Abstract
Previous studies show differentially expressed long non-coding RNA present in the placenta from women with pre-eclampsia, potentially playing a vital role in the pathogenesis of the complication. In a published microarray study, Ribonuclease P RNA component H1 was decreased in leukocytes from women that later developed pre-eclampsia. We hypothesized that Ribonuclease P RNA component H1 decreased during pregnancy in women developing pre-eclampsia and important for the development of the complication. We isolated RNA from extracellular vesicles, leukocytes and plasma using blood samples taken at weeks 22–24 and 36–38 in women who subsequently developed pre-eclampsia and from healthy pregnancy. The expression of Ribonuclease P RNA component H1 was quantified using qPCR. Expression of Ribonuclease P RNA component H1 at 22–24 weeks was further examined to investigate its discriminatory potential of subsequent pre-eclampsia and association with clinical markers. We found lower expression of Ribonuclease P RNA component H1 in leukocytes at 22–24 and 36–38 weeks amongst women who subsequent developed pre-eclampsia compared with those who did not, while increased Ribonuclease P RNA component H1 expression was found in plasma at 36–38 weeks. Pre-eclampsia risk factors could not account for this difference in the Ribonuclease P RNA component H1 expression. Prediction of pre-eclampsia at 22–24 weeks using Ribonuclease P RNA component H1 expression in leukocytes in addition to the screening algorithm used today had a significantly better performance. In conclusion, Ribonuclease P RNA component H1 expression in leukocytes was significantly decreased in women with pre-eclampsia, and the expression at 22–24 weeks associated with the subsequent development of pre-eclampsia. Ribonuclease P RNA component H1 in leukocytes may be a useful biomarker for prediction and/or early detection of pre-eclampsia and an unknown regulator of the signaling affecting immune cells.
Keywords: pre-eclampsia, RPPH1, lncRNA
The lncRNA RPPH1 was decreased in leukocytes while increased in plasma of women developing pre-eclampsia (PE). The expression level of RPPH1 was associated with markers of PE. The RPPH1 expression at 22–24 weeks predicted subsequent PE development.
Graphical Abstract
Graphical Abstract.
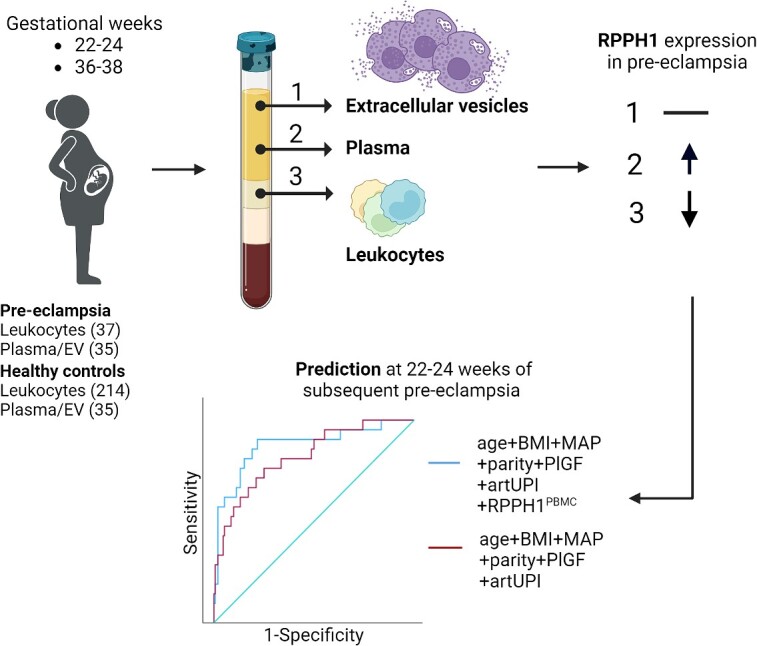
Created with BioRender.com.
Introduction
Pre-eclampsia (PE) is a pregnancy-specific hypertension-syndrome that seriously threatens the safety of mother and infant. Every year, thousands of lives are lost due to this condition, but early diagnosis and follow-up can improve prognosis [1]. Therefore, an accurate biomarker at early gestation would be of great benefit. Unfortunately, current biomarkers are not good enough at predicting PE early and the etiology and pathogenesis also remain unclear. The most promising first trimester screening algorithm today, combining maternal risk factors, mean arterial blood pressure (BP), Doppler ultrasound measured maternal uterine artery pulsatility index (artUPI), and levels of circulating placental growth factor (PlGF), has less utility for term disease, where the greatest burden lies [2, 3]. The two-stage model of PE development includes first stage shallow trophoblast invasion and inadequate spiral artery development leading to second stage with increased production of oxidative stress, inflammatory cytokines, angiotensin 1 autoantibodies, and imbalance in angiogenic/anti-angiogenic factors with high levels of soluble fms-like tyrosine kinase-1 (sFlt-1) causing endothelial dysfunction, systemic maternal inflammation, and clinical features of this disorder [3].
Excessive cell death is a prevalent finding in PE [4] and may promote release of trophoblast debris including extracellular vesicles (EVs) that may activate maternal immune cells and enhance systemic inflammation. These processes may again augment placental cell death by different mechanisms, representing a vicious circle in these women [5]. Indeed, we have recently shown increased ferroptosis and senescence in leukocytes from women developing PE [6, 7] linked to decreased anti-inflammatory capacity and oxidative stress. Long non-coding RNA (lncRNA) is RNA that do not encode proteins [8], yet can have other important functions, such as regulation of epigenetic processes, cell cycle, and differentiation by interacting with RNA or proteins to regulate the expression of target genes [9]. Previous studies have shown a large number of differentially expressed lncRNA present in the placental tissue, in leukocytes and EVs in women with PE [10–12], potentially playing a vital role in the pathogenesis of the disease [13, 14]. However, the impact and function of these lncRNA in PE development and progression is still largely unknown and requires further investigation.
Ribonuclease P RNA component H1 (RPPH1) is an lncRNA and the catalytic RNA component of RNase P, the site-specific endonuclease responsible for the cleavage of pre-tRNA 5′leader sequences and formation of the mature 5′termini of the tRNA sequences [15]. RPPH1 has been found to regulate cell proliferation and expression of inflammatory cytokines [16]. What is more, RPPH1 increased migration and metastasis in cancer cells and silencing RPPH1 promoted apoptosis [17–19]. RPPH1 may interact with additional proteins and specific micro RNAs (micro-RNA sponge) to functionally promote cell growth and proliferation, and recent evidence suggests that these mechanisms drive tumorigenesis and metastasis in a variety of cancer [20]. It also promotes macrophage polarization and may activate related signaling pathways that drive cell proliferation [16, 17]. In summary, RPPH1 appears likely to play an important role in coupling RNA polymerase III transcription to cell growth mechanisms [21]. However, the investigation of RPPH1 expression in plasma, EVs, and leukocytes (peripheral blood mononuclear cells (PBMC)) in the setting of PE has not been studied before.
We found the lncRNA RPPH1 significantly decreased in leukocytes in PE women compared with controls at weeks 22–24 in a pilot microarray [7] and we hypothesized that decreased RPPH1 could promote PE. In the present study, we investigated temporal expression in a larger cohort of leukocytes, in EVs and in plasma comparing PE and controls. The RPPH1 was tested for its association with BP, artUPI, circulating β-galactosidase, advanced oxidative protein levels (AOPP) activity, sFlt-1, and PlGF. RPPH1 was at last investigated for its biomarker potential diagnosing later PE at an early gestation.
Materials and methods
The STORK study, a prospective longitudinal cohort study in which 1031 women of Scandinavian heritage with low-risk singleton pregnancies who gave birth at Oslo University Hospital, Rikshospitalet, Oslo, Norway between 2002 and 2008, was followed throughout pregnancy [22]. Exclusion criteria included the presence of one or more severe chronic diseases (such as pre-gestational diabetes, lung, cardiac, gastrointestinal, and/or renal disease). Each woman had four study-related antenatal visits at 14–16, 22–24, 30–32, and 36–38 weeks’ gestation. In the current study, additional exclusion criteria included gestational diabetes mellitus. A substudy [7] including 214 normotensive controls and 37 PE women were included in this study. For the plasma/EV samples, 35 controls were randomly selected of the 214 normotensive controls and 35 PE were selected of the 37 PE in the substudy. All clinical investigations were conducted in accordance with the principles enshrined in the Declaration of Helsinki. The study was approved by the Regional Committee for Medical Research Ethics of Southern Norway in Oslo, Norway (REK# S-01191a). Written informed consent was obtained from all study participants.
Pre-eclampsia
PE was diagnosed by new-onset hypertension (sustained elevation in BP ≥ 140/90 mmHg) and significant proteinuria (urinary total protein/creatinine ratio > 30 mg/mmol or + 1 on urine dipstick), reflecting the criteria used during the time of data collection between 2002 and 2008. In the STORK study, almost all cases (n = 34) were diagnosed after 34 weeks’ gestation (late-onset PE), three cases before 34 weeks (early-onset PE). No women were treated with low-dose aspirin as this recommendation was not yet implemented in Norway during the period of data collection.
Uterine artery pulsatility index
The ultrasound examinations were done using the same equipment for all participants (Acuson Aspen, Mountain View, CA, USA). The mean artUPI was obtained by bilateral Doppler flow velocity measurements using an abdominal approach. The measurements were performed close to the crossing of the external iliac arteries. The insonation angle was as low as possible. Pulsatility index (PI) is defined as the difference between the peak systolic flow and minimum diastolic flow velocity, divided by the mean velocity recorded throughout the cardiac cycle. The formula for PI = (peak systolic velocity–end diastolic velocity)/mean flow velocity. The PI was calculated as the mean of three heart cycles on each side. For each woman, we used the mean values of the right and left side. Some of the uterine artUPI data were missing (~10 PE and 40 healthy pregnancy in the largest validation (leukocyte) cohort).
Collection, storage, and RNA extraction of maternal leukocytes
Whole blood (8 ml) was drawn (after an overnight fast) directly into ml BD Vacutainer cellular preparation tubes (CPT) Tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) with sodium citrate additives at weeks 22–24 and 36–38. The tubes were centrifuged at room temperature in a horizontal rotor (swing-out head) for 20 min at 1800 RCF (Relative Centrifugal Force) with the use of a FICOLL Hypaque gradient centrifugation and leukocytes (PBMC) were collected by pipetting the mononucleated cell layer and stored at −80°C. RNA was extracted using Magnapure Isolation Kit (Roche Life Science, Penzberg, Germany) at weeks 22–24 and Magmax Isolation Kit (Applied Biosystems, Carlsbad, CA) at weeks 36–38, due to change in instruments at the laboratory, as previously published [23]. Both automated methods used magnetic-bead technology to extract RNA.
Collection, storage, and RNA extraction of maternal EVs
Whole blood was drawn in CPT tubes and plasma collected after removal of the PBMC layer as described above and stored at −80°C. The unthawed plasma stored at −80°C was thawed at room temperature and centrifuged at 3000 g for 5 min (to eliminate residual cellular material, including thrombocyte fragments, but still retain the vast majority of EVs) and total RNA from EVs was isolated from 350 μL plasma using the exoRneasy serum/plasma kit (Qiagen), as previously published [12]. Briefly, the total procedure for isolating RNA from EVs comprises two phases: EVs purification and RNA isolation. In the EV purification stage, precentrifuged sample is mixed with Buffer XBP and bound to an exoEasy membrane affinity spin column (500 g, 1 min). The bound EVs are washed with Buffer XWP (5000 g, 5 min), and then lysed with QIAzol (5000 g, 5 min). In the RNA extraction step, chloroform is added to the QIAzol eluate, and the aqueous phase is recovered and mixed with ethanol. Total RNA binds to the spin column, where it is washed three times and eluted. Purity and concentration of isolated total RNA was measured using Nanodrop ND-1000 Spectrophotometer (Termo Fisher Scientific Inc, USA) and RNA integrity was investigated using Agilent 2100 Bioanalyzer (Agilent Technologies, USA).
Collection, storage, and total RNA extraction from plasma
Whole blood was drawn in CPT tubes and plasma collected after removal of the PBMC layer as described above and stored at −80°C. Total RNA was isolated from 200 μL plasma using the miRneasy serum/plasma kit (Qiagen), as previously published [12]. The spike-in control utilized Caenorhabditis elegans miR-39 miRNA was used based on recommendation from the kit instruction. After addition of qiazol lysis reagent, 3.5 μL of 1.6 × 10^8 copies/μL of mir-39 was added to the mix of qiazol and plasma.
Quantitative real-time polymerase chain reaction
Reverse transcription was performed using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) for PBMC RNA, RT First Strand Kit (Qiagen) for EVs, and miScript II RT Kit (Qiagen) for plasma RNA. RNA quantification was performed using SYBR Green PCR Fast Mix (Quantabio, Beverly, MA) for PBMC, RT2 lncRNA SYBR Green qPCR kit (Qiagen) for EVs, and miScript SYBR Green PCR Kit (Qiagen) for RNA in plasma using the standard curve method on an ABI Prism 7900 (Applied Biosystems). Primers for RPPH1 RT2 lncRNA qPCR Assay (LPH21683A) and Ce_miR-39_1 miScript Primer Assay were from Qiagen. Sequence specific intron spanning oligonucleotide primers for GAPDH (FP: 5′-CCAAGGTCATCCATGACAACTT-3′, RP: 5′-AGGGGCCATCCACAGTCTT-3′), ACTB (FP:5′-AGGCACCAGGGCGTGAT-3′, RP: 5′-TCGTCCCAGTTGGTGACGAT-3′), and RPLP0 (FP:5′-CAGATTGGCTACCCAACTGTT-3′, RP: 5′-GGAAGGTGTAATCCGTCTCCAC-3′) were designed by NCBI Primer Blast. Transcript expression levels were normalized to ACTB and GAPDH and expressed as relative RNA levels in leukocytes, miR-39 for plasma-derived RNA, and RPLP0 in EVs.
Biochemical analysis
Peripheral venous blood was drawn in the morning between 07:30 and 08:30 AM after an overnight fast, collected in tubes with citrate additives, centrifuged for 25 min at 3000 g at 4°C, separated, and stored at −80°C until analysis. Levels of sFlt-1 (catalog# DY321B) and PlGF (catalog#DY264) were measured in duplicate by enzyme immuno-assay with antibodies obtained from R&D Systems (Stillwater, MN), while levels of detection AOPP activity were measured in duplicate using 5 μL plasma, by absorbance readings using kit obtained from Abcam (catalog# ab242295), as previously published [6]. The markers were analyzed in a set-up that combined a CyBi SELMA (CyBio, Jena, Germany), an EL406 washer/dispenser (Biotek, Winooski, VT), and a Synergy H1 microplate reader (Biotek). β-Galactosidase was measured in duplicate by fluorescence, and reported previously [7]. Briefly, 20 μL of citrate plasma and standards recombinant human beta-galactosidase-1 (R&D Systems, Minneapolis, MN) diluted in 50 mM sodium citrate (pH 3.5) and 20 μL of 1.2 mM substrate (4-methylumbelliferyl-β-D-galactopyranoside (Sigma-Aldrich) was loaded into 384-well black plates. The plates were incubated in 37°C for 1 h and the reaction stopped by 40 μL of 0.17 M glycine-carbonate buffer (pH 9.8) and the plates read by the fluorescent plate reader.
Statistical analysis
Data are expressed as mean ± SD when normally distributed and median (25th, 75th percentile) when skewed. Comparison of demographics between women with/without PE was performed using t-test or Mann–Whitney U test, depending on distribution, and Chi-square test for categorical variables. Temporal changes in RPPH1 expression, in PBMC, EVs, and plasma during pregnancy between control and PE women were assessed using linear mixed model analysis with subject as random effect and time and PE diagnosis as fixed effects (also as interaction, time*PE) in addition to age and body mass index (BMI) and are expressed as estimated marginal means (EMM) and 95% confidence intervals (CI). Associations at individual timepoints were also assessed by Spearman correlation analysis. The discriminatory properties of RPPH1 in leukocytes at 22–24 weeks on development of PE were assessed by receiver operating characteristic (ROC) analysis and a cut-off based on Youden index was identified and utilized in logistic regression with adjustment for (a) the propensity score (age, BMI, mean arterial pressure (MAP), parity), (b) the propensity score + PlGF, and (c) the propensity score + PlGF + artUPI. The propensity score in (c) was compared with this score including RPPH1PBMC using ROC analysis. Two-tailed p-values < 0.05 were considered significant. Statistical analyses were conducted using SPSS for Windows, version 28.0 (Chicago, IL).
Results
The characteristics of the STORK study cohort are shown in Table 1. The leukocyte cohort and plasma/EV cohort show similar results. The study revealed significant differences between women who developed PE and the controls; a higher BMI, MAP, and uterine artUPI were found in the PE group, in addition to a lower age and gestation at delivery. Levels of sFlt-1, sFlt-1/PlGF ratio, β-galactosidase, and AOPP were both significantly increased in women who developed PE, as reported previously [6, 7].
Table 1.
Clinical and demographic characteristics of the study population selected from the STORK cohort
| Criteria | Plasma/EV | Leukocytes | ||||
|---|---|---|---|---|---|---|
|
NP (n = 35) |
PE (n = 35) |
p-value |
NP (n = 214) |
PE (n = 37) |
p-value | |
| Age (years) | 32 ± 4 | 30 ± 4 | 0.019 | 32 ± 4 | 30 ± 4 | 0.006 |
| Gestational age at delivery (week) | 40 ± 1 | 39 ± 3 | 0.001 | 40 ± 1 | 38 ± 3 | <0.001 |
| Primiparous n (%) | 19 (54) | 25 (71) | 0.138 | 111 (52) | 26 (70) | 0.043 |
| Uterine artery PI | ||||||
| 22–24 weeks | 0.80 (0.73, 1.0) |
1.01 (0.79, 1.18) |
0.006 | 0.83 (0.72, 0.99) |
1.01 (0.79, 1.18) |
0.010 |
| 36–38 weeks | 0.73 (0.60, 0.87) |
0.84 (0.69, 1.08) |
0.021 | 0.72 (0.59, 0.83) |
0.84 (0.68, 1.01) |
0.014 |
| BMI (kg/m2) | ||||||
| 14–16 weeks | 23.6 (21.2, 26.4) |
27.5 (22.5, 29.8) |
0.007 | 23.5 (21.3, 25.4) |
27.2 (22.8, 30.0) |
<0.001 |
| 22–24 weeks | 24.8 (22.4, 27.6) |
28.1 (23.2, 31.9) |
0.011 | 24.8 (22.5, 26.8) |
28.9 (23.6, 31.8) |
<0.001 |
| 36–38 weeks | 27.4 (25.1, 30.0) |
31.8 (27.1, 34.8) |
0.006 | 27.2 (24.9, 29.5) |
31.8 (27.1, 34.8) |
<0.001 |
| Systolic BP (mmHg) | ||||||
| 22–24 weeks | 110 (102, 115) |
118 (105, 120) |
0.119 | 110 (100, 115) |
114 (104, 120) |
0.069 |
| 36–38 weeks | 115 (100, 120) |
130 (120, 139) |
<0.001 | 110 (105, 120) |
130 (120, 139) |
<0.001 |
| Diastolic BP (mmHg) | ||||||
| 22–24 weeks | 75 (60, 70) |
70 (60, 80) |
0.020 | 65 (60, 70) |
70 (60, 80) |
0.002 |
| 36–38 weeks | 70 (65, 80) |
85 (76, 94) |
<0.001 | 70 (68, 80) |
83 (76, 90) |
<0.001 |
| MAP (mmHg) | ||||||
| 22–24 weeks | 80 (77, 85) |
85 (79, 93) |
0.016 | 80 (73, 85) |
83 (79, 93) |
0.005 |
| 36–38 weeks | 84 (79, 92) |
100 (90, 108) |
<0.001 | 85 (80, 93) |
98 (90, 106) |
<0.001 |
| PlGF (pg/ml) | ||||||
| 22–24 weeks | 216 (154, 301) |
150 (80, 239) |
0.013 | 212 (144, 298) |
150 (94, 257) |
0.013 |
| 36–38 weeks | 172 (69, 245) |
44 (15, 99) |
<0.001 | 132 (61, 272) |
53 (17, 99) |
<0.001 |
| sFlt-1 (ng/ml) | ||||||
| 22–24 weeks | 1.63 (0.98, 1.97) |
1.54 (1.08, 2.23) |
0.597 | 1.41 (1.02, 1.97) |
1.75 (1.14, 2.53) |
0.062 |
| 36–38 weeks | 3.34 (2.54, 5.77) |
6.10 (4.91, 9.33) |
<0.001 | 3.67 (2.54, 5.40) |
6.10 (4.90, 9.33) |
<0.001 |
| sFlt-1/PlGF ratio | ||||||
| 22–24 weeks | 7.0 (4.4, 14.3) |
10.0 (6.4, 21.8) |
0.064 | 6.7 (4.5, 12.0) |
10.1 (6.5, 22.6) |
0.002 |
| 36–38 weeks | 35.2 (8.7, 59.5) |
184.8 (64.8, 732.9) |
<0.001 | 31.6 (10.8, 78.8) |
149.6 (58.1, 732.9) |
<0.001 |
| β-galactosidase (ng/μL) | ||||||
| 22–24 weeks | 8.42 (7.25, 9.28) |
9.69 (7.70, 11.7) |
0.023 | 8.18 (6.92, 9.76) |
9.78 (7.58, 12.5) |
0.009 |
| 36–38 weeks | 10.6 (9.16, 12.2) |
12.3 (9.56, 13.5) |
0.222 | 10.1 (8.40, 12.0) |
12.3 (9.51, 13.5) |
0.068 |
| AOPP activity (μM) | ||||||
| 22–24 weeks | 103 (83, 134) |
115 (99, 142) |
0.056 | 104 (87, 126) |
121 (102, 143) |
0.003 |
| 36–38 weeks | 155 (135, 162) |
182 (156, 194) |
<0.001 | 152 (123, 173) |
183 (156, 195) |
<0.001 |
Data given as mean ± SD when normally distributed and median (25th, 75th percentile) when not normally distributed. NP, normal pregnancy; EV, extracellular vesicles; BMI, body mass index; MAP, mean arterial pressure; BP, blood pressure; PI, pulsatility index; PlGF, placental growth factor; sFlt-1, soluble fms-like tyrosine kinase-1; AOPP, advanced oxidation protein products.
Lower expression of RPPH1 in leukocytes found in women developing PE
The previous published pilot array showed RPPH1 as 1 of the top 10 downregulated transcripts (fold change − 2.3) from leukocytes from PE women at 22–24 weeks [7]. We validated these findings at 22–24 weeks and analyzed the expression also at 36–38 weeks in a larger cohort using qPCR. The expression of RPPH1 increased significantly during pregnancy in both groups (p < 0.001), but was markedly lower in PE women (n = 37) at both timepoints compared with controls (n = 214, Figure 1A), albeit with a larger statistical difference at 22–24 weeks (p < 0.001).
Figure 1.
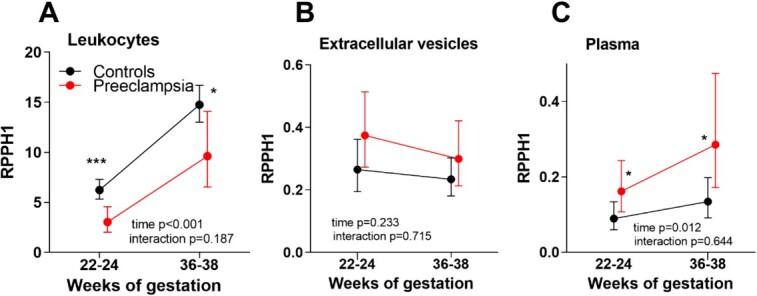
Expression of RPPH1 using qPCR at weeks 22–24 and 36–38 measured in leukocytes (A), EVs (B), and plasma (C) between women who subsequently developed PE and controls (NP). Data, relative expression, are shown as EMM and 95% CI from linear mixed model analysis adjusting for age and BMI. The adjusted p-values (*) represent expression levels comparing RPPH1 between PE and controls at each timepoint, 22–24 and 36–38 weeks, from the linear mixed model analysis. *p-value < 0.05, ***p < 0.001.
No difference in the RPPH1 expression in EVs in women developing PE
The expression of RPPH1 in EVs did not change during pregnancy (Figure 1B). There was no difference in the expression levels of RPPH1 between the groups, PE (n = 35) and controls (n = 35), adjusted for age and BMI, at either weeks 22–24 or 36–38.
Increased expression of RPPH1 in plasma in women developing PE
The expression of RPPH1 in the plasma significantly increased during pregnancy (p = 0.012) however with no interactions between the groups (Figure 1C). However, in contrast to expression in maternal leukocytes, levels were slightly higher in the PE group (n = 35) compared with the control group (n = 35) at weeks 22–24 (p = 0.048) with a larger difference at weeks 36–38 (p = 0.022) in age and BMI adjusted analysis.
Low expression of RPPH1 associated with PE risk factors
Next, we evaluated if RPPH1 expression in leukocytes was associated with maternal clinical variables (age, BMI at inclusion 14–16 weeks, BP, MAP, artUPI, PlGF, and sFlt-1). The main finding was RPPH1 correlated modestly with several factors that affect the chance of developing PE. Thus, at 22–24 weeks, a negative correlation was found with BMI (r = −0.15, p = 0.026), MAP (r = −0.14, p = 0.037), and systolic BP (r = −0.14, p = 0.042). Leukocyte RPPH1 expression was negatively associated with β-galactosidase levels at 22–24 weeks (r = −0.13, p = 0.049). At a higher gestation, weeks 36–38, RPPH1 expression in leukocytes correlated positively with maternal age (r = 0.14, p = 0.048) and negatively with diastolic BP (r = −0.16, p = 0.018). Levels of sFlt-1 was found to be positively correlated to RPPH1 expression in plasma (r = 0.36, p = 0.004), but negatively correlated to the expression of RPPH1 in leukocytes (r = −0.15, p = 0.023). Furthermore, plasma RPPH1 expression was positively associated with β-galactosidase (r = 0.29, p = 0.002, n = 62) and AOPP (r = 0.32, p = 0.010, n = 62) at 36–38 weeks. We found no correlation between RPPH1 and uterine artUPI or PlGF levels at any timepoints. In addition, we found no correlation between RPPH1 expression in EVs with the clinical variables.
Prediction of PE at 22–24 weeks using RPPH1 expression
Finally, we evaluated the discriminatory properties of early, i.e. at weeks 22–24 before signs and symptoms manifest, expression of RPPH1 in plasma, leukocytes, and EVs, in predicting PE through ROC analysis (Figure 2A). A significant association with later PE development was found in leukocytes (PBMC) (AUC 0.68 [CI 0.58–0.78], p = 0.001). No association was found using RPPH1 in EVs or plasma to predict PE at 22–24 weeks. To further examine the relevance of RPPH1 expression in leukocytes, we assessed if having below cut-off levels of RPPH1 (determined by Youden index in ROC analysis) at 22–24 weeks could predict PE in analysis adjusting for established predictors of PE. As shown in logistic regression in Figure 2B, the risk of developing PE is around ~4 times higher when expression of RPPH1 is below the cut-off. We observed no attenuation of the association when including more established PE risk predictors as covariates, and the OR remained ~5.0 in adjusted analysis. Importantly, comparing the propensity scores using age, BMI, parity, MAP, PlGF, and artUPI (AUC 0.82 CI [0.72–0.92], p < 0.001) with the same markers including RPPH1PBMC expression (AUC 0.88 CI [0.78–0.97]) showed a significantly better discrimination at 22–24 weeks of subsequent PE development using the score including RPPH1PBMC (p = 0.032).
Figure 2.
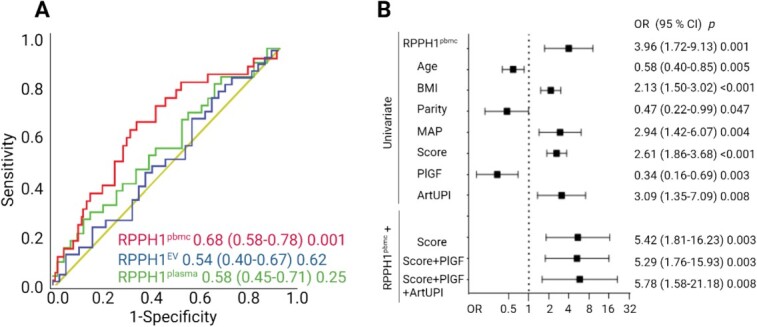
(A) To identify the best predictor of PE, a ROC analysis was performed using data at 22–24 weeks, relative expression using qPCR of RPPH1 in EVs (35 PE, 35 NP), plasma (35 PE, 35 NP), and PBMC (peripheral blood mononuclear cells, leukocytes), (37 PE, 214 NP). (B) Logistic regression analysis of predictors of PE at 22–24 weeks. The top part shows RPPH1-PBMC and established risk factors of PE in univariate analysis. The variables age, BMI, parity, and MAP are included together in a propensity score. The bottom part shows RPPH1 when adjusted for the established PE risk predictors, score (age, BMI, parity, and MAP), PlGF, and artUPI. Age, BMI (14–16 weeks), and the propensity score were transformed to standardized values. RPPH1-PBMC, artUPI, MAP, parity, and PlGF were dichotomized according to Youden index: RPPH1-PBMC cut-of: 5.17, likelihood ratio (LHR):1.9; MAP cut-of: 85.8, LHR: 2.0; artUPI cut-of: 0.93, LHR: 1.9; PlGF cut-of: 167, LHR: 1.8.
Discussion
In this study, we analyzed expression of the lncRNA RPPH1 using blood samples from different gestational timepoints and qPCR to quantify expression in plasma, EVs, and leukocytes. We found that (1) RPPH1 expression increases in leukocytes from 22–24 to 36–38 weeks, but is consistently lower in PE, (2) expression of RPPH1 in plasma is higher in the PE group, (3) below threshold levels RPPH1 at 22–24 weeks was associated with a four times higher odds of subsequent development of PE also when adjusting for established risk markers.
The exact function of RPPH1 is still largely unknown, and what role it plays in the development of PE can only be speculated based on our data. As RPPH1 has been shown to augment migration and metastasis in cancer cells [18, 20, 24] and knockdown promotes apoptosis [17–19], we speculate that the lower expression in leukocytes from PE women could reflect enhanced cell death in PE. We have previously shown increased indices of cell death, ferroptosis, and senescence, in leukocytes from women developing PE [6, 7]. However, the mechanisms by how increased cell death of maternal leukocytes may promote, or represent a proxy, for placental pathology is unknown. RPPH1 was one of the top expressed RNA in a study of placenta biopsies [25] and supports a role for RPPH1 in placental pathology in PE. Furthermore, RPPH1 [26] and sFlt-1 [27] are downregulated in mesenchymal stem cells and upregulated in endothelial cells, respectively, by hypoxia, a main feature of PE development [28]. As syncytiotrophoblasts in the placenta is the major source of sFlt-1 to the circulation of PE women, we further speculate that more RPPH1 may also be secreted by the syncytiotrophoblasts into the circulation in women developing PE, because of increased hypoxia and cell death. Our finding of increased plasma RPPH1 levels in PE positively correlated with sFlt-1 levels at 36–38 weeks may support this.
In the two-stage model in PE development, shallow trophoblast invasion and inadequate spiral artery development are initial events leading to the second stage with increased oxidative stress, inflammation, and increased sFlt-1 in the circulation of the mother. As trophoblast invasion and spiral artery remodeling occurs before 22 weeks of pregnancy when we isolated our initial samples, we can only speculate if RPPH1 is a cause or consequence of PE. The lack of association between uterine artery PI and RPPH1 expression at 22–24 weeks, but correlation with markers reflecting the second stage of PE development such as oxidative stress and senescence markers in the circulation, may suggest the expression of RPPH1 is a consequence of PE. However, as our sample was predominantly late-onset PE where interactions between normal senescence of the placenta and a maternal genetic predisposition to cardiovascular and metabolic disease are central [29], we further speculate that RPPH1 could play a role in the development of late-onset PE.
The opposite expression of RPPH1 in plasma and leukocytes in PE women is intriguing. Support for our finding was a recent study showing increased RPPH1 expression in cell-free RNA isolated from plasma at ≥23 weeks of gestation [30]. Of relevance, a study on senescence-associated mitochondrial ncRNAs revealed that RPPH1 was differentially distributed in the cytoplasm and the mitochondria during cellular senescence [31], and RPPH1 overexpression promoted activation of acidic β-galactosidase as a marker of cellular senescence [31]. Enhanced placental and maternal oxidative stress in PE has been shown in multiple studies and we recently demonstrated increased maternal senescence and oxidative stress in the plasma of PE women using the markers of β-galactosidase and AOPP, respectively [6, 7]. In the present study, leukocyte RPPH1 expression was negatively correlated with β-galactosidase levels at 22–24 weeks while plasma RPPH1 expression was positively associated at 36–38 weeks with β-galactosidase and AOPP, further supporting that RPPH1 may be linked to oxidative stress and cell death in PE, causing release of extracellular RPPH1 in these women. We found no difference in the RPPH1 expression in EVs between controls and PE. EVs in the circulation originate from blood cells, vascular cells, and many other tissues, and accordingly, the cell-free RPPH1 we report in the circulation of pregnant women may be secreted from many different cells and tissues. Apoptotic cells release more EVs than healthy cells, which may be phagocytosed by macrophages and fuse with lysosomes [32]. Thus, the increase in RPPH1 during gestation, regardless of group, may support increased uptake of EVs from the circulation containing RPPH1. As women developing PE have a larger amount of EVs in their circulation we may speculate that some of the EVs will not be taken up by other cells resulting in release of cell-free RPPH1 and increased circulating levels. However, to be noted, there are many different mechanisms of uptake and release of EVs [33].
One of the molecular functions of RPPH1 is related to pre-tRNAs processing and inhibition of RPPH1 expression decreased mature tRNAs [34]. Interestingly, we recently demonstrated decreased expression of the mitochondrial tRNA alanine (MT-TA) in leukocytes of PE women [12]. We may speculate that the decreased tRNA expression in leukocytes of PE women may be impacted by the decrease in RPPH1 expression playing a role in the catalytic activity of RNase P, which catalyzes removal of the 5′ leader from precursor tRNAs to mature tRNAs [21]. Also, exosome derived RPPH1 enhanced macrophage M2 polarization [17] and conversely low RPPH1 induced M1 markers [17]. Thus, the low RPPH1 expression in leukocytes in our study could reflect more pro-inflammatory M1 monocytes in PE women.
Evaluating the discriminatory abilities of RPPH1 in leukocytes at weeks 22–24 revealed only modest prediction of PE. However, having below cut-off levels of RPPH1 nonetheless gave a 4-fold enhanced risk of PE diagnosis, comparable to other established predictors. Importantly, the prediction did not change much when adjusting for known risk markers for PE, suggesting that RPPH1 may reflect other pathways for PE development not accounted for by established biomarkers. Also, when comparing the variables used in the most promising first trimester screening algorithm today, and including RPPH1 expression in leukocytes, the discrimination of later PE diagnosis increased significantly. Thus, the combination of RPPH1 with other markers to predict PE should be explored in future studies to possibly gain a higher prediction of PE at this early timepoint.
Limitation of the study is that we do not have any mechanistic data of RPPH1 function in PE development. Also, the screening algorithm is based on first trimester and we have used samples from 22–24 weeks, we do not have RPPH1 expression data at an earlier timepoint. However, our data are novel investigating RPPH1 in leukocytes from before the women develop any symptoms of PE and the RPPH1 expression/function/marker in relation to PE development has not been performed before. We have not performed any protein analysis to confirm our EVs, however in our recent publication we have investigated RNAs coding for known proteins used to validate EVs using RNA-seq and qPCR, suggesting that our EVs are valid [12]. The lack of a validation study with early sampling is a limitation of the study.
In conclusion, PE women were characterized by low and high expression of the lncRNA RPPH1 in leukocytes and plasma, respectively. Future studies should evaluate if this pattern may promote inflammation, senescence, and cell death in PE development.
Footnotes
† Grant Support: South-Eastern Norway Regional Health Authority, grant number 2023099.
Contributor Information
Dina-Marie Munkelien Myhrer, Faculty of Medicine, University of Oslo, Oslo, Norway.
Monica Frøystad, Faculty of Medicine, University of Oslo, Oslo, Norway; Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
Marie Cecilie Paasche Roland, Department of Obstetrics, Oslo University Hospital, Oslo, Norway; Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway.
Thor Ueland, Faculty of Medicine, University of Oslo, Oslo, Norway; Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway; Thrombosis Research Center (TREC), Division of Internal Medicine, University Hospital of North Norway, Tromsø, Norway.
Tove Lekva, Research Institute of Internal Medicine, Oslo University Hospital, Oslo, Norway.
List of abbreviations
AOPP: Advanced oxidative protein levels
EVs: Extracellular vesicles
lncRNA: Long non-coding RNA
PE: Pre-eclampsia
RPPH1: Ribonuclease P RNA Component H1
artUPI: Uterine artery pulsatility index
Conflict of Interest: The authors have declared that no conflict of interest exists.
Author contributions
DMM, TL, and TU conceptualized and designed the study. TL and MCPR collected data and biospecimens. DMM and TL processed biospecimens and analyzed data. DMM, MF, MCPR, TU, and TL analyzed and interpreted data. DMM and TL drafted the manuscript. DMM, MF, MCPR, TU, and TL revised and approved the final version of the manuscript.
Data availability
The data underlying this article cannot be shared publicly due to ethical restrictions from the Regional Committee for Medical and Research Ethics in South-East Norway. The data will be shared on reasonable request to the corresponding author.
References
- 1. Karrar SA, Hong PL. Preeclampsia. Treasure Island (FL): StatPearls; 2023. [Google Scholar]
- 2. MacDonald TM, Walker SP, Hannan NJ, Tong S, Kaitu'u-Lino TJ. Clinical tools and biomarkers to predict preeclampsia. EBioMedicine 2022; 75:103780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee NMW, Chaemsaithong P, Poon LC. Prediction of preeclampsia in asymptomatic women. Best Pract Res Clin Obstet Gynaecol 2024; 92:102436. [DOI] [PubMed] [Google Scholar]
- 4. Lokeswara AW, Hiksas R, Irwinda R, Wibowo N. Preeclampsia: from cellular wellness to inappropriate cell death, and the roles of nutrition. Front Cell Dev Biol 2021; 9:726513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuster J, Cheng SB, Padbury J, Sharma S. Placental extracellular vesicles and pre-eclampsia. Am J Reprod Immunol 2021; 85:e13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lekva T, Michelsen AE, Roland MCP, Norwitz ER, Estensen ME, Olstad OK, Akkouh IA, Henriksen T, Bollerslev J, Aukrust P, Ueland T. Increased ferroptosis in leukocytes from preeclamptic women involving the long non-coding taurine upregulated gene 1 (TUG1). J Intern Med 2023; 295:181–195. [DOI] [PubMed] [Google Scholar]
- 7. Lekva T, Roland MCP, Estensen ME, Norwitz ER, Tilburgs T, Henriksen T, Bollerslev J, Normann KR, Magnus P, Olstad OK, Aukrust P, Ueland T. Dysregulated non-coding telomerase RNA component and associated exonuclease XRN1 in leucocytes from women developing preeclampsia-possible link to enhanced senescence. Sci Rep 2021; 11:19735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell 2009; 136:629–641. [DOI] [PubMed] [Google Scholar]
- 9. Mattick JS, Amaral PP, Carninci P, Carpenter S, Chang HY, Chen LL, Chen R, Dean C, Dinger ME, Fitzgerald KA, Gingeras TR, Guttman M, et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat Rev Mol Cell Biol 2023; 24:430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He X, He Y, Xi B, Zheng J, Zeng X, Cai Q, Ouyang Y, Wang C, Zhou X, Huang H, Deng W, Xin S, et al. LncRNAs expression in preeclampsia placenta reveals the potential role of LncRNAs contributing to preeclampsia pathogenesis. PloS One 2013; 8:e81437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Long W, Rui C, Song X, Dai X, Xue X, Lu Y, Shen R, Li J, Li J, Ding H. Distinct expression profiles of lncRNAs between early-onset preeclampsia and preterm controls. Clin Chim Acta 2016; 463:193–199. [DOI] [PubMed] [Google Scholar]
- 12. Lekva T, Sundaram AYF, Roland MCP, Asheim J, Michelsen AE, Norwitz ER, Aukrust P, Gilfillan GD, Ueland T. Platelet and mitochondrial RNA is decreased in plasma-derived extracellular vesicles in women with preeclampsia-an exploratory study. BMC Med 2023; 21:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McAninch D, Roberts CT, Bianco-Miotto T. Mechanistic insight into long noncoding RNAs and the placenta. Int J Mol Sci 2017; 18:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong RQ, Nuh AM, Cao HS, Ma M. Roles of exosomes-derived lncRNAs in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2021; 263:132–138. [DOI] [PubMed] [Google Scholar]
- 15. Hannon GJ, Chubb A, Maroney PA, Hannon G, Altman S, Nilsen TW. Multiple cis-acting elements are required for RNA polymerase III transcription of the gene encoding H1 RNA, the RNA component of human RNase P. J Biol Chem 1991; 266:22796–22799. [PubMed] [Google Scholar]
- 16. Zhang P, Sun Y, Peng R, Chen W, Fu X, Zhang L, Peng H, Zhang Z. Long non-coding RNA Rpph1 promotes inflammation and proliferation of mesangial cells in diabetic nephropathy via an interaction with Gal-3. Cell Death Dis 2019; 10:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang ZX, Liu HS, Wang FW, Xiong L, Zhou C, Hu T, He XW, Wu XJ, Xie D, Wu XR, Lan P. LncRNA RPPH1 promotes colorectal cancer metastasis by interacting with TUBB3 and by promoting exosomes-mediated macrophage M2 polarization. Cell Death Dis 2019; 10:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yue K, Ma JL, Jiang T, Yue J, Sun SK, Shen JL, Miao Y. LncRNA RPPH1 predicts poor prognosis and regulates cell proliferation and migration by repressing P21 expression in gastric cancer. Eur Rev Med Pharmacol Sci 2020; 24:11072–11080. [DOI] [PubMed] [Google Scholar]
- 19. Lei B, He A, Chen Y, Cao X, Zhang P, Liu J, Ma X, Qian L, Zhang W. Long non-coding RNA RPPH1 promotes the proliferation, invasion and migration of human acute myeloid leukemia cells through down-regulating miR-330-5p expression. EXCLI J 2019; 18:824–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou J, Shi K, Huang W, Zhang Y, Chen Q, Mou T, Wu Z, Wei X. LncRNA RPPH1 acts as a molecular sponge for miR-122 to regulate Wnt1/beta-catenin signaling in hepatocellular carcinoma. Int J Med Sci 2023; 20:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou S, Van Bortle K. The pol III transcriptome: basic features, recurrent patterns, and emerging roles in cancer. Wiley Interdiscip Rev RNA 2023; 14:e1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roland MC, Friis CM, Voldner N, Godang K, Bollerslev J, Haugen G, Henriksen T. Fetal growth versus birthweight: the role of placenta versus other determinants. PloS One 2012; 7:e39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lekva T, Michelsen AE, Aukrust P, Paasche Roland MC, Henriksen T, Bollerslev J, Ueland T. CXC chemokine ligand 16 is increased in gestational diabetes mellitus and preeclampsia and associated with lipoproteins in gestational diabetes mellitus at 5 years follow-up. Diab Vasc Dis Res 2017; 14:525–533. [DOI] [PubMed] [Google Scholar]
- 24. Zhang Y, Tang L. Inhibition of breast cancer cell proliferation and tumorigenesis by long non-coding RNA RPPH1 down-regulation of miR-122 expression. Cancer Cell Int 2017; 17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deyssenroth MA, Peng S, Hao K, Lambertini L, Marsit CJ, Chen J. Whole-transcriptome analysis delineates the human placenta gene network and its associations with fetal growth. BMC Genomics 2017; 18:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong X, Yuan L, Yang K, Wang X. The HIFIA/LINC02913/IGF1R axis promotes the cell function of adipose-derived mesenchymal stem cells under hypoxia via activating the PI3K/AKT pathway. J Transl Med 2023; 21:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao H, Wang X, Fang B. HIF1A promotes miR-210/miR-424 transcription to modulate the angiogenesis in HUVECs and HDMECs via sFLT1 under hypoxic stress. Mol Cell Biochem 2022; 477:2107–2119. [DOI] [PubMed] [Google Scholar]
- 28. Jahan F, Vasam G, Green AE, Bainbridge SA, Menzies KJ. Placental mitochondrial function and dysfunction in preeclampsia. Int J Mol Sci 2023; 24:4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ 2019; 366:l2381. [DOI] [PubMed] [Google Scholar]
- 30. Moufarrej MN, Vorperian SK, Wong RJ, Campos AA, Quaintance CC, Sit RV, Tan M, Detweiler AM, Mekonen H, Neff NF, Baruch-Gravett C, Litch JA, et al. Early prediction of preeclampsia in pregnancy with cell-free RNA. Nature 2022; 602:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee JW, Chun YL, Kim AY, Lloyd LT, Ko S, Yoon JH, Min KW. Accumulation of mitochondrial RPPH1 RNA is associated with cellular senescence. Int J Mol Sci 2021; 22:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caruso S, Poon IKH. Apoptotic cell-derived extracellular vesicles: more than just debris. Front Immunol 2018; 9:1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu J, Sane S, Kim JE, Yun S, Kim HJ, Jo KB, Wright JP, Khoshdoozmasouleh N, Lee K, Oh HT, Thiel K, Parvin A, et al. Biogenesis and delivery of extracellular vesicles: harnessing the power of EVs for diagnostics and therapeutics. Front Mol Biosci 2023; 10:1330400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin YH, Chen CW, Cheng HC, Liu CJ, Chung ST, Hsieh MC, Tseng PL, Tsai WH, Wu TS, Lai MD, Shih CL, Yen MC, et al. Inhibition of lncRNA RPPH1 activity decreases tumor proliferation and metastasis through down-regulation of inflammation-related oncogenes. Am J Transl Res 2023; 15:6701–6717. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical restrictions from the Regional Committee for Medical and Research Ethics in South-East Norway. The data will be shared on reasonable request to the corresponding author.


