Abstract
The only characteristic of alpha-synuclein (AS) accumulation in the gastrointestinal (GI) tract of Parkinson’s disease (PD) found in pathological studies is the “rostrocaudal gradient,” which describes the more frequent presence of AS accumulation in the upper GI tract than in the lower GI tract. This study aimed to determine the diagnostic accuracy and identify predictors of AS accumulation in the GI tract of PD patients. The frequency of AS accumulation in the GI tract was compared between PD patients (N = 97) who underwent radical GI surgery for cancer and individually matched controls (N = 94). We evaluated AS accumulation in the neural structures using phosphorylated AS immunohistochemistry. A multivariable logistic regression analysis was conducted to determine the predictors of AS accumulation in the GI tract of PD patients. The frequency of AS accumulation was significantly higher in PD patients (75.3%) than in controls (8.5%, p-value < 0.001). The sensitivity and specificity of the full-layer evaluation were 75.3% and 91.5%, respectively. When the evaluation was confined to the mucosal/submucosal layer, the sensitivity and specificity were 46.9% and 94.7%, respectively. The rostrocaudal gradient of AS accumulation was found in PD patients. The duration from symptom onset to surgery was significantly longer in PD patients with AS accumulation (4.9 ± 4.9 years) than in PD patients without AS accumulation (1.8 ± 4.1 years, p-value = 0.005). Both disease duration and rostrocaudal gradient independently predicted the presence of AS accumulation in the GI tract of PD patients. Our study suggests PD-related AS accumulation in the GI tract follows a temporally increasing but spatially static progression pattern.
Subject terms: Parkinson's disease, Diagnostic markers
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by a clinical syndrome of bradykinesia, resting tremor, rigidity, and postural instability. Clinical diagnostic criteria are used to diagnose PD1, and a definitive diagnosis is only possible when the Lewy pathology, the pathological hallmark of PD, is detected in the brain by postmortem neuropathological evaluation2,3. The diagnostic accuracy of the new diagnostic criteria for PD is 92.5% compared with that of pathological diagnosis4. However, a reliable biomarker that reflects the pathology and progression of PD is needed for the precise management of patients in clinical practice and the development of disease-modifying treatments.
Alpha-synuclein (AS) accumulation in the gastrointestinal (GI) tract was first discovered in the esophagus5. Later, the so-called Braak’s hypothesis was proposed that AS accumulation starts in the enteric nervous system of the stomach and subsequently enters the brain via the vagus nerve based on the findings of AS accumulation in the stomach of autopsied subjects with pre-symptomatic Braak stages II and III in the brain2,6. A large-scale autopsy study showed the involvement of AS accumulation in multiple peripheral organs in synucleinopathy7. These studies have led to the search for the role of AS accumulation in the GI tract as an in vivo biomarker of PD.
Many studies have been conducted in the GI tract because it is considered the origin or earliest site of AS accumulation and the tissue is also easily obtainable by routine endoscopic procedures8–18. However, a meta-analysis revealed that immunohistochemistry or immunofluorescence staining on tissues of the GI tract using a primary antibody against phosphorylated AS (pAS) showed mediocre diagnostic accuracy with a pooled sensitivity and specificity of 0.43 and 0.82, respectively19. Moreover, included studies had various ranges of sensitivity and specificity with 0.11–1.0 and 0–1.0, respectively, and significant inconsistency among studies (I2 of sensitivity and specificity, 94.0% and 94.1%, respectively)19. The discrepancy among studies was explained by the small sample size of single-center studies, limited area and depth of biopsied tissue, heterogeneous staining methods, and different definitions of positive staining for AS accumulation in pathological evaluation. Therefore, the meta-analysis presented limited information regarding the true diagnostic accuracy of AS accumulation in the GI tract. To overcome this inevitable limitation of the meta-analysis, a large-scale multicenter study with a verified staining method was required. Moreover, many studies have used biopsied tissues alone or in combination with surgical specimens. we used only surgical specimens, which have many strengths compared to biopsied tissues, such as covering a full depth of the intestinal wall, a large evaluation area, and many neural structures, especially submucosal and myenteric plexuses.
The prion-like progressive nature of AS accumulation has been reported in many animal studies20–23. However, the only characteristic of AS accumulation in the GI tract found in pathological studies is “rostrocaudal gradient,” which describes the more frequent presence of AS accumulation in the upper GI (UGI) tract than in the lower GI (LGI) tract7,14,24,25. The cause of the rostrocaudal gradient in the GI tract is unknown. Some researchers argue that this finding is due to the difference of viable neural structures between the upper and lower GI tract26 or reflection of the brain-to-gut progression of AS aggregates and the vagal nerve distribution in the GI tract27. Moreover, the post-mortem study of Tanei et al. found that AS accumulation in the esophagus was more prevalent in higher pathological stages of the brain28. This implies the temporally progressive nature of AS accumulation in the GI tract, which has not been confirmed in antemortem studies. Therefore, this study aimed to determine the possibility of using AS accumulation as a diagnostic accuracy and evaluate the predictors of AS accumulation in the GI tract of patients with PD.
Results
Selection process
A total of 101 patients with PD and 94 controls matched for age at the time of surgery, sex, and surgical site were selected according to the eligibility criteria (Supplementary Fig. 1). Because only one block was available for nine patients and six controls, 193 blocks from patients with PD and 182 blocks from controls were collected and stained. During the staining process, four slides from the PD patient group were excluded because of poor staining quality. In the pathological evaluation, nine and three slides from the PD patient and control groups, respectively, were excluded because no neural structures were identified by the NF staining. However, no participants were dropped out during this process because the other slide could be evaluated. Finally, 355 pAS-stained slides from 97 patients with PD and 94 matched controls were analyzed.
Clinical characteristics of participants
The clinical characteristics of the participants are summarized in Table 1 (individual data are available in Supplementary Table 1). The matched clinical characteristics did not significantly differ between the patient and control groups. The ages at the final follow-up visit were 75.8 ± 7.0 and 78.6 ± 7.1 years in patients with PD and controls, respectively (p-value = 0.007). Accordingly, the duration from surgery to the final follow-up visit was also significantly longer in the control group (patients vs. controls, 4.8 ± 3.6 vs. 7.7 ± 2.3; p-value < 0.001).
Table 1.
Clinical characteristics of participants
| Characteristic | Patients with PD (N = 97) | Controls (N = 94) | p-value |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 57 (58.8) | 60 (63.8) | 0.472 |
| Female | 40 (41.2) | 34 (36.2) | |
| Age, mean (SD), yr | |||
| At surgery | 71.0 (6.9) | 70.9 (6.8) | 0.913 |
| At the final follow-up visit | 75.8 (7.0) | 78.6 (7.1) | 0.007 |
| At symptom onset | 66.8 (7.9) | ||
| At diagnosis | 68.6 (8.0) | ||
| Duration, mean (SD), yr | |||
| Onset to surgery | 4.2 (4.9) | ||
| Onset to diagnosis | 1.8 (2.3) | ||
| Onset to the final follow-up visit | 9.0 (4.7) | ||
| Surgery to the final follow-up visit | 4.8 (3.6) | 7.7 (2.3) | <0.001 |
| Frequency of patients who underwent surgery before the symptom onset, No. (%) | 12 (12.4) | ||
| HY stage at the year of surgery (available in 29 patients), no. (%) | |||
| 1 | 4 (4.1) | ||
| 2 | 7 (7.2) | ||
| 2.5 | 9 (9.3) | ||
| 3 | 9 (9.3) | ||
| Surgical site, no. (%) | |||
| UGI (stomach + esophagus)a | 55 (56.7) | 53 (56.4) | 0.965 |
| LGI (colon + rectum) | 42 (43.3) | 41 (43.6) | |
PD Parkinson’s disease, HY Hoehn and Yahr, UGI upper gastrointestinal tract, LGI lower gastrointestinal tract.
aNumber of patients who received esophagectomy was 2 and none in patients with PD and controls, respectively.
In the subgroup analyses, 55 patients with PD and 53 matched controls belonged to the UGI subgroup (Supplementary Table 2), and 42 patients with PD and 41 matched controls belonged to the LGI subgroup (Supplementary Table 3). In the UGI subgroup, there was no difference in clinical characteristics between the patient and control groups, except that the duration from surgery to the final follow-up visit of patients with PD was shorter (4.8 ± 3.2 vs. 7.1 ± 2.0; p-value < 0.001). In the LGI subgroup, the patient group had a significantly younger age at the final follow-up visit (75.6 ± 6.8 vs. 79.5 ± 7.6; p-value = 0.016) and shorter duration from surgery to the final follow-up visit (4.8 ± 4.0 vs. 8.5 ± 2.6; p-value < 0.001). In addition, LLGI surgery was more frequent in the patient group (71.4%), whereas RLGI surgery was more frequent in the control group (80.5%, p-value < 0.001).
Frequency of AS accumulation in the GI tract
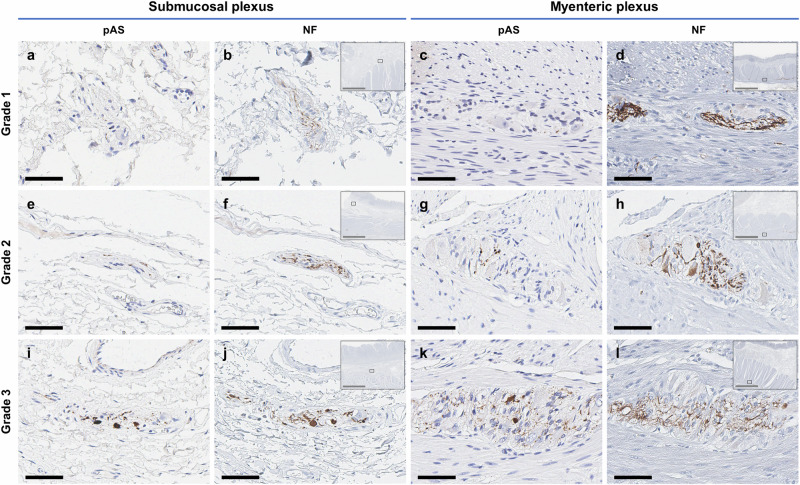
On pathological evaluation, AS accumulation was mostly found in the myenteric and submucosal plexuses (Fig. 1 and Supplementary Fig. 2). The presence of AS accumulation in the neural structure was identified by histologic evaluation and confirmed by NF staining of the next slide. A definite “dots and fibers” pattern was confirmed in the pAS-positive slides, and Lewy body-like aggregation was also found in the slides with semiquantitative grade 3 (Fig. 1I, K).
Fig. 1. Representative images for each grade of AS accumulation of the neural structures within the submucosa and muscularis propria.
a, c, e, g, i, k Representative images of neural structures (ganglia and interganglionic nerve fibers) that were assigned the same grade by all raters. The rating is based on the density of dots and fibers within the myenteric and submucosal plexuses or nerve bundles, as identified via pAS immunostaining (grade 0, negative; grade 1, sparse; grade 2, moderate; and grade 3, frequent). b, d, f, h, j, l For the confirmation of neuronal cells, NF immunostaining is performed in adjacent sections. The low-magnification insets display the anatomic locations of the respective neural structures. All representative slides are sectioned from the stomach except c, d from the rectum. Scale bars: black, 100 μm, and gray, 3 mm. AS alpha-synuclein, pAS phosphorylated AS, NF neurofilament.
Table 2 summarizes the frequency and difference of AS accumulation in the GI tract between the patient and control groups (individual data and semi-quantitative rating results are available in Supplementary Table 1). The frequencies of AS accumulation in the entire GI tract were 75.3% (N = 73) in patients with PD and 8.5% (N = 8) in controls (p-value < 0.001). In the UGI subgroup analysis, the frequencies of AS accumulation were 83.6% and 15.1% in patients and controls, respectively (p-value < 0.001). In the LGI group, the frequencies were 64.3% and 0% in patients and controls, respectively (p-value < 0.001). The rostrocaudal gradient was also confirmed in this study.
Table 2.
Differences of AS accumulation between patients with PD and controls
| Region | Patients with PD | Controls | p-value | |||
|---|---|---|---|---|---|---|
| Total no. | AS+, no. (%) | Total no. | AS+, no. (%) | |||
| Entire GI tract | 97 | 73 (75.3) | 94 | 8 (8.5) | <0.001 | |
| UGI (stomach + esophagus) | 55 | 46 (83.6) | 53 | 8 (15.1) | <0.001 | |
| LGI (colon + rectum) | 42 | 27 (64.3) | 41 | 0 | <0.001 | |
| aRLGI | 12 | 9 (75.0) | 33 | 0 | <0.001 | |
| bLLGI | 30 | 18 (60.0) | 6 | 0 | 0.007 | |
| cOthers | 0 | 0 | 2 | 0 | ||
PD Parkinson’s disease, AS alpha-synuclein, GI gastrointestinal, UGI upper GI tract; LGI lower GI tract, RLGI right LGI, LLGI left LGI.
aRLGI includes surgical specimens acquired by right hemi-colectomy.
bLLGI includes surgical specimens acquired by anterior resection, lower anterior resection, ultra-lower anterior resection, Miles’ operation, Hartmann’s operation, or left hemi-colectomy.
cOthers include surgical specimens acquired by total colectomy and subtotal colectomy.
Detailed distribution of AS accumulation layer by layer in the intestinal wall
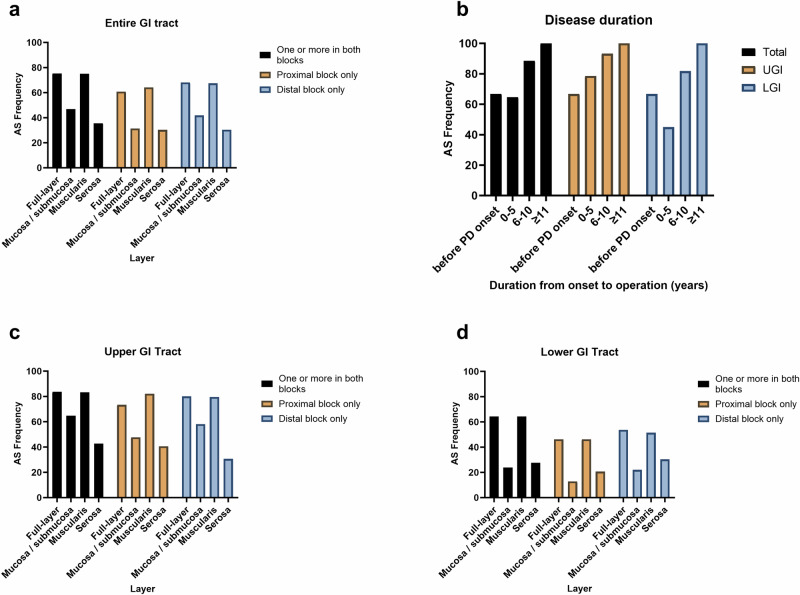
When the presence of AS accumulation in patients with PD was evaluated layer by layer in the gut wall, the frequencies were 46.9%, 75.0%, and 35.6% in the mucosal/submucosal, muscular, and serosal layers, respectively (Fig. 2A, Supplementary Table 4). A similar trend was observed in the controls. Therefore, AS accumulation was mostly found in the muscular layer and was present in all patients with AS+, except for one patient with PD.
Fig. 2. Distribution of AS accumulation according to the layer by layer in the intestinal wall and disease duration of patients with PD.
a Detailed distribution of the frequency of AS accumulation layer by layer in the intestinal wall of patients with PD. The frequency of Lewy pathology is the highest in the muscular layer, mostly found in the myenteric plexus. The concordance of AS accumulation in the proximal and distal blocks in patients with PD is moderate (κ = 0.541, p-value < 0.001). In the control group, the concordance is excellent (κ = 0.927, p-value < 0.001) because most of the controls have AS−. b Distribution of the frequency of AS accumulation in the discretized subgroups of duration from symptom onset to surgery in patients with PD. The frequency of AS accumulation in the GI tract of patients with PD increases from 67% in the before-PD onset group to 100% in the ≥11-year group. In the LGI tract subgroup, the frequency of AS accumulation decreases to 45% in the 0–5-year group but increases thereafter. The duration from symptom onset to surgery is confirmed as an independent predictor of AS accumulation in the GI tract in PD in the multivariate regression analyses, excluding the confounding effect of the surgical site (rostrocaudal gradient). c, d Detailed distribution of the frequency of AS accumulation layer by layer in the upper and lower GI tract in patients with PD. AS accumulation is more frequent in the upper GI tract than in the lower GI tract (rostrocaudal gradient). AS alpha-synuclein, PD Parkinson’s disease, GI gastrointestinal, UGI upper gastrointestinal, LGI lower gastrointestinal.
More detailed data separating the proximal and distal blocks are presented in Supplementary Tables 5–7. In the patient group, there were five patients with AS+ only in the proximal block and 12 in the distal block. Therefore, the concordance of AS accumulation in the proximal and distal blocks in patients with PD was moderate (κ = 0.541, p-value < 0.001). In the control group, the concordance was excellent (κ = 0.927, p-value < 0.001) because most of the controls did not have AS accumulation.
Diagnostic performance of AS accumulation in the GI tract for PD
The diagnostic performance of AS accumulation in the GI tract for PD is summarized in Table 3. When we evaluated the gut specimens with an intact, full layer, the sensitivity and specificity for the diagnosis of PD were 75.3% and 91.5%, respectively. The sensitivity was higher in the UGI tract (83.6%), whereas the specificity was higher in the LGI tract (100%). The overall diagnostic accuracy was excellent in both the UGI (84.3%) and LGI (81.9%) tracts.
Table 3.
Diagnostic accuracy of AS accumulation in the gastrointestinal tract for Parkinson’s disease
| Region | Full layer evaluation (%) | Mucosal/submucosal evaluation (%) | ||||
|---|---|---|---|---|---|---|
| Accuracy | Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | |
| Entire GI tract | 83.3 | 75.3 | 91.5 | 70.5 | 46.9 | 94.7 |
| UGI (stomach + esophagus) | 84.3 | 83.6 | 84.9 | 77.6 | 64.8 | 90.6 |
| LGI (colon + rectum) | 81.9 | 64.3 | 100 | 61.5 | 23.8 | 100 |
AS alpha-synuclein, UGI upper gastrointestinal tract, LGI lower gastrointestinal tract.
We also calculated the diagnostic performance of AS accumulation in the mucosal/submucosal layer, which is a potentially feasible area when the tissue is obtained by endoscopic biopsy. The specificity continued to be excellent, with 90.6% and 100% in the UGI and LGI tracts, respectively. However, the sensitivity was moderate in the UGI tract (64.8%) and poor in the LGI tract (23.8%).
Clinical characteristics related to AS accumulation in the GI tract of patients with PD
Table 4 summarizes the comparison of the clinical characteristics of patients with PD and AS+ (AS+ PD) and patients with PD and AS− (AS− PD). The duration from symptom onset to surgery was significantly longer in the AS+ PD group (4.9 ± 4.9 years) than in the AS− PD group (1.8 ± 4.1 years, p-value = 0.005). The duration from symptom onset to diagnosis was also longer in the AS+ PD group (AS+ PD vs. AS− PD, 2.0 ± 2.6 vs. 1.0 ± 1.0; p-value = 0.006). At the surgical site, specimens from the UGI and LGI tracts were more frequent in the AS+ PD (63.0%) and AS− PD (62.5%) groups, respectively (p-value = 0.029). When the clinical characteristics were compared between the UGI and LGI subgroups in the AS+ PD group, the difference was not statistically significant (Supplementary Table 8). In the control group, the UGI tract was the more frequent surgical site in controls with AS+ (Supplementary Table 9).
Table 4.
Differences of clinical characteristics between PD patients with and without AS accumulation in the GI tract
| Characteristic | Patients with PD | p-value | |
|---|---|---|---|
| AS+ (N = 73) | AS− (N = 24) | ||
| Sex, no. (%) | |||
| Male | 45 (61.6) | 12 (50.0) | 0.315 |
| Female | 28 (38.4) | 12 (50.0) | |
| Age, mean (SD), yr | |||
| At surgery | 71.4 (6.7) | 69.7 (7.4) | 0.308 |
| At the final follow-up visit | 75.9 (6.5) | 75.4 (8.5) | 0.750 |
| At symptom onset | 66.4 (7.5) | 68.0 (8.8) | 0.410 |
| At diagnosis | 68.5 (7.9) | 69.0 (8.5) | 0.790 |
| Duration, mean (SD), yr | |||
| Onset to surgery | 4.9 (4.9) | 1.8 (4.1) | 0.005 |
| Onset to diagnosis | 2.0 (2.6) | 1.0 (1.0) | 0.006 |
| Onset to the final follow-up visit | 9.5 (4.5) | 7.4 (5.1) | 0.063 |
| Surgery to the final follow-up visit | 4.5 (3.4) | 5.7 (3.9) | 0.178 |
| Frequency of patients who underwent surgery before the symptom onset, No. (%) | 8 (11.0) | 4 (16.7) | 0.484 |
| HY stage at the year of surgery (available in 29 patients), No. (%) | |||
| 1 | 3 (4.1) | 1 (4.2) | 0.998 |
| 2 | 5 (6.8) | 2 (8.3) | |
| 2.5 | 7 (9.6) | 2 (8.3) | |
| 3 | 7 (9.6) | 2 (8.3) | |
| Surgical site, No. (%) | |||
| UGI (stomach + esophagus) | 46 (63.0) | 9 (37.5) | 0.029 |
| LGI (colon + rectum) | 27 (37.0) | 15 (62.5) | |
PD Parkinson’s disease, AS alpha-synuclein, HY Hoehn and Yahr, GI gastrointestinal, UGI upper GI, LGI tract lower GI tract.
Predictors of AS accumulation in the GI tract in PD
In summary, the new characteristic features of AS+ PD, in comparison to AS− PD, are shorter durations from symptom onset to surgery and from symptom onset to diagnosis. However, the confounding effect of the surgical site (rostrocaudal gradient) could not be ruled out. The duration from symptom onset to diagnosis was excluded in logistic regression analyses because the time at diagnosis was not meaningful in this retrospective multicenter design. Therefore, a multivariate logistic regression analysis was conducted to confirm the factors predicting AS accumulation in the GI tract in patients with PD (Table 5). Univariate regression analyses showed that the duration from symptom onset to surgery (B = 0.144, p-value = 0.008) and surgical site (B = 1.044, p-value = 0.032) were significant predictors, excluding the duration from symptom onset to diagnosis. To adjust for the confounding effect of the rostrocaudal gradient, two significant predictors from univariate analyses were entered into Model 1 of the multivariate logistic regression analysis. The analysis of Model 1 showed that both the duration from symptom onset to surgery (B = 0.146, p-value = 0.008) and surgical site (B = 1.091, p-value = 0.032) were independent predictors of AS accumulation in the GI tract in patients with PD. We constructed Models 2 and 3 by including all available independent variables from other clinical characteristics. We confirmed that the duration from symptom onset to surgery and surgical site were still significant independent predictors in both models.
Table 5.
Multivariable logistic regression analysis of predictors for AS accumulation in the GI tract in patients with PD
| Predictor | Univariable | Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Exp (B) (95% CI) | p-value | B | Exp (B) (95% CI) | p-value | B | Exp (B) (95% CI) | p-value | B | Exp (B) (95% CI) | p-value | |
| Duration from symptom onset to surgery | 0.144 | 1.155 (1.038, 1.284) | 0.008 | 0.146 | 1.157 (1.038, 1.289) | 0.008 | 0.125 | 1.133 (1.012, 1.269) | 0.031 | 0.129 | 1.138 (1.003, 1.292) | 0.045 |
| Surgical site (UGI vs. LGI) | 1.044 | 2.840 (1.095, 7.366) | 0.032 | 1.091 | 2.976 (1.096, 8.084) | 0.032 | 1.065 | 2.899 (1.042, 8.070) | 0.042 | 1.065 | 2.899 (1.042, 8.070) | 0.042 |
| Duration from symptom onset to diagnosis | 0.373 | 1.452 (0.981, 2.148) | 0.062 | 0.246 | 1.279 (0.869, 1.882) | 0.212 | 0.246 | 1.279 (0.869, 1.882) | 0.212 | |||
| Age at surgery | 0.036 | 1.036 (0.968, 1.110) | 0.306 | 0.005 | 1.005 (0.932, 1.083) | 0.906 | ||||||
| Age at symptom onset | −0.025 | 0.975 (0.919, 1.035) | 0.406 | 0.005 | 1.005 (0.932, 1.083) | 0.906 | ||||||
| Sex (Male vs. Female) | −0.474 | 0.622 (0.246, 1.575) | 0.317 | 0.177 | 1.194 (0.425, 3.352) | 0.737 | 0.177 | 1.194 (0.425, 3.352) | 0.737 | |||
| Nagelkerke R2 | 0.183 | 0.216 | 0.216 | |||||||||
| Cox & Snell R2 | 0.123 | 0.146 | 0.146 | |||||||||
| −2 Log likelihood | 95.799 | 93.285 | 93.285 | |||||||||
| Chi-square | 12.741 | 15.255 | 15.255 | |||||||||
| Chi-square p-value | 0.002 | 0.009 | 0.009 | |||||||||
PD Parkinson’s disease, AS alpha-synuclein, CI confidence interval, GI gastrointestinal, UGI upper GI tract, LGI lower GI tract.
Distribution of the frequency of AS accumulation in the discretized subgroups of duration from symptom onset to surgery in PD
We discretized the duration from symptom onset to surgery to describe the detailed distribution. The PD patient group was divided into four groups with duration from symptom onset as follows: before symptom onset and 0–5, 6–10, and ≥11 years (Fig. 2B and Supplementary Table 10) in consideration of the discretization methods of previous studies29,30. Overall, the frequency of AS accumulation was 66.7% before symptom onset and gradually increased reaching 100% in the ≥11-year group. In the LGI subgroup, the frequency of AS accumulation decreased to 45.0% in the 0–5 year-group but increased thereafter. The frequency of AS accumulation was the expected sensitivity in each period.
Discussion
To the best of our knowledge, this is the largest in vivo neuropathological study in this field. With a multicenter design, we were able to recruit a large number of patients with PD for various durations and prevent selection bias in a single-center study. Individual matching of controls allowed a good comparison of diagnostic accuracy not only in the entire group but also in the subgroup analyses of the UGI and LGI tracts. The collection of full-depth surgical specimens, excluding biopsied tissue, ensured a large evaluation area and an unbiased comparison between patients and controls. Till now, a scarce number of studies have used only surgical specimens14,16. We found that the duration from symptom onset to surgery was an independent predictor of AS accumulation in the GI tract in patients with PD, regardless of the rostrocaudal gradient, age of symptom onset, age at the time of surgery, or sex. This is the first in vivo pathological evidence of the temporally progressive nature of AS accumulation in the GI tract of patients with PD.
Poor sensitivity was a major limitation when using AS accumulation in the GI tract for the diagnosis of patients with PD. The sensitivity and specificity of AS accumulation in the GI tract in this study were 75.3% and 91.5%, respectively, which were better than the pooled sensitivity and specificity of 43% and 82%, respectively, reported in the previous meta-analysis19. Interestingly, the sensitivity of the mucosal/submucosal evaluation was 46.9% in this study, which was similar to the pooled sensitivity in the meta-analysis19. The interpretation and comparison of data from the previous meta-analysis are limited because of the methodological heterogeneity among the included studies. However, the fact that six studies, half of the 12 studies included in the meta-analysis, used biopsied tissue may have a significant impact on the pooled sensitivity19. Moreover, the evaluated area of the mucosal/submucosal layer was much larger than that of biopsy, and AS accumulation was mostly found in the submucosal plexuses and large nerve bundles, which are not obtainable in routine endoscopic biopsy procedures. In addition, the concordance of AS positivity between the proximal and distal blocks in patients with PD was moderate (κ = 0.541), which implies that conventional biopsy would miss the AS-accumulated neural tissues due to the multifocal nature of the GI synucleinopathy. Therefore, this study confirmed the fundamental limit of biopsy as a pathological biomarker of PD, which is consistent with our previous study14.
This study showed better diagnostic accuracy than our previous study, which used the same staining methods14. Higher sensitivity and specificity would be explained by the use of a more conservative definition of pAS positivity (exclusion of a “diffuse” pattern, which was used in the previous study)31,32, avoidance of a selection bias by a large number of participants (97 vs. 33 in the PD patient group), and exclusion of patients with young-onset PD of age at onset ≤ 50, whereas four patients with young-onset PD (12.1%) were included of the previous study14. In another study on the submandibular gland, we reported that AS accumulation was present in 56.2% of patients with PD33 but not in three patients with PRKN gene mutation34. Therefore, a more stringent selection and evaluation of patients with sporadic PD may contribute to the improvement of diagnostic accuracy.
Disease duration, which was analyzed as the variable duration from symptom onset to surgery in this study, was found to be a novel predictor of AS accumulation in the GI tract in PD. This also provides important in vivo evidence of the temporally progressive nature of AS accumulation in the GI tract. This finding is in line with a recent study that reported that the higher the Braak stage in the brain, the higher the positive rate of AS accumulation in the esophagus (Braak stages 0–3 vs. 4–6, 11% vs. 32%)28. Previous small-number and biopsy-based in vivo studies attempted to evaluate the temporal pattern of AS accumulation in the GI tract but reported no significant relationship between the frequency of AS accumulation and disease duration8,35.
The temporally increasing nature of AS accumulation in the GI tract has important implications for future studies in this field. To increase the sensitivity of detecting AS accumulation in biopsied tissue from peripheral organs, alternative methods, such as pathological AS seed amplification36 or confirmation-specific AS antibody37, have been used. However, a recent meta-analysis reported that pathological AS seed amplification, which is thought to be a promising method for detecting peripheral synucleinopathy, has a pooled sensitivity of 88% for the diagnosis of PD but 64% for idiopathic rapid eye movement sleep behavior disorder (RBD), which is a well-known prodromal synucleinopathy36. Differences in the pathological progression of AS accumulation according to disease duration may be an under-recognized influencing factor. Therefore, future studies should consider the temporally progressive nature of AS accumulation when developing new methods using peripheral tissues in PD. The possibility of detecting AS accumulation in the peripheral organs during the early or prodromal stages is likely to be low. This is a major limitation and obstacle to overcome during biomarker development using tissue of the GI tract. In addition, this result suggests that the temporal relationship of the frequency of AS accumulation in other tissues, such as the skin or submandibular gland, should be evaluated through a large-scale study.
The unexpected higher frequency (66.7%) in “before symptom onset” period than in “0–5” years after symptom onset (45.5%) in the LGI subgroup can be explained as follows. First, we may include PD patients who are more likely to have AS accumulation in the colon in “before symptom onset” period. Some researchers suggested that several clinical characteristics such as prodromal RBD, hyposmia, or symmetric symptoms may be related to the initiation of AS pathology in the GI tract, so-called “body-first” PD38,39. However, we could not have those detailed clinical data in this study. Second, nerve fibers in the colon may be already lost in the early stage after the symptom onset in patients with PD26. If it is true, as the disease duration increases, the frequency of AS accumulation in the colon is expected to decrease. However, our study showed an increase in AS accumulation after symptom onset in the LGI subgroup. Moreover, there were many adequate neural structures in the nerve plexuses in the colon of PD patients with more than 10 years of disease duration. Finally, this may be a coincidental finding because only 12 patients with PD underwent surgery before the onset of symptoms. Prospective colon biopsy studies with detailed clinical evaluation are necessary to elucidate this result.
This study has several limitations. First, we could not evaluate and follow up the controls with AS+. We organized and have been following neurologically normal participants who had undergone surgery on the stomach because of early gastric cancer in a prospective cohort study (EGC-PPD cohort study)40. Therefore, we investigated whether the controls with AS+ participated in the cohort; however, they did not. Second, the acquisition of detailed clinical information, including GI symptoms, RBD, or Parkinsonian symptom severity and laterality, was difficult because of the retrospective multicenter design. Therefore, clinicopathological correlation was insufficient in this study. Finally, we did not evaluate other Parkinsonian syndromes, such as multiple system atrophy or progressive supranuclear palsy. Therefore, the interpretation of diagnostic accuracy of AS accumulation in the GI tract is limited. The role of AS accumulation as a pathologic biomarker remains to be determined in the following studies comparing with other diseases.
This study revealed that AS accumulation in the GI tract of patients with PD was present more prevalently than that estimated in a meta-analysis using previous studies. The results confirmed higher accuracy of AS accumulation in the UGI tract than in the LGI tract for the diagnosis of PD. This implies that the stomach is the possible location for future studies using AS accumulation in the GI tract. However, conventional immunohistochemistry of biopsy tissues does not have sufficient diagnostic accuracy. Finally, our study suggests PD-related AS accumulation in the GI tract follows a temporally increasing but spatially static progression pattern.
Methods
Study design and participants
This multicenter, matched case-control study was conducted at six tertiary hospitals in the Republic of Korea. The study design was approved by the Institutional Review Boards (IRBs) of all participating hospitals (IRB No. of Seoul National University Hospital [SNUH], principal research institute. H-1807-161-962). A waiver of informed consent met the requirements and was granted.
We reviewed the medical records of eligible patients at each hospital and selected those who were diagnosed with PD and underwent radical surgery in the GI tract for cancer treatment between 2004 and 2019. The inclusion criteria for the patient group were as follows: (1) clinical diagnosis of PD evaluated by experienced movement disorder specialists. Because of the retrospective multicenter design, the application of the diagnostic criteria for PD was not possible. However, movement disorder experts of the participating hospitals have at least more than 15 years of experience in the management of patients with PD. The diagnostic accuracy of the expert’s clinical diagnosis is better than the diagnostic criteria;4 (2) clinical diagnosis of PD secured at the medical record of a final follow-up visit more than 1 year after the onset of symptoms; (3) age at symptom onset > 50 years, (4) radically resected surgical specimen of the GI tract available at the pathology bank of each hospital; and (5) permission to use the specimen for research purposes by participants. The exclusion criteria were as follows: (1) clinical diagnosis of other parkinsonian syndromes, such as Parkinson plus syndrome or secondary parkinsonism; (2) known genetic causes; (3) presence of any inflammatory disease in the surgical specimen; (4) presence of metastatic cancer; and (5) chemotherapy or radiotherapy prior to the surgery.
Controls were screened in the pathology database of SNUH and individually matched for age at the time of surgery, sex, and surgical site. The eligibility criteria for the control group were as follows: (1) absence of parkinsonism, dementia, or other neurological diseases in the medical record, which could potentially affect the results; (2) absence of any inflammatory disease in the surgical specimen; (3) absence of metastatic cancer; and (4) no chemotherapy or radiotherapy prior to surgery.
Clinical evaluation
Clinical characteristics, including sex, age at the time of surgery, age at symptom onset of PD, age at diagnosis of PD, age at the final follow-up visit to the clinic, and surgical site, were identified by chart review. The symptom onset of PD was defined as the onset year of any of the motor symptoms of PD. In addition, the Hoehn and Yahr (HY) stage in the year of surgery was also acquired in patients with PD, if possible41,42. Duration from Parkinsonian symptom onset to surgery, onset to diagnosis, and onset to the final follow-up visit, and surgery to the final follow-up visit were calculated from the identified age information.
Selection of the specimens
We collected two formalin-fixed paraffin-embedded blocks per patient, one in the proximal margin and the other in the distal margin of the archived surgical specimens of the GI tract, which were the most distant normal areas from the cancer lesions as in previous studies14,43, from the pathology bank of each hospital. Two serial 3-μm slides per collected block were sectioned for the purpose of pAS and neurofilament (NF) immunostaining. Therefore, a total of four slides per patient were used in this study. Sectioned slides were delivered to SNUH within 2 days for immunohistochemistry.
Immunohistochemistry
The collected slides were de-waxed, rehydrated, and incubated with primary antibodies on automated machines as previously described14,43. A primary antibody against pAS (1/1000 anti-pAS at serine 129 monoclonal Ab [EP1536Y]; Abcam ab51253, Cambridge, UK) was used in conjunction with the Leica Bond-III system, in accordance with the manufacturer’s instruction. Bound antibodies were detected using the Bond Polymer Refine Detection system (Leica Biosystems, Wetzlar, Germany). The next slide was stained with a primary antibody against NF (1/2000 anti-NF monoclonal Ab; DAKO clone 2F11, California, USA) using the Ventana BenchMark XT system. The pAS antibody used in this study has frequent non-specific staining in the vessel wall31,32. Confirmation of neural structures with NF staining can resolve the shortcomings of pAS staining, which was proven to be a reliable method in our previous study43.
Pathological evaluation
All stained slides were scanned using a Leica Slide Scanner (Aperio GT 450 DX; Leica Biosystems, Germany), randomized, and anonymized. Digital slides were evaluated using pathology slide viewing software (Aperio ImageScope ver. 12.4; Leica Biosystems, Germany).
The presence of AS accumulation was conservatively defined as in previous studies32,43: (1) pAS immunohistochemistry showing definite and clear positive staining patterns such as “dots and fibers” or “Lewy body-like staining” pattern and (2) localization in neural structures confirmed with the histologic inspection and presence of positive NF staining43. Positive findings were semi-quantitatively rated as grade 1, 2, or 3, corresponding to sparse, moderate, or frequent, respectively.
To avoid observer bias, all raters were blinded to the participants’ clinical information. A neuropathologist (S.-I.K.) and neurologist (C.W.S.) independently examined the slides. The neurologist (C.W.S.) participated in previous studies on GI synucleinopathy14,31,43, and both raters completed a training program for the pathological evaluation of peripheral AS pathology in the Systemic Synuclein Sampling Study35. Any discrepancies between the two raters were resolved in a consensus meeting with the independent investigators (S.-H.P. and B.S.J.).
Statistical analysis
For descriptive analysis, quantitative and categorical variables are reported as means ± standard deviations and frequencies (%), respectively. The normality of variables was evaluated using the Kolmogorov–Smirnov test. When the normality assumption was satisfied, Student’s t-test and Pearson’s chi-square test were used to analyze continuous and categorical variables, respectively. If the assumption was not satisfied, the corresponding non-parametric analyses were conducted. Cohen’s kappa analysis was used to evaluate the concordance between the slides of the proximal and distal blocks of one patient.
Considering the rostrocaudal gradient7,14,24,25, subgroup analyses were conducted by dividing the entire group into the UGI (stomach and esophagus) and LGI (colon and rectum) subgroups according to the surgical site. The LGI subgroup was further subdivided into the right LGI (RLGI) and left LGI (LLGI) subgroups because the colon was significantly long. The RLGI subgroup included patients whose surgical specimens were acquired via right hemicolectomy, whereas the LLGI subgroup included patients who underwent anterior resection, lower anterior resection, ultralow anterior resection, Miles’ operation, Hartmann’s operation, or left hemi-colectomy. Patients who had surgical specimens acquired by total colectomy or subtotal colectomy were categorized into the “others” LGI subgroup.
The clinical characteristics were further compared between patients with positive AS (AS+) and negative (AS−) AS accumulation to identify the features related to AS accumulation in the GI tract of the patient and control groups. Multivariate logistic regression analysis was conducted to confirm whether the factors associated with AS accumulation were independent predictors of AS accumulation in the GI tract. Significant variables in the univariable analyses were included in Model 1 of the multivariable analysis. We constructed Models 2 and 3 to adjust the duration from symptom onset to diagnosis, sex, and age at surgery or age at symptom onset. Age at surgery and age at symptom onset were not included simultaneously in a multivariable analysis due to multicollinearity with duration from symptom onset to surgery. Age at the final follow-up visit and the related duration variables were not included in the logistic regression analysis. In addition, the HY stage was not entered because data were available in only 29 patients. Finally, an exploratory discretized descriptive analysis was conducted for the duration from symptom onset to surgery. We performed Fisher’s exact test to evaluate group differences. Statistical significance was defined as a two-sided p-value of <0.05. All statistical analyses were performed using SPSS version 26.0.0.0 (IBM Corp., Armonk, NY, USA).
Supplementary information
Acknowledgements
The training program for the Systemic Synuclein Sampling Study (S4) was provided courtesy of Prof. Beach. This study was supported by a research grant from the National Research Foundation of Korea (grant no. 2018R1D1A1B07041440).
Author contributions
Study conception and design: C.W.S., BS.J. Data acquisition: C.W.S., S.-I.K., S.-H.P., J.-M.K., J.-Y.L., S.J.C., J.W.K., T.-B.A., K.W.P., H.-J.L., S.-H.K., Y.-S.S., H.-J.K., H.-K.Y. Data analysis: C.W.S., S.-I.K., S.-H.P., J.H.S., C.Y.L., H.-J.K. Interpretation of data: C.W.S., S.-I.K., S.H.P., J.H.S., C.Y.L., H.-J.K., B.S.J. Drafting the manuscript: C.W.S. Review and revision of the manuscript: C.W.S., S.-I.K., S.-H.P., J.-M.K., J.-Y.L., S.J.C., J.W.K., T.-B.A., K.W.P., J.H.S., C.Y.L., H.-J.L., S.-H.K., Y.-S.S., H.-J.K., H.-K.Y., B.S.J.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chaewon Shin, Seong-Ik Kim.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-024-00766-3.
References
- 1.Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord.30, 1591–1601 (2015). 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 2.Braak, H. et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging24, 197–211 (2003). 10.1016/S0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 3.Attems, J. et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol.141, 159–172 (2021). 10.1007/s00401-020-02255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virameteekul, S., Revesz, T., Jaunmuktane, Z., Warner, T. T. & De Pablo-Fernández, E. Clinical diagnostic accuracy of Parkinson’s disease: where do we stand? Mov. Disord.38, 558–566 (2023). 10.1002/mds.29317 [DOI] [PubMed] [Google Scholar]
- 5.Qualman, S. J., Haupt, H. M., Yang, P. & Hamilton, S. R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology87, 848–856 (1984). 10.1016/0016-5085(84)90079-9 [DOI] [PubMed] [Google Scholar]
- 6.Braak, H., de Vos, R. A., Bohl, J. & Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett.396, 67–72 (2006). 10.1016/j.neulet.2005.11.012 [DOI] [PubMed] [Google Scholar]
- 7.Beach, T. G. et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol.119, 689–702 (2010). 10.1007/s00401-010-0664-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebouvier, T. et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE5, e12728 (2010). 10.1371/journal.pone.0012728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebouvier, T. et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut57, 1741–1743 (2008). 10.1136/gut.2008.162503 [DOI] [PubMed] [Google Scholar]
- 10.Shannon, K. M. et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord.27, 709–715 (2012). 10.1002/mds.23838 [DOI] [PubMed] [Google Scholar]
- 11.Visanji, N. P. et al. Colonic mucosal a-synuclein lacks specificity as a biomarker for Parkinson disease. Neurology84, 609–616 (2015). 10.1212/WNL.0000000000001240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes, L. et al. Similar alpha-Synuclein staining in the colon mucosa in patients with Parkinson’s disease and controls. Mov. Disord.31, 1567–1570 (2016). 10.1002/mds.26702 [DOI] [PubMed] [Google Scholar]
- 13.Chung, S. J. et al. Alpha-synuclein in gastric and colonic mucosa in Parkinson’s disease: limited role as a biomarker. Mov. Disord.31, 241–249 (2016). 10.1002/mds.26473 [DOI] [PubMed] [Google Scholar]
- 14.Shin, C. et al. Fundamental limit of alpha-synuclein pathology in gastrointestinal biopsy as a pathologic biomarker of Parkinson’s disease: comparison with surgical specimens. Parkinsonism Relat. Disord.44, 73–78 (2017). 10.1016/j.parkreldis.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Ruffmann, C. et al. Detection of alpha-synuclein conformational variants from gastro-intestinal biopsy tissue as a potential biomarker for Parkinson’s disease. Neuropathol. Appl. Neurobiol.44, 722–736 (2018). 10.1111/nan.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan, F. et al. Gastrointestinal nervous system alpha-synuclein as a potential biomarker of Parkinson disease. Medicine97, e11337 (2018). 10.1097/MD.0000000000011337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrenschee, M. et al. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun.5, 1 (2017). 10.1186/s40478-016-0408-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Punsoni, M. et al. Enteric pathologic manifestations of alpha-synucleinopathies. Appl. Immunohistochem. Mol. Morphol.27, 543–548 (2019). 10.1097/PAI.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 19.Tsukita, K., Sakamaki-Tsukita, H., Tanaka, K., Suenaga, T. & Takahashi, R. Value of in vivo alpha-synuclein deposits in Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord.34, 1452–1463 (2019). 10.1002/mds.27794 [DOI] [PubMed] [Google Scholar]
- 20.Challis, C. et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci.23, 327–336 (2020). 10.1038/s41593-020-0589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jan, A., Gonçalves, N. P., Vaegter, C. B., Jensen, P. H. & Ferreira, N. The prion-like spreading of alpha-synuclein in Parkinson’s disease: update on models and hypotheses. Int. J. Mol. Sci.22, 8338 (2021). 10.3390/ijms22158338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmqvist, S. et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol.128, 805–820 (2014). 10.1007/s00401-014-1343-6 [DOI] [PubMed] [Google Scholar]
- 23.Van Den Berge, N. et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol.138, 535–550 (2019). 10.1007/s00401-019-02040-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelpi, E. et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord.29, 1010–1018 (2014). 10.1002/mds.25776 [DOI] [PubMed] [Google Scholar]
- 25.Annerino, D. M. et al. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol.124, 665–680 (2012). 10.1007/s00401-012-1040-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirayama, M., Nishiwaki, H., Hamaguchi, T. & Ohno, K. Gastrointestinal disorders in Parkinson’s disease and other Lewy body diseases. NPJ Parkinsons Dis.9, 71 (2023). 10.1038/s41531-023-00511-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borghammer, P. The brain-first vs. body-first model of Parkinson’s disease with comparison to alternative models. J. Neural Transm. (Vienna)130, 737–753 (2023). 10.1007/s00702-023-02633-6 [DOI] [PubMed] [Google Scholar]
- 28.Tanei, Z. I. et al. Lewy pathology of the esophagus correlates with the progression of Lewy body disease: a Japanese cohort study of autopsy cases. Acta Neuropathol.141, 25–37 (2021). 10.1007/s00401-020-02233-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stokholm, M. G., Danielsen, E. H., Hamilton-Dutoit, S. J. & Borghammer, P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson’s disease patients. Ann. Neurol.79, 940–949 (2016). 10.1002/ana.24648 [DOI] [PubMed] [Google Scholar]
- 30.Halliday, G., Hely, M., Reid, W. & Morris, J. The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol.115, 409–415 (2008). 10.1007/s00401-008-0344-8 [DOI] [PubMed] [Google Scholar]
- 31.Shin, C. et al. Alpha-synuclein staining in non-neural structures of the gastrointestinal tract is non-specific in Parkinson disease. Parkinsonism Relat. Disord.55, 15–17 (2018). 10.1016/j.parkreldis.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 32.Beach, T. G. et al. Multicenter assessment of immunohistochemical methods for pathological alpha-synuclein in sigmoid colon of autopsied Parkinson’s disease and control subjects. J. Parkinson’s Dis.6, 761–770 (2016). 10.3233/JPD-160888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin, J. et al. Submandibular gland is a suitable site for alpha synuclein pathology in Parkinson disease. Parkinsonism Relat. Disord.58, 35–39 (2019). 10.1016/j.parkreldis.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 34.Shin, J. H. et al. Negative α-synuclein pathology in the submandibular gland of patients carrying PRKN pathogenic variants. Parkinsonism Relat. Disord.81, 179–182 (2020). 10.1016/j.parkreldis.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 35.Chahine, L. M. et al. In vivo distribution of α-synuclein in multiple tissues and biofluids in Parkinson disease. Neurology95, e1267–e1284 (2020). 10.1212/WNL.0000000000010404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo, D. et al. Diagnostic value of α-synuclein seeding amplification assays in α-synucleinopathies: a systematic review and meta-analysis. Parkinsonism Relat. Disord.104, 99–109 (2022). 10.1016/j.parkreldis.2022.10.007 [DOI] [PubMed] [Google Scholar]
- 37.Emmi, A. et al. Duodenal alpha-synuclein pathology and enteric gliosis in advanced Parkinson’s disease. Mov. Disord.38, 885–894 (2023). 10.1002/mds.29358 [DOI] [PubMed] [Google Scholar]
- 38.Borghammer, P. et al. A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. npj Parkinson’s Dis.8, 166 (2022). 10.1038/s41531-022-00436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borghammer, P. The α-synuclein origin and connectome model (SOC model) of Parkinson’s disease: explaining motor asymmetry, non-motor phenotypes, and cognitive decline. J. Parkinson’s Dis.11, 455–474 (2021). 10.3233/JPD-202481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin, C. et al. Prospective cohort study of patients with early gastric cancer to detect prodromal Parkinson disease (EGC-PPD): a study protocol and baseline characteristics. J. Clin. Neurosci.66, 26–32 (2019). 10.1016/j.jocn.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 41.Goetz, C. G. et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord.19, 1020–1028 (2004). 10.1002/mds.20213 [DOI] [PubMed] [Google Scholar]
- 42.Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology17, 427–442 (1967). 10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- 43.Shin, C. et al. Gastric synucleinopathy as prodromal pathological biomarker in idiopathic REM sleep behaviour disorder. J. Neurol. Neurosurg. Psychiatry92, 450–451 (2021). 10.1136/jnnp-2020-324743 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.