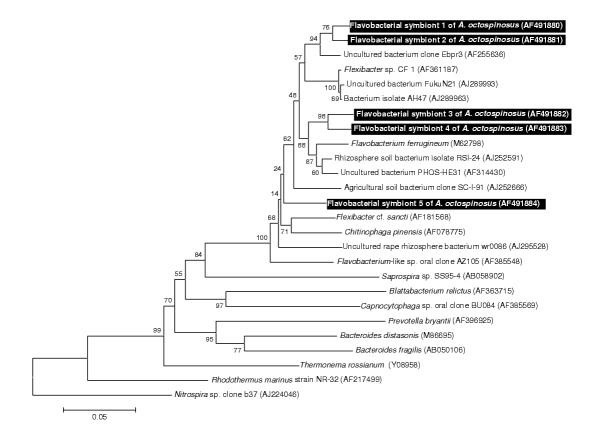
A total of nine strains of Gram-positive bacteria with high G+C content (Fig 3) was identified, all belonging to the Actinomycetales: superfamilies Pseudonocardinae (4 strains), Propionibacterina (3 strains), and Micrococcinae (2 strains). The single sequence belonging to the Gram-positive bacteria with low G+C content clustered within the insect pathogenic family Entomoplasmataceae (Fig 4) and five flavobacterial strains clustered together with various representatives of the Flexibacter and Flavobacterium group, none of which are known associates of insects.
Figure 3.
Neighbour joining tree for Gram positive bacteria with high G+C content based on partial sequences of the 16S rRNA gene The tree was rooted using Fusobacterium equorum (EMBL AJ295750) as an outgroup sequence. See legend of Fig. 1 for further details.
Figure 4.
Neighbour joining tree for Gram positive bacteria with low G+C content based on partial sequences of the 16S rRNA gene The tree was rooted using Bacillus subtilis (EMBL AB065370). See legend of Fig. 1 for further details.
Discussion
It is difficult to predict the fitness effects of an associated microorganism (i.e. wheter it is a mutualist, a parasite, or just a commensal or ingested food item) from its phylogenetic affiliation. Closely related microorganisms often reside in a totally different ecological niche (eg. Bartonella pathogenic proteobacteria are closely related to root nodulating Rhizobiaceae, [41]. Only in some cases, where a complete monophyletic group of microorganisms has a similar life style, reasonable predictions can be made about the fitness effects of additional microbes clustering within such groups. Possible examples in this study include: (1) A subset of five proteobacterial strains clearly clustering within the Wolbachia group of reproductive parasites of arthropods (Fig 2). Wolbachia is a maternally transmitted bacterium that manipulates the reproduction of its arthropod host [42-44]. These bacteria often force their hosts to produce broods of mostly female offspring, the sex that maximises Wolbachia transmission due to its exclusively maternal inheritance. Wolbachia infections are present in more than half of the ant species studied so far, but are unusually diverse in the leafcutter ants [17,18,22,45,46]. (2) A second case where the phylogeny is informative concerns the fungal strain that clusters within a group of Cordyceps and Beauveria entomopathogens (Fig. 1). Cordyceps species are known to infect a variety of insects [47] and can have severe mortality and morbidity consequences for ant hosts [48,49]. A small subset of Cordyceps species jumped to truffles hosts instead of insects [47], but all known Cordyceps species are virulent and mostly obligatory pathogens. Since all Cordyceps have an obligatory and potentially virulent parasitic life history, the most parsimonous assumpion is that new fungi clustering within this group are also parasitic. Secondary loss of a parasitic life might have occurred but would require numerous adaptations, since the biology of these microorganisms is evolutionary tuned to be parasitic. We also sequenced an 18S rRNA gene fragment of a Cordyceps fruiting body from a Camponotus atriceps worker that was found in the same sampling site (Fig 1). Although horizontal transmission of Cordyceps has been documented before [47], we did not find any evidence for horizontal transmission. The Cordyceps originating from Camponotus was not more closely to the one found in A. octospinosus than those from hosts in other environments, indicating that there is no link between proximity and relatedness of infecting strains. (3) The single Gram positive bacterium with high GC content we found in A. octospinosus, clusters with sequences representing the genera Entomoplasma and Mesoplasma (Fig 4), both of which consist mostly of insect associated parasites [50,51]. It is therefore also in this case very likely that the strain we found in A. octospinosus is also a parasite. (4) A total of 15 clones were almost identical in sequence and clustered with the Leucoagaricus garden symbionts cultivated as a major food resource by other Attine ants [52,53]. This implies that fragments of the Acromyrmex octospinosus mutualistic fungus were present as food particles in the ant guts. As a consequence, our PCR's are also likely to have amplified other microorganisms that occur in the fungus garden.
For the remaining sequences isolated in this study, no SSU rRNA sequences are currently present in Genbank that give a clear idea about their effects in Acromyrmex octospinosus. They may be just neutral passengers in the gut, or they may be parasites or mutualists for which the rRNA genes of closely related symbionts have not yet been determined. These bacterial associates with unclear effect include: (1) two of the fungal sequences having plant pathogens (resp. Didymella and Rhizoctonia) among their closest relatives. If the clustering of these fungi within plant pathogenic taxa remains stable when more sequences become available through GenBank (which is not certain given the considerable distances to their currently known closest relatives), the most probable explanation for their occurrence inside the ants is that they are passengers and food in the gut, originating from harvested plant material. The last fungal associate, related to the human skin pathogen Fonseceae pedrosoi leaves us no clue as to its effects on the ants. We are almost certain that this is not a artifact, as all laboratory work was done under a laminar flow hood and wearing gloves and because this microorganism was not retrieved in the analysis of the Camponotus tissue. (2) Of the remaining proteobacteria, the distant relative of the water bacterium Caulobacter may be another environmental contaminant, as this bacterium belongs to the Rhizobiaceae, which are almost all plant symbionts [54]. Other Rhizobiaceae have been described as symbionts of the ant Tetraponera binghami[14], but only in association with a specialized organ for nitrogen recycling, which is not present in leafcutter ants. The E. coli relatives (assuming a stable clustering near E. coli) are most probably mild gut parasites, as this lifestyle is typical for the genus Escherichia[55]. A close relative, E. blattae has been described from the gut of a cockroach [56], confirming that insects can be hosts of these bacteria. Unfortunately, the SSU rRNA gene of E. blattae has not been sequenced yet, so that we cannot determine how our sequences relate to it. (3) Several of the nine Actinomycetal associates of A. octospinosus are related to soil bacteria. These may be nuisances removed from the nest and carried by the ants in their infrabuccal pocket. As no phylogenetically conserved lifestyles seem to exist in this group of bacteria, however, they may equally well be genuine mutualists or pathogens harboured by the ants. Some actinomycetes have been described that reside on these ants' cuticle and produce an antibiotic against garden pests [15,16]. None of the sequences we isolated in this study, however, were closely related to these cuticular symbionts (C. Currie, pers. comm.). All other known insect-associated actinomycetes (gut associates of Culex mosquitos, [57], and of termites, [58] belong to the Streptomycetinae, a subfamily to which none of our sequences belongs. In conclusion, from the information currently present we cannot say what effect the isolated actinomycete strains may have on A. octospinosus. (4) The relatives of the five identified Flavobacteria come from diverse environments and leave us no idea about the possible effect of the identified strains. Most of the related Genbank Flavobacteria were actually isolated from water or soil samples, so that we cannot exclude that some of our sequences were contaminants from nest particles that were stored in the infrabuccal pockets of the ants.
We did not amplify any DNA sequences using primers designed to target Microsporidia, Parabasalidea and Diplomonadidae, Spirochetes and Fibrobacter. Known microsporidian insect associates include Thelohania and Vairimorpha parasites of fire ants [26,59,60], Nosema spp. parasitising numerous bee species and a variety of termite associates (reviewed in [61]). Several hindgut symbionts of termites belong to the Parabasalidea [33] and the Spirochetes [33]. The absence of any bacteria from the latter Fibrobacter control group, which is known to contain only ruminal bacteria [39], is an extra confirmation of the specificity of our primer sets [40]. The absence of any representative of these groups is unlikely to be a technical artifact since the group-specific primer pairs were designed using all known representatives in GenBank of the respective groups and given the elaborate amplification tests using several positive controls per group in Van Borm and Boomsma (2002). Our analysis thus suggests that three groups known to contain insect-associated microorganism are lacking or very rare (below the detection threshold), so that they are unlikely to be important in the investigated population of Acromyrmex octospinosus.
Conclusions
The major merits of the screening approach documented here is its high sensitivity and specificity, which allowed us to identify several microorganisms that are promising candidates for further study of their interactions with Acromyrmex leafcutter ants or their gardens. However, a disadvantage associated with the sensitivity of our approach is the co-amplification of a number of microorganisms that may be nothing more than food or biologically irrelevant gut passengers. Further research, including microorganism-specific PCR screenings and in situ hybridisation will be needed to clarify the importance of the microorganisms found in this study for the functioning of the ant-fungus symbiosis.
Materials and Methods
Sampling and DNA extraction
Workers of 14 colonies of Acromyrmex octospinosus were collected in Gamboa, Panama in April 2000, and preserved in 95% ethanol. The sampled colonies included AO101, AO103, AO104 (only 3 small workers sampled), AO107, AO108, AO110, AO115, AO116, AO117, AO120, AO121, AO122, AO127, and AO130 (voucher specimens of workers from these colonies were deposited in the collection of the Lab. of Entomology, K.U. Leuven). To minimise the risk of cross-contamination, the pre-extraction treatment and all DNA extraction procedures were performed in a laminar flow hood under sterile conditions. Nine workers (3 small workers, 3 medium sized workers, and 3 large workers) of each colony were externally sterilised by immersion in 70% ethanol, followed by two rinses in double distilled water and exposure for 2 h to UV radiation (250 nm). The entire ants were subsequently ground after freezing in liquid nitrogen, and their DNA was extracted by 3 h incubation at 55°C and 20 min boiling in 500 μl of an auto-claved 10 % Biorad Chelex 100 resin solution. The resulting extract thus contained DNA from all microorganisms within the ant's body (i.e. not only parasites and symbionts, but also food and micro-organisms in the gut). To sample the total microbial diversity of the studied population, 5 μl of each of the 120 (9 workers sampled from 13 colonies + 3 workers sampled from 1 colony) individual extracts was taken and joined in a pooled sample. All samples were centrifuged and stored at -20°C until use.
Group-specific amplification of SSU rDNA
SSU ribosomal RNA encoding sequences were specifically amplified for 9 microbial groups (9) using the primer pairs, control templates, and conditions identified by Van Borm and Boomsma [40]. Primer information will be available on request from the authors. For each reaction, 10 μl of the amplification product was electophorised together with a 100 bp length standard on 1% agarose minigels. For groups that yielded a positive result (meaning that at least one representative of the group was present in our pooled sample), the group-specific band was excised from the agarose gel and DNA was extracted from the gel fragment (GFX™ PCR DNA and Gel Band Purification Kit, Amersham Pharmacia Biotech Inc.).
Cloning
The purified PCR amplification products were subsequently ligated into a pCR®2.1-TOPO vector (Invitrogen Topo™ TA Cloning Kit). The vectors were transformed in chemically competent E. coli cells (Invitrogen Topo™ TA Cloning Kit), plated on selective agar plates containing ampicillin and incubated overnight at 37°C. The resulting clones were suspended in 50 μl double distilled water. Positive transformants were determined by PCR, using primers M13F (5' GTA AAA CGA CGG CCA G 3') and M13R (5' CAG GAA ACA GCT ATG AC 3') provided by the manufacturer. PCR amplification reactions were carried out in 25 μl reaction mixtures containing 0.8 μM of each primer, 0.2 mM of each dNTP, 1.5 mM MgCl2, 1 μl of the suspended clone, 0.3 U of Taq DNA polymerase (AmpliTaq, Perkin Elmer Cetus) and PCR buffer specified by the manufacturer. PCR was performed with an initial denaturation at 97°C for 5 min, followed by 30 cycles consisting of 95°C for 30 sec, 60°C for 45 sec and 72°C for 1 min, and a final extension at 72°C for 10 min.
Sequencing
DNA was purified from the M13-PCR product (GFX™ PCR DNA and Gel Band Purification Kit, Amersham Pharmacia Biotech Inc.). The number of clones sequenced for each of the groups is given in Table 1. Sequencing reactions contained 4 pmol of each IRD (infrared-dye)-labelled M13 primer, 5 U of SequiTherm Excel™II DNA polymerase (Epicentre Technologies) and buffer prescribed by the manufacturer. The PCR reaction was performed with an initial denaturation at 95°C for 5 min, followed by 30 cycles of 95°C for 30 sec, 55°C for 15 sec and 70°C for 1 min, and a final extension at 70°C for 10 min. Subsequently, reactions were transferred to a Polyacrylamide gel in a LI-COR® automated sequencer.
Figure 5.
Neighbour joining tree for flavobacterial strains based on partial sequences of the 16S rRNA gene The tree was rooted using Nitrospira sp. (EMBL AJ224046) as an outgroup sequence. See legend of Fig. 1 for further details.
Phylogenetic analysis
The partial SSU rDNA sequences of each microbial group were aligned using the CLUSTAL W program [62] followed by manual refinements. The two closest relatives of each sequence found in a BLAST similarity search and several representative species found in Genbank [63] were included in the alignments. Using the MEGA2.0 software [64], a neighbour-joining tree was calculated for each group from a Kimura 2-parameter based distance matrix with pairwise deletion of insertions and deletions. Bootstrap analysis testing the reliability of the clades in the phylogeny included 1000 pseudoreplications. Each tree was rooted using an outgroup sequence (see figures for details), except for the Fungi where the tree was rooted using the Basidiomycota group as a sister group of the Ascomycota.
Authors' contributions
SVB carried out the molecular laboratory work and the phylogenetic analysis, and participated in the fieldwork. JB designed and coordinated the study. JJB participated in the design of the sampling scheme, in the fieldwork and advised on the analysis. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
We thank J. Longino for identifying the Camponotus atriceps and C. Currie for advise and comments. This work was supported by grant no. 981085 from the Institute for the Promotion of Innovation by Science and Technology in Flanders (IWT) to SVB. The research collaboration between the Universities of Leuven and Copenhagen has been supported by subcontracts under the EU-TMR network "Social Evolution" and the EU-IHP network "INSECTS".
Contributor Information
Steven Van Borm, Email: steven.vanborm@bio.kuleuven.ac.be.
Johan Billen, Email: johan.billen@bio.kuleuven.ac.be.
Jacobus J Boomsma, Email: JJBoomsma@zi.ku.dk.
References
- Woese CR. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. Journal of Bacteriology. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RI, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Bateson MM, Weller R, Ruff-Roberts AL. Ribosomal RNA analysis of microorganisms as they occur in nature. In: Marshall KC, editor. Advances in Microbial Ecology. Vol. 12. New York: Plenum Press; 1992. pp. 219–286. [Google Scholar]
- Schmidt TM, DeLong EF, Pace NR. Analysis of a marine picoplankton community by 16S rRNA gen cloning an sequencing. Journal of Bacteriology. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon J-J, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–2813. doi: 10.1128/aem.63.7.2802-2813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barns SM, Fundyga RE, Jeffries MW, Pace NR. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:1609–1613. doi: 10.1073/pnas.91.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RI, Springer N, Ludwig W, Görtz H-D, Schleifer K-H. Identification in situ and phylogeny of uncultured endosymbionts. Nature. 1991;351:161–164. doi: 10.1038/351161a0. [DOI] [PubMed] [Google Scholar]
- Watt C, Dobson AP, Grenfell BT. Glossary. In: Grenfell BT, Dobson AP, editor. Ecology of infectious diseases in natural populations. Cambridge: Cambridge University Press; 1995. pp. 510–521. [Google Scholar]
- Herre EA. Laws governing species interactions? Encouragement and caution from figs and their associates. In: Keller L, editor. Levels of selection in evolution. Princeton, New Jersey: Princeton University Press; 1999. pp. 209–237. [Google Scholar]
- Clark MA, Moran NA, Baumann P, Wernegreen JJ. Cospeciation between bacterial endosymbionts (Buchnera) and a recent radiation of aphids (Uroleucon) and pitfalls of testing for phylogenetic congruence. Evolution. 2000;54:517–525. doi: 10.1111/j.0014-3820.2000.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Sauer C, Stackebrandt E, Gadau J, Hölldobler B, Gross R. Systematic relationships and cospeciation of bacterial endosymbionts and their carpenter ant host species: proposal of the new taxon Candidatus Blochmannia gen.nov. International Journal of Systematic and Evolutionary Microbiology. 2000;50:1877–1886. doi: 10.1099/00207713-50-5-1877. [DOI] [PubMed] [Google Scholar]
- Peloquin JJ, Miller SG, Klotz SA, Stouthammer R, Davis LR, Klotz JH. Bacterial endosymbionts from the genus Camponotus (Hymenoptera: Formicidae). Sociobiology. 2001;38:695–708. [Google Scholar]
- Van Borm S, Buschinger A, Boomsma JJ, Billen J. Tetraponera ants have gut-symbionts related to nitrogen-fixing symbionts. Submitted. 2002. [DOI] [PMC free article] [PubMed]
- Currie CR. A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu Rev Microbiol. 2001. [DOI] [PubMed]
- Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. doi: 10.1038/19519. [DOI] [Google Scholar]
- Van Borm S, Wenseleers T, Billen J, Boomsma JJ. Wolbachia in leafcutter ants: a widespread symbiont that may induce male killing or incompatible matings. Journal of Evolutionary Biology. 2001;14:805–814. doi: 10.1046/j.1420-9101.2001.00321.x. [DOI] [Google Scholar]
- Van Borm S, Wenseleers T, Billen J, Boomsma JJ. Cloning and sequencing of wsp encoding gene fragments reveals a diversity of co-infecting Wolbachia strains in Acromyrmex leafcutter ants. Mol Phylogenet Evol. 2002;Submitted doi: 10.1016/s1055-7903(02)00298-1. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Kinship, recognition, disease and intelligence: constraints of social evolution. In: Ito Y, Brown JL, Kikkawa J, editor. Animal Societies: Theories & Facts. Tokyo: Japan Scientific Societies Press; 1987. pp. 81–102. [Google Scholar]
- Sherman PW, Seeley TD, Reeve HK. Parasites, pathogens, and polyandry in social Hymenoptera. Am Nat. 1988;131:602–610. doi: 10.1086/284809. [DOI] [PubMed] [Google Scholar]
- Shykoff JA, Schmid-Hempel P. Parasites and the advantage of genetic variability within social insect colonies. Proceedings of the Royal Society of London Series B Biological Sciences. 1991;243:55–58. [Google Scholar]
- Wenseleers T, Ito F, Van Borm S, Huybrechts R, Volckaert F, Billen J. Widespread occurrence of the micro-organism Wolbachia in ants. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1447–52. doi: 10.1098/rspb.1998.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder D, Deppisch H, Obermayer M, Krohne G, Stackebrandt E, Hölldobler B, Goebel W, Gross R. Intracellular endosymbiotic bacteria of Camponotus species (carpenter ants) : systematics, evolution and ultrastructural characterization. Mol Microbiol. 1996;21:479–489. doi: 10.1111/j.1365-2958.1996.tb02557.x. [DOI] [PubMed] [Google Scholar]
- Sameshima S, Hasegawa E, Kitade O, Minaka N, Matsumoto T. Phylogenetic comparison of endosymbionts with their host ants based on molecular evidence. Zoological Science. 1999;16:993–1000. [Google Scholar]
- Moser BA, Becnel JJ, Maruniak J, Patterson RS. Analysis of the ribosomal DNA sequences of the microsporidia Thelohania and Vairimorpha of fire ants. J Inv Pathol. 1998;72:152–159. doi: 10.1006/jipa.1998.4776. [DOI] [PubMed] [Google Scholar]
- Moser BA, Becnel JJ, DF W. Morphological and molecular characterisation of the Thelohania solenopsae complex (Microsporidia: Thelohaniidae). J Inv Pathol. 2000;75:174–177. doi: 10.1006/jipa.1999.4895. [DOI] [PubMed] [Google Scholar]
- Gatehouse HS, Malone LA. The ribosomal RNA gene region of Nosema apis (Microspora): DNA sequence for small and large subunit rRNA genes and evidence of a large tandem repeat unit size. J Inv Pathol. 1998;71:97–105. doi: 10.1006/jipa.1997.4737. [DOI] [PubMed] [Google Scholar]
- Govan VA, Allsopp MH, Davison S. A PCR detection method for rapid identification of Paenibacillus larvae. Appl Environ Microbiol. 1999;65:2243–5. doi: 10.1128/aem.65.5.2243-2245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan VA, Brozel V, Allsopp MH, Davison S. A PCR detection method for rapid identification of Melissococcus pluton in honeybee larvae. Appl Environ Microbiol. 1998;64:1983–1985. doi: 10.1128/aem.64.5.1983-1985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries I, Feng F, daSilva A, Slemenda SB, Pieniazek NJ. Nosema ceranae n. sp. (Microsporidia, Nosematidae), morphological and molecular characterisation of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). European Journal of Protistology. 1996;32:256–365. [Google Scholar]
- Shoemaker DD, Ross KG, Keller L, Vargo EL, Werren JH. Wolbachia infections in native and introduced populations of fire ants (Solenopsis spp.). Insect Mol Biol. 2000;9:661–673. doi: 10.1046/j.1365-2583.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- Wenseleers T, Sundström L, Billen J. Deleterious Wolbachia in the ant Formica truncorum. Proceedings of the Royal Society of London, Series B. 2002;296:623–629. doi: 10.1098/rspb.2001.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Ohkuma M, Moriya S, Noda S, Ohtoko K. Molecular phylogenetic identification of the intestinal anaerobic microbial community in the hindgut of the termite, Reticulitermes speratus, without cultivation. Extremophiles. 1998;2:155–161. doi: 10.1007/s007920050055. [DOI] [PubMed] [Google Scholar]
- Ohkuma M, Ohtoko K, Iida T, Tokura M, Moriya S, Usami R, Horikoshi K, Kudo T. Phylogenetic identification of hypermastigotes, Pseudotrichonympha, Spirotrichonympha, Holomastigotoides, and parabasalian symbionts in the hindgut of termites. J Eukaryot Microbiol. 2000;47:249–259. doi: 10.1111/j.1550-7408.2000.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Ohkuma M, Noda S, Horikoshi K, Kudo T. Phylogeny of symbiotic methanogens in the gut of the termite Reticulitermes speratus. FEMS Microbiol Lett. 1995;134:45–50. doi: 10.1016/0378-1097(95)00379-J. [DOI] [PubMed] [Google Scholar]
- Ohkuma M, Kudo T. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus. Applied and Environmental Microbiology. 1996;62:461–468. doi: 10.1128/aem.62.2.461-468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braumann A, Dore J, Eggleton P, Bignell D, Breznak JA, Kane MD. Molecular phylogenetic profiling of prokaryotic communities in guts of termites with different feeding habits. FEMS Microbiol Lett. 2001;35:27–36. doi: 10.1111/j.1574-6941.2001.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Friedrich MW, Schmitt-Wagner D, Lueders T, Brune A. Axial differences in community structure of Cremnarchaeota amnd Euryarchaeota in the highly compartimentalized gut of the soil-feeding termite Cubitermes orthognathus. Appl Environ Microbiol. 2001;67:4880–4890. doi: 10.1128/AEM.67.10.4880-4890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery L, Flesher B, Stahl D. Transfer of Bacteroides succinogenes (Hungate) to Fibrobacter gen. nov. as Fibrobacter succinogenes comb. nov. and description of Fibrobacter intestinalis sp. nov. International Journal of Systematic Bacteriology. 1988;38:430–435. [Google Scholar]
- Van Borm S, Boomsma JJ. Group-specific PCR amplification of SSU rRNA encoding gene fragments from 12 microbial taxa. Molecular Ecology Notes. 2002.
- Ihler GM. Bartonella bacilliformis : dangerous pathogen slowly emerging from deep background. FEMS Microbiology Letters. 1996;144:1–11. doi: 10.1016/0378-1097(96)00307-2. [DOI] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Hoffmann AA, Werren JH. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford: Oxford University Press; 1997. [Google Scholar]
- Stouthamer R, Breeuwer JA, Hurst GD. Wolbachia pipientis : microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Wenseleers T, Schoeters E, Billen J, Wehner R. Distribution and comparative morphology of the cloacal gland in ants (Hymenoptera: Formicidae). Int J Insect Morphol Embryol. 1998;27:121–128. doi: 10.1016/S0020-7322(97)00026-3. [DOI] [Google Scholar]
- Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Nikoh N, Fukatsu T. Interkingdom host jumping underground: phylogenetic analysis of entomoparasitic fungi of the genus Cordyceps. Molecular Biology and Evolution. 2000;17:629–638. doi: 10.1093/oxfordjournals.molbev.a026341. [DOI] [PubMed] [Google Scholar]
- Evans HC, Samson RA. Cordyceps spp. and their anamorph pathogenic on ants (Formicidae) in tropical forest ecosystems. I. The Cephalotes (Myrmicinae) complex. Transactions of the British Mycological Society. 1982;79:431–453. [Google Scholar]
- Evans HC, Samson RA. Cordyceps spp. and their anamorph pathogenic on ants (Formicidae) in tropical forest ecosystems. II. The Camponotus (Formicinae) complex. Transactions of the British Mycological Society. 1984;82:127–150. [Google Scholar]
- Tully JG, Whitcomb RF, Hackett KJ, Williamson DL, Laigret F, Carle P, Bove JM, Henegar RB, Ellis NM, Dodge DE, Adams J. Entomoplasma freundtii sp. nov., a new species from a green tiger beetle (Coleoptera: Cicindelidae). International Journal of Systematic Bacteriology. 1998;48:1197–1204. doi: 10.1099/00207713-48-4-1197. [DOI] [PubMed] [Google Scholar]
- Tully JG, Whitcomb RF, Hackett KJ, Rose DL, Henegar RB, Bove JM, Carle P, Williamson DL, Clark TB. Taxonomic descriptions of eight new non-sterol-requiring mollicutes assigned to the genus Mesoplasma. International Journal of Systematic Bacteriology. 1994;44:685–693. doi: 10.1099/00207713-44-4-685. [DOI] [PubMed] [Google Scholar]
- Chapela IH, Rehner SA, Schultz TR, Mueller UG. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691. [DOI] [PubMed] [Google Scholar]
- Weber NA. Symbiosis between fungus-growing ants and their fungus. The American Philosophical Society. 1955. pp. 153–157.
- Yanagi M, Yaasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiology Letters. 1993;107:115–120. doi: 10.1016/0378-1097(93)90364-8. [DOI] [PubMed] [Google Scholar]
- Krieg NR, Holt JG. Bergey's manual of systematic bacteriology. Baltimore/London: Williams & Wilkins; 1984. [Google Scholar]
- Burgess NRH, McDermott SN, Withing J. Aerobic bacteria occuring in the hind-gut of the cockroach Blatta orientalis. J Hyg (Lond) 1973;71 doi: 10.1017/s0022172400046155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthi V, Hoti SL. Microbial flora in gut of Culex quinquefasciatus breeding in cess pits. Southeast Asian Journal of Tropical Medicine and Public Health. 1992;23:312–317. [PubMed] [Google Scholar]
- Pasti MB, Pometto ALr, Nuti MP, Crawford DL. Lignin-solubilizing ability of actinomycetes isolated from termite (Termitidae) gut. Applied and Environmental Microbiology. 1990;56:2213–2218. doi: 10.1128/aem.56.7.2213-2218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briano JA, Patterson RS, Becnel JJ, Cordo HA. The black imported fire ant, Solenopsis richteri, infected with Thelohania solenopsae: intracolonial prevalence of infection and evidence for transovarial transmission. J Inv Pathol. 1996;67:178–179. doi: 10.1006/jipa.1996.0026. [DOI] [Google Scholar]
- Moser BA, Becnel JJ, Maruniak J, Patterson RS. Analysis of the ribosomal DNA sequences of the microsporidia Thelohania and Vairimorpha of fire ants. J Inv Pathol. 1998;72:154–9. doi: 10.1006/jipa.1998.4776. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Parasites in social insects. Princeton: Princeton University Press; 1998. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:6008–6013. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DA. GenBank. Nucleic Acids Res. 2000;28:15–18. doi: 10.1093/nar/28.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tumara K, Jakobsen IB, Nei M. MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics. 2002. [DOI] [PubMed]