Abstract
We investigated the biomarker profile of neurodegeneration, Alzheimer’s and Lewy body pathology in the CSF of 148 polysomnography-confirmed patients with isolated REM sleep behavior disorder (IRBD), a condition that precedes Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). We assessed misfolded α-synuclein (AS) by RT-QuIC assay, amyloid-beta peptides (Aβ42 and Aβ40), phosphorylated tau (p-tau), and total tau (t-tau) by CLEIA and neurofilament light chain (NfL) by ELISA. We detected AS in 75.3% of patients, pathologically decreased Aβ42/Aβ40 ratio in 22.5%, increased p-tau in 15.5%, increased t-tau in 14.9%, and elevated NfL in 14.7%. After a mean follow-up of 2.48 ± 2.75 years, 47 (38.1%) patients developed PD (n = 24) or DLB (n = 23). At CSF collection, AS positivity [HR 4.05 (1.26–12.99), p = 0.019], mild cognitive impairment [3.86 (1.96–7.61), p < 0.001], and abnormal DAT-SPECT [2.31 (1.09–4.91), p < 0.030] were independent predictors of conversion to PD and DLB. Among the other CSF markers, only elevated p-tau/Aβ42 was predictive of conversion, although only to DLB and not as an independent variable. In IRBD, CSF AS assessment by RT-QuIC provides an added value in defining the risk of short-term conversion to PD and DLB independent of clinical and instrumental investigations. Positive Alzheimer's disease (AD) pathology markers and elevated NfL occur in a subgroup of patients, but p-tau/Aβ42 is the only marker that predicts short-term conversion to DLB. Longer follow-up is needed to assess if AD biomarkers predict the later development of PD and DLB in IRBD.
Subject terms: Predictive markers, Neurodegeneration
Introduction
The synucleinopathies Parkinson’s disease (PD), dementia with Lewy bodies (DB) and multiple system atrophy (MSA) are neurodegenerative disorders characterized by misfolded α-synuclein (AS) accumulation in the nervous system1. The severity and extent of AS deposits in vulnerable regions are thought to be responsible for the core clinical manifestations of these diseases, namely parkinsonism and dementia1. The comorbid Alzheimer’s disease (AD) pathology, documented by postmortem studies in DLB and, to a lesser extent, in PD, is also thought to contribute to the development of cognitive decline2,3.
There is strong evidence indicating that isolated REM sleep behavior disorder (IRBD) is a prodromal clinical condition often progressing to the synucleinopathies PD and DLB and, less frequently, MSA4–6. Thus, IRBD patients constitute a candidate population to enter in neuroprotective trials addressing the early disease stages of synucleinopathies7.
Neuropathological studies in subjects with IRBD revealed a high burden of AS deposits in the brainstem8–11. We have observed that in IRBD patients who develop PD with associated dementia (PDD) and DLB, the neuropathological substrate consists of widespread AS, but comorbid AD changes are also frequently found12.
The study of cerebrospinal fluid (CSF) in living individuals allows the evaluation of biomarkers specific to synucleinopathies, AD, and neuronal degeneration. In the CSF of most PD and DLB patients, seed amplification assays (SAAs), such as real-time quaking-induced conversion (RT-QuIC), detect AS13. The core CSF biomarker profile of AD includes decreased levels of Aβ42, the 42-amino acid isoform of amyloid-beta and of its relative ratio with Aβ40, the 40-amino acid isoform, and increased hyperphosphorylated tau (p-tau) and total tau (t-tau) concentrations, reflecting the presence of amyloid plaques, tau-related AD pathology and neuronal degeneration in the brain14. This CSF AD profile can also be detected with increasing prevalence in PD, PDD, and DLB patients15–19. In the CSF and blood, increased neurofilament light chain (NfL) levels are indicative of myelinated axonal injury and are considered predictors of cognitive decline and disease progression in several neurodegenerative conditions including PD18.
SAAs detect AS in the CSF of 75–90% of the IRBD patients, a finding supporting the notion that this parasomnia represents the prodromal stage of the synucleinopathies20–23. In IRBD, there is limited data on AD biomarkers and NfL levels; abnormalities in these markers could have prognostic implications24,25. Our study aimed to assess the prevalence and clinical significance of the CSF biomarker profile of neurodegeneration in living IRBD patients.
Results
We retrospectively evaluated 148 IRBD patients, 113 (76.4%) men, who underwent a lumbar puncture at the mean age of 69.1 ± 9.7 years. Demographic, clinical and biomarker data are reported in Tables 1–3. There was a significant association between age at lumbar puncture with AS positivity, increased levels of NfL (r = 0.396, p < 0.0001), t-tau (r = 0.280, p = 0.0007), and p-tau (r = 0.344, p < 0.0001), and reduced Aβ42/Aβ40 ratio (r = −0.207, p = 0.014).
Table 1.
Demographic, clinical, and CSF biomarkers data of the patients with IRBD
| All patients | Positive α-synuclein | Negative α-synuclein | P value | |
|---|---|---|---|---|
| N (%) | 148a | 110 (75.3) | 36 (24.7) | – |
| Female, n (%) | 35 (23.6) | 27 (24.5) | 8 (22.2) | >0.99 |
| Age at LP, yrs | 69.1 ± 9.7 | 70.9 ± 6.5 | 65.4 ± 10.1 | 0.0002 |
| Age at IRBD diagnosis, yrs | 66.7 ± 7.6 | 67.8 ± 6.5 | 63.4 ± 9.7 | 0.0021 |
| Time from IRBD diagnosis to LP, yrs | 1.7 (0.5–4.2) | 2.1 (0.5–4.5) | 0.8 (0.4–3.4) | 0.126 |
| Prodromal PD rate at LP, % | 82.1 ± 28.3 | 89.0 ± 22.9 | 59.4 ± 32.3 | <0.0001 |
| DAT-SPECT abnormal/tested, n (%) | 84/145 (57.9) | 73/108 (67.6) | 9/35 (25.7) | <0.0001 |
| UPSIT-40 score | 19.0 (14.0–25.0) | 17.0 (13.0–22.0) | 27.0 (22.0–30.0) | <0.0001 |
| Mild cognitive impairment at LP, n (%) | 33 (22.3) | 26 (23.6) | 6 (16.7) | 0.488 |
| A + T + N + , n (%) | 13 (9.3) | 10 (9.4) | 3 (8.8) | >0.99 |
| A + T + , n (%) | 16 (11.4) | 12 (11.3) | 4 (11.8) | >0.99 |
| A + T-, n (%) | 16 (11.4) | 13 (12.3) | 3 (8.8) | 0.761 |
| Aβ42, pg/mL | 570 (405–808) | 573 (403–821) | 574 (415–822) | 0.761 |
| Aβ42/Aβ40 | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.8 (0.6–0.9) | 0.653 |
| p-tau, pg/mL | 38 (27–49) | 39 (28–50) | 35 (25–44) | 0.271 |
| p-tau/Aβ42 | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | 0.473 |
| t-tau, pg/mL | 280 (217–353) | 290 (219–380) | 257 (212–335) | 0.308 |
| NfL, pg/mL | 550 (405–761) | 620 (459–802) | 485 (344–665) | 0.0045 |
Data are expressed as mean ± SD, median (interquartile range), number, and percentage, as appropriate. Aβ positivity (A+) was defined by CSF Aβ42/Aβ440 ratio < 0.65; tau positivity (T+) by CSF p-tau181 > 60 pg/ml; and neurodegeneration positivity (N+) by CSF t-tau > 450 pg/ml.
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, IRBD isolated REM sleep behavior disorder, PD Parkinson disease, Aβ42 42 amino acid isoform of amyloid-beta, Aβ40 40 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta, T tau, N neurodegeneration, NfL neurofilament light chain, t-tau total tau protein.
aα-synuclein status was available in 146 patients while Alzheimer’s disease core biomarkers and NfL values were available in 142 individuals.
Table 3.
IRBD cohort stratified according to NfL values tested in 142 patients
| NfL low | NfL intermediate | NfL high | P value | |
|---|---|---|---|---|
| N (%) | 47 (33.1) | 48 (33.8) | 48 (33.1) | – |
| Female, n (%) | 16 (34.0) | 8 (16.7) | 10 (20.8) | 0.116 |
| Age at LP, years | 65.7 ± 9.0a | 69.7 ± 5.9 | 72.8 ± 7.3 | <0.0001 |
| Age at IRBD diagnosis, yrs | 63.2 ± 8.1b | 66.2 ± 6.1c | 70.3 ± 6.9 | <0.0001 |
| Prodromal PD rate at LP, % | 75.2 ± 30.0c | 77.4 ± 33.0 | 91.5 ± 17.4 | 0.0076 |
| DAT-SPECT tested/abnormal, n (%) | 23/44 (52.3) | 24/47 (51.1) | 32/48 (66.7) | 0.234 |
| UPSIT-40 score | 22 (16.0–27.5) | 19 (13.8–24.5) | 17.5 (14.0–23.0) | 0.182 |
| Converted during follow-up, n (%) | 12 (25.5) | 17 (35.4) | 15 (31.3) | 0.578 |
| Converted to DLB/PD (%) | 4/8 (33.3/66.7) | 10/7 (58.8/41.2) | 8/7 (53.3/46.7) | 0.381 |
| Time LP to conversion, yrs | 1.4 (0.5–4.1) | 2.3 (0.6–5.3) | 1.3 (0.3–3.7) | 0.143 |
| Mild cognitive impairment, n (%) | 10 (21.3) | 9 (18.8) | 13 (27.1) | 0.604 |
| AS positivity, n (%) | 29 (63.0) | 37 (77.1) | 40 (83.3) | 0.069 |
| A+T+, n (%) | 0 (0.0)a | 5 (10.9) | 11 (24.4) | 0.0008 |
| A+T-, n (%) | 6 (13.0) | 7 (15.2) | 3 (6.7) | 0.420 |
| Aβ42, pg/mL | 544 (381–703) | 604 (429–812) | 733 (429–921) | 0.156 |
| Aβ40/Aβ42 | 0.8 (0.7–0.9) | 0.8 (0.6–0.9) | 0.8 (0.5–0.9) | 0.627 |
| p-tau, pg/mL | 31 (25–39)b,c | 44 (30–50) | 46 (35–82) | <0.0001 |
| p-tau/Aβ42 | 0.6 (0.4–0.7) | 0.6 (0.4–0.9) | 0.7 (0.4–1.2) | 0.145 |
| t-tau, pg/mL | 238 (191–290)b,d | 305 (212–368) | 341 (249–550) | <0.0001 |
Data are expressed as mean ± SD, median (interquartile range), number, and percentage, as appropriate. NfL tertiles are defined as follow: low = 129–465 pg/ml; intermediate = 466–677 pg/ml; high = 678–1469 pg/ml. Aβ positivity (A+) was defined by CSF Aβ42/Aβ40 ratio < 0.65; and tau positivity (T+) by CSF p-tau181 > 60 pg/ml.
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, IRBD isolated REM sleep behavior disorder, PD Parkinson disease, DLB dementia with Lewy bodies, AS α-synuclein; Aβ42 42 amino acid isoform of amyloid-beta; Aβ40 40 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta, T tau, NfL neurofilament light chain, t-tau total tau protein.
avs. high p < 0.001.
bvs. high p < 0.0001.
cvs. high p < 0.01.
d vs. intermediate p < 0.05.
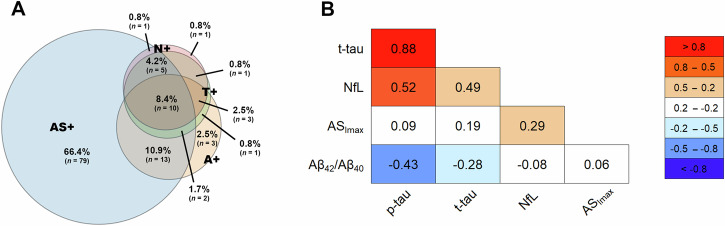
We detected AS positivity in 75.3% of the patients, decreased Aβ42/Aβ40 ratio (A+) in 22.5%, increased p-tau levels (T+) in 15.5%, high t-tau levels (N+) in 14.9% and increased NfL in 14.7%. The profile A+T− was found in 11.4% of the patients, A+T+ in 11.4%, and A+T+N+ in 9.3% (Table 1 and Fig. 1). One hundred four (73.2%) IRBD patients were A-T-.
Fig. 1. Overlap and correlations between abnormal biomarker results in IRBD.
Schematic representation of concomitant pathological profiles (A) and correlations among biomarkers (B). Biomarker positivity was defined as follows: A+ = Aβ positivity (Aβ42/Aβ40 < 0.65), T+ = tau positivity (p-tau181 > 60 pg/ml), N+ = neurodegeneration positivity (t-tau > 450 pg/ml), AS+ = misfolded α-synuclein positivity by SAA. For correlations, t-tau, p-tau, and NfL values were log-transformed. Pearson’s r values are shown. AS misfolded α-synuclein, ASImax the maximum peak of SAA fluorescence curve, Aβ42 42 amino acid isoform of amyloid-beta, Aβ40 40 amino acid isoform of amyloid-beta, p-tau tau protein phosphorylated at residue 181, NfL neurofilament light chain, t-tau total tau protein, SAA seed amplification assay.
When compared with patients with AS negativity, those with AS positivity were older, showed reduced DAT-SPECT uptake and hyposmia more frequently, greater likelihood of prodromal PD, and increased levels of NfL (Table 1). There were no significant differences in the percentage of patients with the A+T+ or A+T− profile between the AS positive and AS negative groups (Table 1). Seven patients with AS negativity had positive AD biomarkers in the CSF (four were A+T+, and three were A+T−).
The AD biomarker status A+T+ was positively associated with age, female sex, and NfL levels (Table 2).
Table 2.
IRBD cohort stratified according to AD core biomarker results tested in 142 patients
| Normal Aβ- (A-) | Abnormal Aβ (A+) | P value | Abnormal Aβ and p-tau (A+/T+) | P value | |
|---|---|---|---|---|---|
| N (%) | 110 (77.5) | 32 (22.5) | – | 16 (11.3) | – |
| Female, n (%) | 23 (20.9) | 12 (37.5) | 0.055 | 8 (50.0) | 0.025 |
| Age at LP, yrs | 68.5 ± 8.2 | 72.9 ± 6.1 | 0.0046 | 73.9 ± 6.2 | 0.0120 |
| Age at IRBD diagnosis, yrs | 65.5 ± 7.5 | 70.8 ± 6.8 | 0.0006 | 72.3 ± 7.0 | 0.0009 |
| Time IRBD diagnosis to LP, yrs | 1.5 (0.5–2.3) | 1.7 (0.4–3.5) | 0.391 | 0.6 (0.3–1.9) | 0.080 |
| Prodromal PD rate at LP, % | 80.6 ± 29.9 | 91.2 ± 15.9 | 0.254 | 89.7 ± 15.0 | 0.969 |
| Abnormal DAT-SPECT, n (%)a | 62 (57.9) | 20 (62.5) | 0.646 | 10 (62.5) | 0.792 |
| UPSIT-40 score | 19.0 (14.0–26.0) | 16.5 (14.0–21.0) | 0.128 | 17.0 (14.5–22.0) | 0.327 |
| Mild cognitive impairment at LP, n (%) | 21 (19.1) | 11 (34.4) | 0.069 | 3 (18.8) | 0.974 |
| AS positivity, n (%)a | 81 (75.0) | 25 (78.1) | 0.817 | 12 (75.0) | >0.99 |
| Aβ42, pg/mL | 638 (474–899) | 387 (318–449) | <0.0001 | 409 (332–541) | 0.0001 |
| Aβ40/Aβ42 | 0.9 (0.8–0.9) | 0.5 (0.4–0.5) | <0.0001 | 0.4 (0.4–0.5) | <0.0001 |
| p-tau, pg/mL | 35 (26–44) | 57 (46–85) | <0.0001 | 85 (71–100) | <0.0001 |
| p-tau/Aβ42 | 0.55 (0.4–0.7) | 1.5 (1.2–2.3) | <0.0001 | 2.1 (1.4–2.7) | <0.0001 |
| t-tau, pg/mL | 254 (201–331) | 365 (298–559) | <0.0001 | 545 (453–545) | <0.0001 |
| NfL, pg/mL | 524 (393–743) | 664 (501–784) | 0.096 | 749 (667–879) | 0.019 |
Data are expressed as mean ± SD, median (interquartile range), number, and percentage, as appropriate. Aβ positivity (A+) was defined by CSF Aβ42/Aβ40 ratio < 0.65; and tau positivity (T+) by CSF p-tau181 > 60 pg/ml. For group comparisons, the normal Aβ (A-) group was used as reference.
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, IRBD isolated REM sleep behavior disorder, PD Parkinson disease, AS α-synuclein, Aβ42 42 amino acid isoform of amyloid-beta, Aβ40 40 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, NfL neurofilament light chain, t-tau total tau protein.
aDAT imaging and α-synuclein status in the normal Aβ group were available in 107 and 108 cases, respectively.
NfL levels were associated with those of t-tau (r = 0.492, p < 0.001) and showed higher values in patients with positive AD biomarkers (A+T+) compared to patients with negative AD biomarkers (A−T−) and, to a lesser extent, in those with AS positivity compared to those with AS negativity (Table 3). However, these differences were no longer statistically significant after correcting for age.
Two IRBD patients had very high NfL levels above the range usually found in PD18. One patient with a very high NfL level (3900 pg/mL) was AS positive and had low Aβ42/Aβ40 and a normal p-tau level. The other patient (NfL of 1484 pg/mL) had AS negativity, and the AD biomarkers were within the normal ranges.
After a mean follow-up of 2.48 ± 2.75 (range, 0.6–12.7) years from the time of lumbar puncture and 5.3 years from IRBD diagnosis, 47 (31.8%) patients developed PD (n = 24) or DLB (n = 23).
At the time of the lumbar puncture, 33 (22.3%) IRBD patients had coexistent mild cognitive impairment. Of these 33 patients, six converted to PD (out of 24 total PD converters), 15 converted to DLB (out of 23 total DLB converters), and 12 remained disease-free at the end of the current study (out of 100 total non-converters) (Table 4).
Table 4.
Baseline characteristics of IRBD patients stratified according to clinical follow-up in non-converters, PD converters, and DLB converters
| Non-converters | Converted to PD | Converted to DLB | P value | |
|---|---|---|---|---|
| N | 100 | 24 | 23 | |
| Female, n (%) | 22 (22.0) | 7 (29.2) | 6 (26.1) | 0.731 |
| Age at LP, yrs | 67.7 ± 10.6 | 71.7 ± 6.1 | 73.5 ± 5.8 | 0.0104a |
| Age at IRBD diagnosis, yrs | 65.3 ± 10.1 | 68.6 ± 5.5 | 68.9 ± 7.2 | 0.126 |
| Time from IRBD diagnosis to LP, yrs | 1.4 (0.4–3.6) | 1.9 (0.5–4.5) | 4.3 (0.5–6.5) | 0.0218a |
| Prodromal PD rate at LP, % | 76.2 ± 31.5 | 92.7 ± 16.6 | 95.7 ± 10.5 | 0.0005b,c |
| DAT-SPECT abnormal/tested, n (%) | 48/97 (49.5) | 20/24 (83.3) | 15/23 (65.2) | 0.0079c |
| UPSIT-40 score | 21.0 (15.5–27.0) | 19.0 (14.5–22.5) | 15.0 (12.3–17.8) | 0.0139a |
| Mild cognitive impairment at LP, n (%) | 12 (12.0) | 6 (25.0) | 15 (65.2) | <0.0001d,e |
| AS positivity, n (%) | 68 (68.0) | 20 (86.9) | 21 (95.5) | 0.0095b |
| A+T+N+, n (%) | 8 (8.4) | 2 (8.7) | 3 (13.0) | 0.786 |
| A+T+, n (%) | 10 (10.6) | 3 (13.0) | 3 (13.0) | 0.907 |
| A+T-, n (%) | 18 (18.9) | 6 (26.1) | 8 (34.6) | 0.224 |
| Aβ42/Aβ40 | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.7 (0.5–0.8) | 0.055 |
| p-tau, pg/mL | 37 (28–47) | 35 (26–50) | 41 (34–51) | 0.212 |
| p-tau/Aβ42 | 0.6 (0.4–0.9) | 0.6 (0.6–0.9) | 0.8 (0.6–1.5) | 0.0008e |
| t-tau, pg/mL | 292 (219–376) | 236 (182–329) | 285 (247–363) | 0.112 |
| NfL, pg/mL | 542 (412–751) | 540 (339–751) | 606 (507–794) | 0.489 |
Follow-up information was available in 147 patients, CSF AD core biomarkers in 95 non-converters, 23 PD converters, and 23 DLB converters; CSF NfL in 98 non-converters, 22 PD converters, and 22 DLB converters.
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, IRBD isolated REM sleep behavior disorder, PD Parkinson disease, DLB dementia with Lewy bodies, AS misfolded α-synuclein, Aβ42 42 amino acid isoform of amyloid-beta, Aβ40 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta, T tau, NfL neurofilament light chain, t-tau total tau protein.
aDLB converters vs non-converters, p < 0.05.
bDLB converters vs non-converters, p < 0.01.
cPD converters vs non-converters, p < 0.01.
dDLB converters vs non-converters, p < 0.0001.
eDLB converters vs PD converters, p < 0.001.
There was a trend toward a higher prevalence of A+ status in patients who converted to DLB than in those converting to PD and in those who remained disease-free (p = 0.055). However, the CSF AD profile A+T+ distribution did not differ significantly among the above-mentioned groups (Table 4). As compared with non-converters, patients who converted to DLB were older and had a higher mean prodromal PD rate, a lower UPSIT-40 score, and a greater prevalence of mild cognitive impairment status and AS positivity. Patients who converted to PD had a higher mean prodromal PD rate and abnormal DAT-SPECT uptake than non-converters and also a lower prevalence of mild cognitive impairment than those who converted to DLB (Table 4).
On univariable Cox regression, the variables significantly associated with a greater likelihood of developing DLB or PD (i.e., either one of the two) at the time of lumbar puncture were older age, the presence of mild cognitive impairment, abnormal DAT-SPECT, high prodromal PD rate, and AS positivity (Table 5). Besides the above-mentioned variables, increased p-tau/Aβ42 ratio and abnormal UPSIT-40 score (but not DAT-SPECT) predicted phenoconversion in the analysis considering only the conversion to DLB (Table 6). Differently, only abnormal DAT-SPECT and a high prodromal PD rate predicted phenoconversion in the analysis limited to PD converters (Table 7).
Table 5.
Univariable and multivariable Cox regression analyses for prognostic factors of conversion to combined PD and DLB
| Univariable analysis | HR (95% CI) | P value | |
|---|---|---|---|
| Age at LP | 1.05 (1.00–1.10) | 0.030 | |
| Female sex | 1.60 (0.83–3.07) | 0.158 | |
| Mild cognitive impairment at LP | 2.81 (1.58–5.02) | <0.001 | |
| Prodromal PD rate at LP | 1.03 (1.01–1.06) | 0.001 | |
| Abnormal DAT-SPECT | 2.66 (1.37–5.16) | 0.004 | |
| UPSIT-40 score | 0.94 (0.88–1.00) | 0.053 | |
| AS positivity | 4.51 (1.60–12.7) | 0.004 | |
| t-tau value | 0.99 (0.99–1.00) | 0.283 | |
| p-tau/Aβ42 ratio | 1.17 (0.85–1.61) | 0.339 | |
| A+ | 1.55 (0.83–2.92) | 0.169 | |
| A + T+ | 0.99 (0.42–2.37) | 0.999 | |
| NfL |
Low tertile Intermediate tertile High tertile |
Ref. 1.85 (0.86–3.99) 1.92 (0.89–4.13) |
Ref. 0.118 0.098 |
| Multivariable analysisa | HR (95% CI) | P value | |
|---|---|---|---|
| Age | 1.03 (0.98–1.09) | 0.251 | |
| Mild cognitive impairment at LP | 3.86 (1.96–7.61) | <0.001 | |
| Abnormal DAT-SPECT | 2.31 (1.09–4.91) | 0.030 | |
| AS positivity | 4.05 (1.26–12.99) | 0.019 | |
p values indicating statistically significant differences between groups are highlighted in bold.
Ref. reference group, LP lumbar puncture, PD Parkinson disease, AS α-synuclein, Aβ42 42 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta, T tau, NfL neurofilament light chain, t-tau total tau protein.
aIn the multivariable analysis, the prodromal PD rate was not included since it accounts for other variables (e.g., age, reduced DAT-SPECT uptake) already included in the model.
Table 6.
Univariable and multivariable Cox regression analysis for prognostic factors of short-term conversion to DLB
| Univariate analysis variable (s) | HR (95% CI) | P value |
|---|---|---|
| Age at LP | 1.08 (1.01–1.16) | 0.024 |
| Female sex | 1.49 (0.57–3.87) | 0.412 |
| Mild cognitive impairment at LP | 6.52 (2.75–15.44) | <0.001 |
| Prodromal PD rate at LP | 1.05 (1.01–1.09) | 0.017 |
| Abnormal DAT-SPECT | 1.78 (0.74–4.25) | 0.197 |
| UPSIT-40 score | 0.89 (0.81–0.98) | 0.031 |
| AS positivity | 9.18 (1.22–68.7) | 0.031 |
| t-tau value | 1.00 (0.99–1.00) | 0.774 |
| p-tau/Aβ42 ratio | 1.46 (1.01–2.11) | 0.046 |
| A+ | 2.31 (0.99–5.36) | 0.050 |
| A+T+ | 1.02 (0.30–3.46) | 0.975 |
| Neurofilament light chain | 1.00 (0.99–1.00) | 0.187 |
| Multivariate analysis variable (s)a | HR (95% CI) | P value |
|---|---|---|
| Age | 0.97 (0.89–1.07) | 0.606 |
| Mild cognitive impairment at LP | 11.23 (3.06–41.21) | <0.0001 |
| UPSIT-40 score | 1.01 (0.89–1.15) | 0.835 |
| p-tau/Aβ42 ratio | 1.25 (0.67–2.37) | 0.482 |
| AS positivity | 9.40 (0.88–100.65) | 0.064 |
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, PD Parkinson disease, AS α-synuclein, Aβ42 42 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta; T tau; t-tau total tau protein.
aIn the multivariable analysis, the prodromal PD rate was not included since it accounts for other variables (i.e., age, etc.) that are already included in the model.
Table 7.
Univariable Cox regression analysis for prognostic factors of short-term conversion to PD
| Univariate analysis variable(s) | HR (95% CI) | P value |
|---|---|---|
| Age at LP | 1.03 (0.96–1.09) | 0.414 |
| Female sex | 1.70 (0.70–4.18) | 0.243 |
| Mild cognitive impairment at LP | 1.16 (0.46–2.94) | 0.749 |
| Prodromal PD rate at LP | 1.03 (1.00–1.06) | 0.034 |
| Abnormal DAT-SPECT | 4.35 (1.48–12.75) | 0.007 |
| UPSIT-40 score | 0.98 (0.90–1.08) | 0.642 |
| AS positivity | 2.95 (0.87–10.02) | 0.083 |
| t-tau value | 0.99 (0.99–1.00) | 0.071 |
| p-tau/Aβ42 ratio | 0.73 (0.35–1.55) | 0.422 |
| A+ | 0.97 (0.36–2.63) | 0.959 |
| A+T+ | 0.98 (0.29–3.32) | 0.973 |
| Neurofilament light chain | 0.99 (0.99–1.00) | 0.673 |
p values indicating statistically significant differences between groups are highlighted in bold.
LP lumbar puncture, PD Parkinson disease, AS α-synuclein, Aβ42 42 amino acid isoform of amyloid-beta, p-tau phosphorylated tau protein, A amyloid-beta, T tau, t-tau total tau protein.
Multivariable analyses confirmed AS positivity, mild cognitive impairment, and reduced DAT-SPECT uptake as independent predictors of conversion to DLB or PD (i.e., both conversions considered) (Table 5). Even after excluding the patients with mild cognitive impairment, AS positivity remained an independent predictor of conversion (HR 4.33, 95% CI 1.02–18.42, p = 0.047). Finally, mild cognitive impairment remained the only independent predictor of conversion in the multivariable analysis limited to DLB converters (Table 6), whereas there was no independent predictor in the multivariable analysis limited to PD converters (Table 7).
Kaplan-Meier curve analysis assessing the effect of time from the lumbar puncture to conversion in IRBD patients stratified for the AS status demonstrated a significant rise (p = 0.004) in the slopes of conversion over time in the AS positive group (41/45, 91.1% AS positive converters with a median time to conversion of five years; 4/45, 8.9%, AS negative converters with a median time to conversion of nine years) independent of AD status (Fig. 2). Specifically, the analysis showed that after five years of follow-up from lumbar puncture, the estimated rate of phenoconversion to PD and DLB was 50% in the AS+ patients and 15% in the AS- patients.
Fig. 2. Predictive value of CSF AS from the time of lumbar puncture in the conversion from IRBD to PD or DLB.
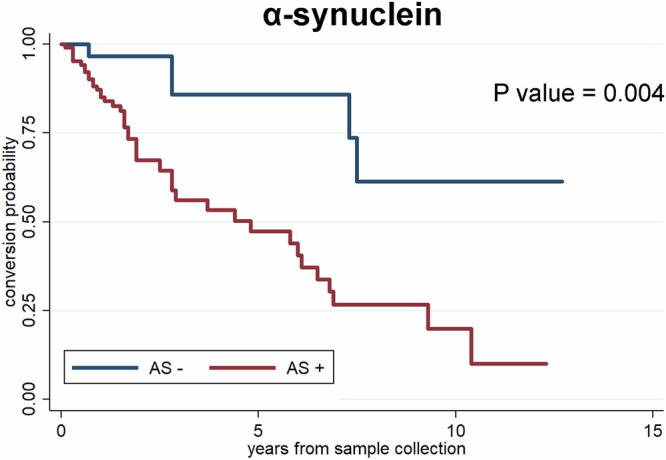
Survival curves in patients with IRBD according to the AS status (negative = blue; positive = red). CSF cerebrospinal fluid, IRBD isolated REM sleep behavior disorder, AS misfolded α-synuclein.
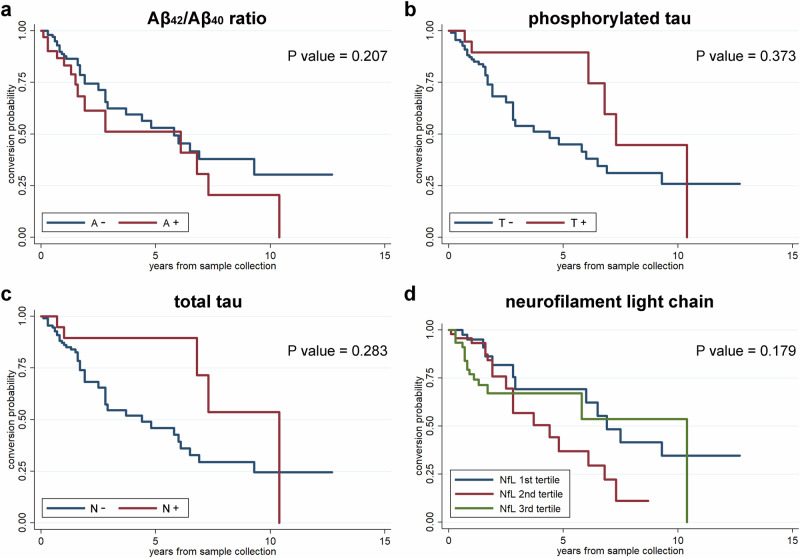
Kaplan-Meier curves showed that patients with abnormal Aβ42/Aβ40 ratio (p = 0.207), p-tau (p = 0.373), t-total (p = 0.283) and NfL levels (p = 0.179) had no short-term increased risk of developing PD and DLB (Fig. 3).
Fig. 3. Predictive value of CSF AD core markers and NfL in the conversion from IRBD to PD or DLB.
Survival curves in patients with IRBD according to the amyloid-beta (a), tau (b), and neurodegeneration (c) status (negative = blue; positive = red), and NfL value tertiles (d).
Discussion
In the present study, we confirmed and expanded previous findings on CSF biomarkers in IRBD and their predictive value on the short-term conversion to PD or DLB. We extended the AS RT-QuIC analysis to a larger cohort and added the evaluation of AD core markers and NfL in the CSF. Moreover, we studied the predictive values of biomarkers and associated demographic and clinical findings to estimate the risk of conversion using the whole cohort and a longer longitudinal observation compared to a previous study20.
The AS RT-QuIC results confirmed that our assay detects the misfolded protein in about 75% of patients with IRBD and that compared to the AS negative group, patients with AS positivity were older and showed a higher likelihood of prodromal PD (e.g., hyposmia and abnormal DAT-SPECT). Questions arise regarding the nature of the IRBD patients who are AS negative and about the fact that the percentage of AS negativity between different studies varied significantly20–22. In a recent neuropathological case series, we demonstrated a clear association between RT-QuIC sensitivity in the CSF and the stage and burden of Lewy body pathology in the brain26. In patients with neocortical or limbic Lewy body stages, the sensitivity to detect AS in the CSF was 100% but was significantly lower in patients at the brainstem stage (60.8%). According to these findings, the AS negative IRBD patients may have a lower burden of synuclein pathology in the brain, probably confined to the lower brainstem structures that modulate muscle atonia in REM sleep. Alternatively, it is also plausible that some IRBD patients had a different neurobiological pathogenesis unrelated to Lewy body pathology, particularly those with AS negativity and positive AD markers. Clinical follow-up and longitudinal CSF assessments of AS may elucidate the underlying etiology in these IRBD patients.
Although the neuropathological hallmark of PD and DLB is the presence of intraneuronal Lewy bodies and neurites, where abnormal AS aggregates are the main component1, autopsy studies of PD, PDD and DLB patients have shown coexisting AD pathology in 20–70% of the patients, particularly in those who had cognitive impairment2,3,27. Moreover, approximately 5% of patients with PD, 10% with PDD and 25–40% of those with DLB show the core CSF AD profile16–18. In PD and DLB, reduced CSF Aβ42 levels predict cognitive impairment and a more aggressive disease course and correlate with postmortem β-amyloid plaques in the brain16,17,19. In our study, the proportions of IRBD patients with reduced Aβ42 levels, increased p-tau levels, and increased t-tau levels were similar to those described in PD patients of similar age and lower than those seen in DLB patients16. Altogether, this observation combined with the equal rate of conversion to PD and DLB in our cohort indicate that IRBD is a prodromal feature of the full spectrum of Lewy body disease and that the relatively high conversion rate to DLB in these patients seems to be largely independent of the association with AD pathology.
NfL proteins are cytoskeletal proteins expressed in neurons. In neurodegenerative diseases such as amyotrophic lateral sclerosis, increased NfL levels in both CSF and plasma are the result of nonspecific axonal damage28. In PD patients, NfL levels are similar to those of healthy controls but may predict motor and cognitive decline in those patients with increased levels25. CSF NfL levels are elevated in DLB and MSA patients and in individuals with pure autonomic failure who eventually develop MSA18,29. We found that in IRBD the CSF NfL levels are associated with age, abnormal AD biomarkers and AS positivity. However, given the higher mean age of the patients with positive AD markers and AS positivity, the association between the neurodegenerative markers did not hold after correction for age due to the small number of IRBD patients that we found having AD pathology in the CSF.
At the end of our study, 47 (31.8%) patients developed PD or DLB after a mean follow-up of 2.5 years. At the time of lumbar puncture, the short-term risk of developing PD and DLB was associated with demographic and clinical variables and biomarker changes such as older age, a high prodromal PD score, mild cognitive impairment, abnormal DAT-SPECT, and CSF AS positivity. Abnormal CSF levels of Aβ42, p-tau, t-tau, and NfL were not predictors of short-term conversion to PD or DLB. Nevertheless, an elevated p-tau/Aβ42 ratio increased the risk of conversion to DLB. These findings suggest that AS is the pathologic substrate that mainly drives phenoconversion, but AD pathology may also contribute to precipitating the short-term onset of cognitive decline in a subset of IRBD patients. Our findings in patients converting to DLB are in line with previous literature showing that mild cognitive impairment, reduced cortical cholinergic activity demonstrated by PET imaging and slowness in the background EEG activity predict the development of dementia rather than parkinsonism, whereas abnormal DAT-SPECT, hyposmia and dysautonomia do not distinguish between dementia-first and parkinsonism first phenoconverters6. The mean patient follow-up in the present study was relatively short, of only 2.5 years. However, even when we only considered the patients with a follow-up longer than 5 years, we did not see a significant change in the percentage of AS-positive patients (8/10, 80% vs 41/45, 91.1%), likely because the few (n = 4) AS-negative converters developed PD or DLB at variable times after the LP (0.7, 2.8, 7.3, and 7.5 years, respectively). Patients with IRBD may develop either PD, DLB, or MSA with time. The conversion to MSA is much less common than to PD and DLB, as it only accounts for about 5% of all IRBD cases that develop a synucleinopathy6. In our current study, none of our IRBD patients developed MSA. It can be speculated that our IRBD patients with very high NfL levels in the CSF represent prodromal MSA patients, as MSA advances faster and is a more aggressive condition than PD, PDD, and DLB18,29. Follow-up of our two IRBD patients with 3900 pg/mL and 1484 pg/mL NfL concentrations will elucidate whether they develop MSA.
Our study has some limitations. First, there was no postmortem confirmation of our findings. It should be noted that a major drawback of autopsy studies is that they involve patients with end-stage disease, whereas, in our study, we examined living subjects with IRBD who are in an earlier stage of the neurodegenerative process. Thus, postmortem and CSF antemortem assessments of the presence and severity of AS and AD pathologies may not be comparable. Second, we could not evaluate all the biomarkers in eight (5.7%) patients because the available CSF did not contain enough volume for all the measurements. Third, the study was retrospective, and the biomarkers were not assessed longitudinally to determine, for example, whether AS and AD negativities changed to positivity over time. Fourth, our mean follow-up was 2.5 years. It can be speculated that longer follow-up will show that baseline Aβ42, p-tau, and t-tau concentrations may predict the late development of dementia. Finally, patients had different follow-up durations ranging from six months to 12 years between CSF collection and the time of the study.
Our study also has strengths. First, we evaluated a well-characterized IRBD cohort. Second, in all the instances, the diagnosis of IRBD was confirmed by video-polysomnography, which revealed increased electromyography activity in REM sleep linked to dream-enacting behaviors30. Third, patients underwent a comprehensive assessment that included DAT-SPECT and the UPSIT-40 smell test at the time of lumbar puncture. This allowed us to calculate the likelihood ratio of prodromal PD in each patient. Fourth, all the CSF measurements were performed blindly to the subject’s condition.
In conclusion, our IRBD study showed that CSF contains AS in an important number of patients (75%), while AD biomarkers are found in a subset (15–22%). Although the main pathological driver in IRBD is AS pathology, AD pathology may likely contribute to the late development of cognitive decline.
As the IRBD represents the prodromal stage of PD and DLB, in vivo identification of CSF biomarkers could be useful for understanding the underlying neuropathological process during its earliest phase and for selecting patients for future neuroprotective clinical trials, not only to target AS but also to implement anti-amyloid and anti-tau therapies.
Methods
Participants
We assessed CSF biomarkers of neurodegeneration (AS, Aβ42, Aβ40, p-tau, t-tau, and NfL) in 148 IRBD patients diagnosed at the Hospital Clinic of Barcelona, Spain. The diagnosis of IRBD was made by the current diagnostic criteria, including video-polysomnography, in subjects without dementia or parkinsonism30. Some IRBD patients had coexistent mild cognitive impairment as they had: (1) complaints of subjective cognitive decline in at least one relevant cognitive domain (attention, memory, visual perception, language, executive function, and praxis), (2) objective impairment in at least one test in one or more cognitive domains as evident by scores 1.5 standards below the appropriate norms adjusted for age and education, and 3) preserved activities of daily living.
Lumbar punctures were performed between March 2008 and March 2023. At the time of lumbar puncture, patients underwent demographic and clinical history, neurological examination, the UPSIT-40 smell test, and DAT-SPECT, which were used to calculate the likelihood ratio of prodromal PD in each patient31. At the time of this study, when the biomarkers were measured in the CSF (May 2023), we reviewed whether patients who underwent lumbar puncture were disease-free or if they were diagnosed with PD, DLB or MSA according to current clinical criteria32–34. The CSF of 40 individuals without RBD at video-polysomnography served as a blind element for the personnel at the Istituto delle Scienze Neurologiche di Bologna, Italy, who examined the presence of biomarkers in the samples from the IRBD patients.
CSF biomarker analyses
CSF was collected during a standardized lumbar puncture procedure. Storage and shipment from Barcelona, Spain, to Bologna, Italy, were performed as described previously22. All CSF samples were analyzed by researchers blinded to the individual clinical condition. As previously reported, the presence of AS seeding activity was analyzed by an in-house RT-QuIC assay and dichotomised as AS-positive (present) or AS-negative (absent)13,22. All other biomarkers were measured by commercially available kits. The levels of Aβ42, Aβ40, p-tau181, and t-tau were measured by an automated chemiluminescent enzyme immunoassay (CLEIA) on the Lumipulse G600II platform (Fujirebio, Gent, Belgium). The mean intra-assay and interassay CVs for these markers were <8%. The Aβ42/Aβ40 and p-tau/Aβ42 ratios were also calculated., NfL was measured by a validated ELISA assay (NfL ELISA kit, IBL, Hamburg, Germany), as described previously18. The biomarker levels were analyzed either as continuous values or dichotomised as positive (abnormally increased or decreased) or negative (within the normal range) according to our in-house following cutoffs: abnormally positive when Aβ42/Aβ40 ratio was <0.65, p-tau was >60 pg/mL, t-tau was >450 pg/mL, and NfL was >550 pg/ml19. Patients were classified according to the A/T/N framework based on the combined Aβ42/Aβ40 ratio (A), p-tau (T), and t-tau (N) dichotomized results as abnormal (+) and normal (−).
Statistical analysis
Data are presented as mean (standard deviation), median (interquartile interval), or number (percentage), as appropriate. The normality of the distribution of continuous variables was assessed using the Kolmogorov‒Smirnov test and Shapiro‒Wilk test. The data were compared using the Student’s t-test, the Mann‒Whitney U test, the Kruskal-Wallis test (followed by the Dunn post hoc test), the X2 test, and Fisher’s exact test. We used NfL levels as the dependent variable in a multivariable general linear model to test the association with other biomarkers correcting for age. We used the Kaplan-Meier method to estimate the cumulative time-dependent probability of conversion to PD and DLB for the prognostic analysis. The time of entry into the analysis was the date of lumbar puncture, and the time of the endpoint was the date of phenoconversion to a neurodegenerative disease or the date of the last follow-up visit in the disease-free IRBD patients. We performed univariable and multivariable Cox regression models to study prognostic factors in the IRBD group of patients who developed either PD or DLB. The results are presented as hazard ratios (HRs) and 95% CI. The assumption of proportional hazard was assessed by Schoenfeld residuals. Differences were considered significant at a two-sided p < 0.05.
Analyses were performed with SPSS version 27.0 and Stata SE 14.2.
The ethical committees of HCB (HCB/2022/1089) and Area Vasta Emilia Centro (EM1173–2021–20226) approved this study. All participants provided written informed consent.
Acknowledgements
Gerard Mayà was funded by BBVA-Baselga grant. The study was supported by the grant #NextGenerationEU (NGEU), funded by the Italian Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006). The publication of this article was supported by the "Ricerca Corrente" funding from the Italian Ministry off Health.
Author contributions
A.I., A.M.L., M.B., P.P., and S.B. contributed to the conception and design of the study. A.I., A.M., A.M.L., A.T., C.G., C.Z., G.M., J.S., M.B., M.R., M.S., P.P., S.B., and S.D. contributed to the acquisition and analysis of the data. A.I., A.M.L., C.Z., M.B., P.P., and S.B. contributed to drafting the text and preparing the figures. All authors read and approved the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Alex Iranzo, Email: airanzo@clinic.cat.
Piero Parchi, Email: piero.parchi@unibo.it.
References
- 1.Goedert, M., Jakes, R. & Spillantini, M. G. The synucleinopathies: twenty years on. J. Parkinsons Dis.7, S51–S69 (2017). 10.3233/JPD-179005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aarsland, D. et al. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim.7, 1–21 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Colom-Cadena, M. et al. Regional overlap of pathologies in Lewy body disorders. Neuropathol. Exp. Neurol.76, 216–224 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Jellinger, K. A. Multiple system atrophy. A clinicopathological update. Free Neuropathol. 1, 1-17 (2020). [DOI] [PMC free article] [PubMed]
- 5.Iranzo, A., Santamaria, J. & Tolosa, E. Idiopathic rapid eye movement sleep behavior disorder: diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol.15, 405–419 (2016). 10.1016/S1474-4422(16)00057-0 [DOI] [PubMed] [Google Scholar]
- 6.Postuma, R. B. et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behavior disorder: a multicenter study. Brain142, 744–759 (2019). 10.1093/brain/awz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Videnovic, A. et al. Clinical trials in REM sleep behavior disorder. Challenges and opportunities. J. Neurol. Neurosurg. Psychiatry91, 740–749 (2020). 10.1136/jnnp-2020-322875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchiyama, M. et al. Incidental Lewy body disease in a patient with REM sleep behavior disorder. Neurology45, 709–712 (1995). 10.1212/WNL.45.4.709 [DOI] [PubMed] [Google Scholar]
- 9.Boeve, B. F. et al. Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease. Sleep Med.8, 60–64 (2007). 10.1016/j.sleep.2006.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaig, C. et al. Pathologically description of a nonmotor variant of multiple system atrophy. J. Neurol. Neurosurg. Psychiatry79, 1399–1400 (2008). 10.1136/jnnp.2008.145276 [DOI] [PubMed] [Google Scholar]
- 11.Iranzo, A. et al. Neuropathology of prodromal Lewy body disease. Mov. Disord.29, 410–415 (2014). 10.1002/mds.25825 [DOI] [PubMed] [Google Scholar]
- 12.Maya, G. et al. Neuropathological alterations in subjects initially diagnosed by polysomnography with isolated REM sleep behavior disorder. J. Sleep. Res31, 78 (2022). [Google Scholar]
- 13.Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol.140, 49–62 (2020). 10.1007/s00401-020-02160-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blennow, K., Biscetti, L., Eusebi, P. & Parnetti, L. Cerebrospinal fluid markers in Alzheimer’s disease and Parkinson’s disease. From pathophysiology to clinical practice. Mov. Disord.31, 836–847 (2016). 10.1002/mds.26656 [DOI] [PubMed] [Google Scholar]
- 15.Farotti, L., Paoletti, F. P., Simoni, S. & Parnetti, L. Unraveling pathophysiological mechanisms of Parkinson’s disease: contribution of CSF biomarkers. Biomark. Insights15, 1–9 (2020). 10.1177/1177271920964077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Steenoven, I. et al. Cerebrospinal fluid Alzheimer’s disease biomarkers across the spectrum of Lewy bodies diseases: results from large multicenter cohort. J. Alzheimers Dis.54, 287–295 (2016). 10.3233/JAD-160322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemstra, A. W. et al. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry88, 113–118 (2017). 10.1136/jnnp-2016-313775 [DOI] [PubMed] [Google Scholar]
- 18.Quadalti, C. et al. Neurofilament light chain and α-synuclein RT-QuIC as differential diagnostic biomarkers in parkinsonisms and related syndromes. NPJ Parkinsons Dis.7, 93 (2021). 10.1038/s41531-021-00232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin, D. J. et al. Evolution of Alzheimer’s disease cerebrospinal fluid biomarkers in early Parkinson’s disease. Ann. Neurol.88, 574–587 (2020). 10.1002/ana.25811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iranzo, A. et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behavior disorder: a longitudinal observational study. Lancet Neurol.20, 203–212 (2021). 10.1016/S1474-4422(20)30449-X [DOI] [PubMed] [Google Scholar]
- 21.Poggiolini, I. et al. Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain145, 584–595 (2022). 10.1093/brain/awab431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iranzo, A. et al. Misfolded α-Synuclein assessment in the skin and CSF by RT-QuIC in isolated REM sleep behavior disorder. Neurology100, e1944–e1954 (2023). 10.1212/WNL.0000000000207147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Concha-Marambio, L. et al. Accurate detection of α-Synuclein seeds in cerebrospinal fluid from isolated rapid eye movement sleep behavior disorder and patients with Parkinson’s disease in the DeNovo Parkinson (DeNoPa) cohort. Mov. Disord.38, 567–578 (2023). 10.1002/mds.29329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes, M. et al. Cerebrospinal-fluid biomarkers for predicting phenoconversion in patients with isolated rapid-eye movement sleep behavior disorder. Sleep47, zsad198 (2024). 10.1093/sleep/zsad198 [DOI] [PubMed] [Google Scholar]
- 25.Aamodt, W. W. et al. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov. Disord.36, 2945–2950 (2021). 10.1002/mds.28779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bentivenga, G. M. et al. Performance of a seed amplification assay for misfolded alpha-synuclein in cerebrospinal fluid and brain tissue in relation to Lewy body disease stage and pathology burden. Acta Neuropathol.147, 18 (2024). 10.1007/s00401-023-02663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin, D. J. et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol.16, 55–65 (2017). 10.1016/S1474-4422(16)30291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol.76, 1035–1048 (2019). 10.1001/jamaneurol.2019.1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain predict phenoconversion of pure autonomic failure. Ann. Neurol.89, 1212–1220 (2021). 10.1002/ana.26089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Academy of Sleep Medicine. International Classification Of Sleep Disorders. 3rd edn. test revision. (American Academy of Sleep Medicine, 2023).
- 31.Heinzel, S. et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord.34, 1464–1470 (2019). 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 32.Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord.30, 1591–1601 (2015). 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 33.McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology89, 88–100 (2017). 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wenning, G. K. et al. The Movement Disorder Society Criteria for the diagnosis of multiple system atrophy. Mov. Disord.37, 1131–1148 (2022). 10.1002/mds.29005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.