Graphical Abstract
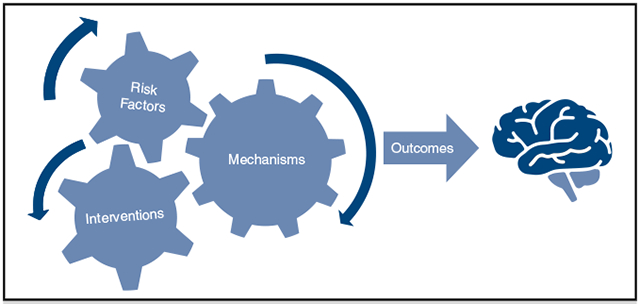
Interaction of risk factors, interventions, and mechanism producing cognitive outcomes.
Keywords: neurocognitive decline, cardiovascular disease, cardiac surgery, cardiopulmonary bypass, quality of life, microemboli, inflammation
Neurocognitive decline (NCD) is common after patients undergo cardiac surgery and has important implications for acute and long-term clinical outcomes and patient quality of life.1 Patients with atherosclerotic cardiovascular disease have an increased risk of developing cognitive impairment or dementia simply as a comorbidity of their primary disease process.2-5 It is well documented that approximately 40% to 50% of patients who undergo cardiac surgery develop measurable NCD in the early postoperative period (Table 1)1,6,9-12,14-17 Critical questions for cardiothoracic surgeons are the degree of disability patients suffer as a result of this early NCD, whether patients can be identified before surgery using a biomarker or cerebral imaging, and whether there are any interventions that could decrease that risk. Another question of interest is how long the dysfunction might persist and whether this will affect the patient long-term with regard to quality of life.
TABLE 1.
Incidence of neurocognitive decline
| Study | N | Postoperative date | Incidence | Neuropsychological test battery | Definition of neurocognitive decline |
|---|---|---|---|---|---|
| Hernandez et al6 | 201 | 4 Days | 52%-62% | Trails Digit Span VIGIL Grooved Pegboard Rey-Osterrieth Controlled oral word association Hopkins verbal learning WRAT-3 |
20% or greater decline in at least 20% of tests |
| 6 Months | 44%-47% | Brixton Spatial Anticipation Test | |||
| Newman et al7 | 261 | At discharge | 53% | Short Story of Randt Memory Test Digit Span of WAIS-R Benton Revised Visual Retention |
Decline of 1 SD or more in any 1 of 4 domains |
| 6 Weeks | 36% | Test | |||
| 6 Months | 24% | Digit Symbol of WAIS-R | |||
| 5 Years | 42.4% | Trail Making Test | |||
| Ramlawi et al8 | 42 | 4 Days | 40.5% | Trail Making A and B Hopkins Verbal Learning Test Digit Span Boston Naming Test Semantic Fluency Phonemic Fluency Wechsler Test of Adult Reading Stroop |
Decline of 1 SD from baseline on 25% of tasks |
| Patel et al9 | 103 | 6-8 Weeks | 43%-50% | Trail Making Tests A and B Grooved Pegboard Wechsler Memory Scale WAIS |
Drop of z-score greater than 1 SD from baseline |
| Slater et al10 | 265 | Before discharge | 60% | MMSE Saccadic eye movement Trail Making Test A and B HVLT Trial 1-3, B-C Grooved Pegboard Stroop Color and Word |
Decline of 1 SD or more on 1 or more test |
| Colak et al11 | 181 | 7 Days | 40.3% | MMSE Color Trail Test 1 Grooved Pegboard Test |
MMSE decline 3 or more points. CTT1 or GP decline of 1 SD |
| Glumac et al12 | 161 | 6 Days | 29%* | MMSE Rey Auditory Verbal Learning Test Wechsler Memory Scale Symbol Digit Modalities Test PsychE |
Reliable change index equal or less than −1.96 or a Z-score equal or less than −1.96 on at least 1 test |
| Ottens et al13 | 291 | 1 Month | 7.2%* | Corsi Blocks Rey Auditory Verbal Learning Grooved Pegboard Trail Making Test A and B |
Reliable change index equal or less than −1.96 or a Z-score equal or less than −1.96 on at least 2 tests |
| 12 Months | 3.5%* | WAIS Digit Span | |||
| Grigore et al14 | 165 | 6 Weeks | 45% | Short Story module Randt Memory Test Digit Span WAIS Modified Visual Reproduction Test (Wechsler Memory Scale) Digit Symbol WAIS Trail Making Test A and B |
Decline of 1 SD in 1 of 4 factors |
| Phillips-Bute et al15 | 551 | 6 Week | 41% | Randt Memory Test Digit Span, WAIS-R domains Modified Visual Reproduction Test (Wechsler Memory Scale) Digit Symbol, WAIS-R |
Decline of 1 SD in 1 or more domains |
| 1 Years | 36.8% | Trail Making A&B | |||
| Relander et al16 | 100 | 1 Week | 71% | Rivermead Behavioral Memory test Auditory Verbal Learning Test Rey Visual Learning Test Digit Span Letter Cancellation Test Trail Making Test A and B Stroop Test Verbal Phonemic Fluency Verbal Categorical Fluency Finger Tapping, both hands Similarities, WAIS-R Block Design, WAIS-R |
Z-score less than −2 in 1 domain |
| 3 Months | 47% | MMSE |
VIGIL, Continuous performance test; WRAT-3, Wide Range Achievement Test III; WAIS-R, Wechsler Adult Intelligence Scale-Revised; SD, standard deviation; WAIS, Wechsler Adult Intelligence Scale; MMSE, Mini-Mental State Examination; HVLT, Hopkins Verbal Learning Test; CTT1, Color Trail Test 1; GP, Grooved Pegboard. *Control group from experiments that also had an intervention group.
One of the key limitations in NCD research is the heterogeneous definitions used to describe NCD. In general the term NCD describes patients who experience a negative change in neurocognitive function from preoperative to postoperative examination. This can be a decline in memory, diminished function of a specific cognitive skill, or global decline in cognitive function. Importantly, this is a separate phenomenon from stroke in that there are not focal neurologic deficits. Unlike delirium, which is a diagnosis on the basis of a 1-time assessment, NCD requires pre- and postoperative testing. Thus it is primarily identified in the setting of research studies. Some have defined it as a simple quantified negative change in score. This method can be subject to test and retest variability, and a floor or ceiling effect.18 Others have used a reliable change index to incorporate standard deviation of error to control for some of these effects. One proposed change in vocabulary is to describe change in cognitive function within the first 30 days as delayed neurocognitive recovery and only use NCD to describe changes after 30 days.18 This is an attempt to differentiate patients who exhibit an acute postoperative decline with subsequent recovery and patients who experience persistent long-term decline.
Cerebral imaging has been studied as a tool to diagnose NCD after cardiac surgery. The results have been mixed. A systematic review of 13 small studies suggested a potential relationship between new brain lesions and NCD, however the studies used a wide variety of neuropsychological testing batteries and different timing of those tests, which makes it difficult to draw any firm conclusions.19 Additionally in an analysis of data from a prospective randomized trial, despite 55% of patients being found to have new brain lesions on postoperative magnetic resonance imaging, there was no correlation with NCD 1 month after surgery.20 Although cerebral imaging would be a useful objective tool to diagnosis NCD, it is not currently used and would need more consistent evidence supporting it.
The etiology of NCD is multifactorial with proposed mechanisms including microembolism, cerebral hypoperfusion, and systemic inflammation.21,22 The exact contribution from each of these factors is still the subject of ongoing research and likely depends on the individual patient and which operation they undergo. In most cases, in the absence of overt stroke, NCD is relatively mild and not apparent to the surgeon, patient, or their family. One might ask if it is not clinically apparent in most patients, is it even an issue? NCD is associated with worsened clinical outcomes including prolonged hospitalization, excessive operative mortality, higher hospitalization costs, and altered quality of life.1,23 There are preoperative clinical and genomic factors that can assist surgeons in counseling their patients, intraoperative factors that surgeons can control, and postoperative risk factors that can be managed.
MECHANISMS OF NEUROLOGIC INJURY
Microemboli
Microemboli are thought to contribute to NCD either through translocation of air emboli, fat emboli, or other particulates, from the aorta into the cerebral circulation where they can obstruct flow through small vessels and cause local ischemia. Macroemboli do this in a more obvious way by causing strokes and areas of ischemia that can be detected via magnetic resonance imaging. Transcranial doppler has been used to estimate the quantity of microemboli and macrobubbles that enter the brain during cardiac surgery. Despite the fact that patients who underwent valve surgeries experienced more microemboli and 7 times as many macroemboli compared with patients who underwent coronary artery bypass grafting (CABG), there was no significant relationship with postoperative NCD.9 This would suggest that microembolization is not the primary driving cause behind NCD, however, the study was small and would need to be studied in a larger population before drawing firm conclusions.
Neuronal Ischemia
Neuronal ischemia can result from diminished cerebral blood flow, systemic oxygen desaturation, or decreased oxygen-carrying capacity. Heart failure causes decreased cerebral blood flow and is associated with cognitive impairment.24-26 Decreased cardiac output before or after surgery might exacerbate neurologic injury. A study using transcranial doppler to estimate blood flow velocity through the middle cerebral artery after induction of anesthesia and during cardiopulmonary bypass showed a correlation between a decreased blood flow velocity at either time point and development of acute postoperative NCD.27 A reduction in blood flow could cause ischemic injury throughout the brain if present for a prolonged period of time. The duration of diminished blood flow needed to cause NCD is yet to be studied.
Hemoglobin plays a critical role in oxygen delivery and thus acute blood loss anemia could potentially contribute to NCD. An association has been documented between lower preoperative hematocrit levels and NCD, although there was no relationship shown between red cell transfusion, 6-hour postoperative hematocrit, or postoperative day 4 hematocrit and NCD.23 This suggests that patients with lower baseline hematocrit levels might be more susceptible to neurocognitive injury possibly because decreased hematocrit is a marker of generalized poor health. It does not support the theory that acute blood loss anemia is a cause of NCD for most patients.
Inflammation
A growing body of evidence supports the relationship between inflammation and NCD, although there is some contradictory research.28 There are a myriad of potential mechanisms by which systemic inflammation might alter neurocognitive function including: hyperglycemia, cytokine and complement activation, and blood–brain barrier (BBB) disruption.
Diabetes and Insulin Resistance
Diabetes is an inflammatory disease and it is hypothesized that patients with diabetes or perioperative hyperglycemia might be at increased risk of systemic inflammation and thus increased risk of NCD. This is on the basis of evidence of greater incidence of baseline cognitive impairment in patients with either diabetes, poor glycemic control and diabetes, or longer duration of diabetes.29 The literature is mixed regarding the effect of insulin resistance, either in the context of preexisting diabetes or acute perioperative hyperglycemia.30,31 In a study in which standard metabolic care was compared with coadministration of insulin and glucose to maintain normoglycemia, a difference only in the verbal portion of cognitive testing was shown.32 Because glucose is the primary energy substrate for neuronal function, an elevated serum glucose level might be beneficial to maintain neurologic function during surgery. It should be strongly emphasized that at least moderate perioperative control of glucose has been documented to reduce the incidence of non-neurologic complications after cardiovascular and other surgical procedures.33
Cytokine and Complement Activation
Cerebral inflammation in cardiac surgery might be a result of the general inflammatory process due to the trauma of major surgery, cardiopulmonary bypass, or ischemia–reperfusion injury. Inflammatory mediators are able to cross the BBB either directly via active transport mechanisms or indirectly via stimulation of the vagus nerve.34 Cytokines and complement factors acting within the central nervous system have a deleterious effect on cognitive function.34,35 When administered peripherally to treat cancer or chronic hepatitis C cytokines such as interferon α, interleukin (IL)-1β, and IL-6, have been shown to produce changes in cognition and mood.34,36,37 Complement activation during surgery might cause downstream oxidative damage in the central nervous system similar to that seen in dementia.38 Systemic inflammatory response syndrome, notable for marked leukocytosis, is negatively associated with cognitive function after surgery, particularly in the elderly population. Unfortunately, the effects of systemic inflammatory response syndrome can last for years after the event.39
Elevated postoperative levels of IL-6 have been associated with increased risk of 1-year readmission and mortality among cardiac surgery patients.40 Increased circulating levels of inflammatory markers and cytokines including C-reactive protein, IL-1β, IL-6, IL-10, and S-100β in the perioperative period have been associated with NCD.17,41 Patients with NCD might demonstrate differences in genomic expression pathways postoperatively compared with patients without NCD including pathways associated with systemic inflammation, antigen presentation, and cellular adhesion.8 Pathways found to differ preoperatively include activation of T-cell maturation, cytokine signaling, cell death, and oxidative stress.42
BBB Disruption
The BBB might be disrupted as a result of cardiopulmonary bypass. There is a correlation between the degree of permeability of the BBB and the degree of cognitive decline.43 The mechanism behind this relationship is not well documented, but some believe that systemic postsurgical inflammation leading to cerebral inflammation might be an underlying cause. Previous testing of cerebrospinal fluid in patients who underwent cardiac surgery has shown alterations in cerebrospinal fluid to serum albumin ratios suggesting BBB disruption, increased spinal fluid IL-6 and IL-8 suggesting cerebral inflammation, and increased S-100B and glial fibrillary acid protein suggesting glial cell injury.44,45
RISK FACTORS
It is important to give patients and their families an assessment of the risks of stroke and NCD before any cardiovascular operation. There are several clinical predictors of NCD. These include the burden of atherosclerotic cardiovascular disease, advancing age, diabetes, depression, heart failure, a history of stroke, carotid artery stenosis, and baseline cognitive impairment (Table 2).1,46 Although the increased expression of various genes after cardiac surgery has been associated with NCD,8 knowing this after surgery does not help the surgeon counsel the patients as to the risk of NCD before surgery. However, a recent study did show that the preoperative gene expression profile might be able to help predict postoperative NCD.42 This might be a useful tool to guide discussion of risk preoperatively in addition to other clinical risk factors. However, this hypothesis will need to be assessed in a larger clinical trial.
TABLE 2.
Relative strength of risk factors
| Risk factor | Type of evidence supporting risk factor | Reference |
|---|---|---|
| Burden of atherosclerosis | Meta-analysis | Greaves et al46 |
| Advancing age | Prospective cohort study | Newman et al7; Greaves et al46 |
| Lower level of education | Prospective cohort study | Newman et al7 |
| Baseline cognitive impairment | Prospective cohort study | Newman et al7 |
| Depression | Meta-analysis | Greaves et al46 |
| History of stroke | Meta-analysis | Greaves et al46 |
| Valve surgery | Multiple small prospective cohort studies | Ebert et al47; Hudetz et al48 |
| Baseline anemia | Prospective cohort study | Gorvitovskaia et al23; Shayan et al49 |
| Carotid artery stenosis | Meta-analysis | Greaves et al46 |
| Diabetes | Meta-analysis. Prospective cohort studies | Greaves et al46; Puskas et al30 |
There are numerous intraoperative factors to consider when discussing the risk for postoperative NCD. Longer intubation times and crossclamping the aorta multiple times or excessive aortic manipulation are all associated with NCD.46,50 Studies on the effect of using different types of anesthesia, volatile versus intravenous, show mixed results with regard to the development of NCD.21 The use of corticosteroids in cardiac surgery to reduce the risk of NCD has been studied by multiple groups with no difference in outcomes compared with placebo.12,13 The use of cardiotomy suction with processing of blood has shown mixed results in relationship to NCD.51,52 Although anemia has been shown to be a risk factor for postoperative NCD, intraoperative red blood cell transfusions do not appear to alleviate the chance of NCD.23 Extreme hemodilution (hematocrit 15%-18% vs 27%) during cardiopulmonary bypass is associated with NCD.53 A lower average intraoperative nadir in hemoglobin, which is lower in patients with preoperative anemia (7.7 g/dL) than in patients without anemia (8.75 g/dL), has been associated with postoperative neurocognitive changes.51 Similarly a low nadir oxygen delivery on bypass is associated with NCD.54
In a study that compared cognitive outcomes of CABG versus valve replacement surgery it was shown that patients with valve surgery had a greater incidence of NCD.47 The etiology of this difference is unclear, although valve patients had longer crossclamp times and length of intensive care unit stay, which might explain the finding. Heparin coating on cardiopulmonary bypass tubing decreases the level of complement activation, which correlates with decreased neurologic injury.38 However, a trial on the use of a complement-blocking drug, pexelizumab, did not show a significant decrease in NCD over use of placebo.55 The data regarding temperature on cardiopulmonary bypass, hypothermia versus normothermia, and the development of NCD in patients who underwent CABG are mixed.56,57 Importantly, slower rewarming from hypothermia has been reported to decrease NCD.14,58 Cerebral desaturation is a risk factor for NCD and prolonged hospital stay after CABG.10 In some studies, patients with cerebral oximetry monitoring intraoperatively and protocols to correct for hypoxia had decreased incidence of NCD, whereas other studies have not shown a benefit.10,11,59 Hyperoxia does not improve NCD over normoxia and might in some cases increase neurologic injury.60
Data are mixed in comparisons of neurocognitive outcomes for on-pump CABG versus off-pump CABG.6,61 A meta-analysis of 8 trials incorporating 892 patients with reported neurocognitive outcomes showed little convincing evidence that NCD was different for off-pump and on-pump approaches during CABG.62 Thus, it might be the case that the overall trauma and inflammation of the operation contributes equally in off-pump and on-pump CABG, and that cardiopulmonary bypass is not the primary driver of inflammation, and that other factors play a greater role in the development of NCD in this setting.
Changes in cerebral blood flow and its regulation during and after cardiac surgery likely plays a role in the NCD observed after cardiac surgery. Significant changes in vasomotor regulation have been shown in the brain and various other organs after surgery using cardiopulmonary bypass in vitro63-67 and in vivo.65,68 It is likely that these changes in vasomotor regulation, permeability, and cellular signaling might contribute to cerebral malperfusion during and after surgery.69 Intraoperative mean arterial pressure alone is likely insufficient as a measurement of cerebral perfusion. However, in some studies, there is an indirect correlation between mean arterial pressure of cardiopulmonary bypass and the incidence of NCD after cardiac surgery.70 In one study patients were randomized to either personalized mean arterial pressure goals on the basis of cerebral autoregulation testing or usual practice blood pressure management and showed decreased incidence of delirium and improved postoperative cognitive testing scores with personalized goals.71
There are a few factors in the postoperative period that have been shown to affect cognitive function. Acute kidney injury (AKI) is known to cause the release of inflammatory mediators that might affect the brain and have downstream effects including diminished neurologic function.72 Furthermore, it has been recently shown that patients who experience AKI during hospitalization are significantly more likely to develop dementia even after controlling for other various comorbidities.73 Even mild inflammation from AKI is correlated with early NCD.
LONG-TERM OUTCOMES AND QUALITY OF LIFE
The long-term prognosis of NCD holds importance for general patient health and quality of life.1,15 NCD is associated with prolonged hospital stay.23 Among patients who undergo surgical aortic valve replacement with postoperative stroke or delirium, both distinct albeit related neurologic processes from NCD, have increased hospital length of stay and decreased quality of life.74 Most patients who suffer NCD after cardiac surgery have a normalization of function with prevalence reduced from 40% at postoperative day 4 to 2.5% at 3 months.17 Acute postoperative NCD correlates with long-term cognitive deterioration at 6 years postoperation.7,16 This suggests that although most patients recover, there are important long-term implications for patients with persistent NCD. These findings have been challenged by work showing that patients with coronary artery disease (CAD) have worse baseline neurocognitive performance and decline faster than healthy patients over time, but that there is not a significant difference between patients with CAD who underwent surgery and those with CAD who did not undergo surgery.61,75,76 Comparing patients with CAD who do versus do not have surgery is an imperfect comparison in that patients who undergo surgery probably had worse disease.
Many diseases of cognitive dysfunction, including Alzheimer’s disease and vascular dementia, begin slowly years before formal diagnosis, and become more prevalent with age. Thus it is possible that many patients who undergo cardiac surgery have already begun to develop subclinical cognitive decline or dementia but have not yet been clinically diagnosed. Alzheimer’s disease can begin showing biomarker changes in cerebrospinal fluid up to 25 years before symptom onset.77 One study showed an increase in amyloid β peptides present in cerebrospinal fluid after cardiopulmonary bypass.45 Perhaps there is an enhanced susceptibility to neurologic injury among this and the aged population.78
One concern regarding long-term NCD is that it might be a risk factor for development of dementia even many years after surgery. A study that included all patients in Sweden who underwent CABG over a 23-year period examined the long-term risk for developing dementia compared with matched control participants. The control patients and the CABG patients had an increased risk for development of all-cause dementia with no significant difference between groups. However the subgroup analysis yielded more nuanced findings. CABG patients younger than 75 years were at an increased risk and CABG patients older than 75 years were at a decreased risk for developing all-cause dementia compared with age-matched control participants.79 Another study showed a greater prevalence of acute postoperative NCD in younger patients, although most returned toward baseline by the 1-month follow-up.80 Younger patients were also shown to have higher levels of perioperative inflammatory markers.80 Perhaps a heightened inflammatory response in younger patients could be a risk factor for acute postoperative NCD and predispose patients to development of dementia.
OPPORTUNITIES TO INTERVENE
The first logical place to intervene in the incidence of NCD is minimizing risk factors that the surgical team is able to manage (Table 3). These would include avoiding prolonged intubation, using heparin-coated cardiopulmonary bypass tubing, and maintaining adequate blood flow during cardiopulmonary bypass by maintaining mean arterial pressure on cardiopulmonary bypass above the lower limit of cerebral autoregulation. Furthermore, NCD might be reduced or prevented with appropriate intraoperative blood glucose control, slowly rewarming from hypothermia and avoiding hyperthermia, avoiding excessive aortic manipulation and multiple episodes of aortic crossclamping, appropriate monitoring of cerebral oximetry, and treating instances of hypoxia. Instituting factors that might avoid postoperative AKI have been determined to lessen the incidence of NCD. Additionally, several of the preoperative risk factors for NCD are modifiable including: diabetes, depression, baseline anemia, and carotid artery stenosis. If patients with preoperative anemia are at risk for a lower intraoperative hemoglobin nadir, then it might be worth investigating whether avoiding the nadir in these patients can decrease NCD. Perhaps optimization of baseline depression preoperatively can improve neurocognitive outcomes. Another potential intervention that might be indicated is the initiation of a program of postoperative cognitive assessment and referral to rehabilitation services in the same way that patients are routinely evaluated by physical therapy for functional status before discharge and connected with appropriate outpatient support services. Perhaps this type of rehabilitation could prevent acute changes from becoming long-term deficits. A better understanding of causes, incidence, and treatment of NCD after cardiac surgery might help lessen the incidence of NCD and its clinical effect.
TABLE 3.
Interventions
| Type of study | Summary | Reference | |
|---|---|---|---|
| Evidence-based interventions | |||
| Avoiding prolonged intubation times | Meta-analysis | Strongest intraoperative continuous risk factor in meta-analysis | Greaves et al46 |
| Heparin-coated bypass tubing | RCT | Non–heparin-coated group showed changes in executive function and attention | Baufreton et al38 |
| Maintaining adequate blood flow during CPB | Prospective cohort | Lower middle cerebral artery blood flow in patients who developed NCD | Bukauskiene et al27 |
| Intraoperative glucose control | RCT | Small improvement in verbal learning and recall | Schricker et al32 |
| Slower rewarming from hypothermia | RCT | Slower rewarming associated with less NCD using multivariate analysis | Grigore et al14 |
| Avoiding multiple aortic crossclamps | RCT | Multiple aortic crossclamp had 30% new NCD at 6 mo vs 9% for single crossclamp | Hammon et al50 |
| Cerebral oximetry monitoring and avoiding hypoxia | RCT | NCD incidence at 7 d was 52% without cerebral oximetry vs 28%11 | Slater et al10; Colak et al11; Uysal et al59 |
| Personalized MAP goals | RCT | Improved memory testing at 4 to 6 wk in patients with personalized MAP goal | Hogue et al71 |
| Avoiding postoperative AKI | Retrospective cohort | Increased development of dementia compared with matched controls (HR, 1.88) | Tsai et al73 |
| Inconclusive evidence | |||
| Type of anesthesia | Literature review | No strong evidence supporting use of specific type of anesthesia to reduce NCD | Lomivorotov et al21 |
| Off-pump vs on-pump CABG | RCT, prospective cohort, and meta-analysis | Mixed results. No significant difference in meta-analysis | Hernandez et al6; Selnes et al61; Marasco et al62 |
| Use of cardiotomy suction with cell saver processing | RCT | No difference in NCD among groups with and without blood processing | Rubens et al51; Djaiani et al52 |
| Appear to not affect NCD | |||
| Corticosteroids | RCT | High-dose corticosteroids do not appear to reduce risk of NCD | Glumac et al12; Ottens et al13 |
| Red blood cell transfusions | Prospective cohort study | Blood transfusion does not appear to reduce risk of NCD | Gorvitovskaia et al23 |
| Complement blockade | RCT | No effect of complement blockade with pexelizumab on NCD | Mathew et al55 |
| Hyperoxygenation | RCT | No difference in NCD between intraoperative normoxia and hyperoxia patients | Shaefi et al60 |
RCT, Randomized control trial; CPB, cardiopulmonary bypass; NCD, neurocognitive decline; MAP, mean arterial blood pressure; AKI, acute kidney injury; HR, hazard ratio; CABG, coronary artery bypass grafting.
SUMMARY
Acute NCD is common after cardiac surgery, and has an effect on immediate and long-term patient outcomes and quality of life. At the moment it is challenging to identify the patients who are going to develop NCD or the patients for whom short-term NCD will persist into long-term NCD. There have been numerous risk factors examined, although the evidence supporting a relationship with NCD is mixed for many of them. Many risk factors attempt to capture a patient’s baseline vulnerability that place them at a higher risk for NCD. At this point in time the etiology of NCD appears to be multifactorial and successful interventions or treatments will likely also need to be multifactorial. Surgeons can counsel patients regarding the risk of NCD on the basis of preoperative risk factors, attempt to optimize modifiable factors, and then mitigate intraoperative risks. In addition to the existing research attempting to identify mechanisms, clinical risk factors, and intraoperative interventions, the field of NCD research would benefit from further work on how to support and treat patients when they have developed NCD.
CENTRAL MESSAGE
Acute neurocognitive decline after cardiac surgery is common, has an effect on immediate and long-term outcomes, and is multifactorial in etiology.
PERSPECTIVE
Neurocognitive decline is common after patients undergo cardiac surgery and has important implications for acute and long-term clinical outcomes and patient quality of life.The etiology is multifactorial with proposed mechanisms including embolization, neuronal ischemia, and inflammatory mechanisms. Future interventions will likely need to address multiple mechanisms.
Acknowledgments
Funding for this research was provided by the National Heart, Lung, and Blood Institute (R01HL46716 and R01HL128831; Dr Sellke).
Abbreviations and Acronyms
- AKI
acute kidney injury
- BBB
blood–brain barrier
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- NCD
neurocognitive decline
Footnotes
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Newman MF, Grocott HP, Mathew JP, White WD, Landolfo K, Reves JG, et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke. 2001;32:2874–81. 10.1161/hs1201.099803 [DOI] [PubMed] [Google Scholar]
- 2.Deckers K, Schievink SHJ, Rodriquez MMF, van Oostenbrugge RJ, van Boxtel MPJ, Verhey FRJ, et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PLoS One. 2017;12:e0184244. 10.1371/journal.pone.0184244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toledo C, Andrade DC, Diaz HS, Inestrosa NC, Del Rio R. Neurocognitive disorders in heart failure: novel pathophysiological mechanisms underpinning memory loss and learning impairment. Mol Neurobiol. 2019;56:8035–51. 10.1007/s12035-019-01655-0 [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung HE, Chen J, Ghosalkar D, Christensen JL, Chu AJ, Mantsounga CS, et al. Aortic valve calcification is associated with future cognitive impairment. J Alzheimers Dis Rep. 2021;5:337–43. 10.3233/ADR-200253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez F, Brown JR, Likosky DS, Clough RA, Hess AL, Roth RM, et al. Neurocognitive outcomes of off-pump versus on-pump coronary artery bypass: a prospective randomized controlled trial. Ann Thorac Surg. 2007;84:1897–903. 10.1016/j.athoracsur.2007.07.036 [DOI] [PubMed] [Google Scholar]
- 7.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. 10.1056/NEJM200102083440601 [DOI] [PubMed] [Google Scholar]
- 8.Ramlawi B, Otu H, Rudolph JL, Mieno S, Kohane IS, Can H, et al. Genomic expression pathways associated with brain injury after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2007;134:996–1005. 10.1016/j.jtcvs.2007.01.096 [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Banahan C, Janus J, Horsfield MA, Cox A, Marshall D, et al. Neurological impact of emboli during adult cardiac surgery. J Neurol Sci. 2020;416: 117006. 10.1016/j.jns.2020.117006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44; discussion: 44-5. 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 11.Colak Z, Borojevic M, Bogovic A, Ivancan V, Biocina B, Majeric-Kogler V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg. 2015;47:447–54. 10.1093/ejcts/ezu193 [DOI] [PubMed] [Google Scholar]
- 12.Glumac S, Kardum G, Sodic L, Bulat C, Covic I, Carev M, et al. Longitudinal assessment of preoperative dexamethasone administration on cognitive function after cardiac surgery: a 4-year follow-up of a randomized controlled trial. BMC Anesthesiol 2021;21:129. 10.1186/s12871-021-01348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottens TH, Dieleman JM, Sauër AMC, Peelen LM, Nierich AP, de Groot WJ, et al. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121:492–500. 10.1097/ALN.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 14.Grigore AM, Grocott HP, Mathew JP, Phillips-Bute B, Stanley TO, Butler A, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94:4–10, table of contents. 10.1097/00000539-200201000-00002 [DOI] [PubMed] [Google Scholar]
- 15.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, et al. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–75. 10.1097/01.psy.0000221272.77984.e2 [DOI] [PubMed] [Google Scholar]
- 16.Relander K, Hietanen M, Rantanen K, Ramo J, Vento A, Saastamoinen K, et al. Postoperative cognitive change after cardiac surgery predicts long-term cognitive outcome. Brain Behav. 2020;10:e01750. 10.1002/brb3.1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramlawi B, Rudolph JL, Mieno S, Khabbaz K, Sodha NR, Boodhwani M, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg. 2006;244:593–601. 10.1097/01.sla.0000239087.00826.b4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–61. 10.1093/arclin/acr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Wu X, Wang W, Jin L. Cognitive dysfunction after off-pump versus on-pump coronary artery bypass surgery: a meta-analysis. J Int Med Res. 2012;40:852–8. 10.1177/147323001204000303 [DOI] [PubMed] [Google Scholar]
- 20.Lewis C, Levine A, Balmert LC, Chen L, Sherwani SS, Nemeth AJ, et al. Neurocognitive, quality of life, and behavioral outcomes for patients with covert stroke after cardiac surgery: exploratory analysis of data from a prospectively randomized trial. Anesth Analg. 2021;133:1187–96. 10.1213/ANE.0000000000005690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomivorotov VV, Moroz G, Abubakirov M, Osinsky R, Landoni G. Volatile and intravenous anesthetics for brain protection in cardiac surgery: does the choice of anesthesia matter? J Cardiothorac Vasc Anesth. 2022;36:567–76. 10.1053/j.jvca.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 22.Miniksar ÖH, Çiçekçioğlu F, Kılıç M, Honca M, Miniksar DY, Gocmen AY, et al. Decreased brain-derived neurotrophic factor levels may predict early perioperative neurocognitive disorder in patients undergoing coronary artery bypass surgery: a prospective observational pilot study. J Clin Anesth. 2021;71:110235. 10.1016/j.jclinane.2021.110235 [DOI] [PubMed] [Google Scholar]
- 23.Gorvitovskaia AY, Scrimgeour LA, Potz BA, Sellke NC, Ehsan A, Sodha NR, et al. Lower preoperative hematocrit, longer hospital stay, and neurocognitive decline after cardiac surgery. Surgery. 2020;168:147–54. 10.1016/j.surg.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoth KF, Poppas A, Ellison KE, Paul RH, Sokobin A, Cho Y, et al. Link between change in cognition and left ventricular function following cardiac resynchronization therapy. J Cardiopulm Rehabil Prev. 2010;30:401–8. 10.1097/HCR.0b013e3181e1739a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–3. 10.1161/hs1101.098360 [DOI] [PubMed] [Google Scholar]
- 26.Sauvé MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail. 2009;15:1–10. 10.1016/j.cardfail.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 27.Bukauskienė R, Širvinskas E, Lenkutis T, Benetis R, Steponavičiūtė R. The influence of blood flow velocity changes to postoperative cognitive dysfunction development in patients undergoing heart surgery with cardiopulmonary bypass. Perfusion. 2020;35:672–9. 10.1177/0267659120906045 [DOI] [PubMed] [Google Scholar]
- 28.Westaby S, Saatvedt K, White S, Katsumata T, van Oeveren W, Halligan PW. Is there a relationship between cognitive dysfunction and systemic inflammatory response after cardiopulmonary bypass? Ann Thorac Surg. 2001;71:667–72. 10.1016/S0003-4975(00)02405-X [DOI] [PubMed] [Google Scholar]
- 29.Rawlings AM, Sharrett AR, Albert MS, Coresh J, Windham BG, Power MC, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC Study. Diabetes Care. 2019;42:1248–54. 10.2337/dc19-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84:1467–73. 10.1016/j.athoracsur.2007.06.023 [DOI] [PubMed] [Google Scholar]
- 31.Scrimgeour LA, Ikeda I, Sellke NC, Shi G, Feng J, Cizginer S, et al. Glycemic control is not associated with neurocognitive decline after cardiac surgery. J Card Surg. 2022;37:138–47. 10.1111/jocs.16102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schricker T, Sato H, Beaudry T, Codere T, Hatzakorzian R, Pruessner JC. Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PLoS One. 2014;9:e99661. 10.1371/journal.pone.0099661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bláha J, Mráz M, Kopecký P, Stritesky M, Lips M, Matias M, et al. Perioperative tight glucose control reduces postoperative adverse events in nondiabetic cardiac surgery patients. J Clin Endocrinol Metab. 2015;100:3081–9. 10.1210/jc.2015-1959 [DOI] [PubMed] [Google Scholar]
- 34.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–56. 10.1046/j.1532-5415.2002.50619.x [DOI] [PubMed] [Google Scholar]
- 35.Rudolph JL, Ramlawi B, Kuchel GA, McElhaney JE, Xie D, Sellke FW, et al. Chemokines are associated with delirium after cardiac surgery. J Gerontol A Biol Sci Med Sci. 2008;63:184–9. 10.1093/gerona/63.2.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amodio P, De Toni EN, Cavalletto L, Mapelli D, Bernardinello E, Del Piccolo F, et al. Mood, cognition and EEG changes during interferon alpha (alpha-IFN) treatment for chronic hepatitis C. J Affect Disord. 2005;84:93–8. 10.1016/j.jad.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 37.Cheung YT, Lim SR, Ho HK, Chan A. Cytokines as mediators of chemotherapy-associated cognitive changes: current evidence, limitations and directions for future research. PLoS One. 2013;8:e81234. 10.1371/journal.pone.0081234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baufreton C, Allain P, Chevailler A, Etcharry-Bouyx F, Corbeau JJ, Legall D, et al. Brain injury and neuropsychological outcome after coronary artery surgery are affected by complement activation. Ann Thorac Surg. 2005;79:1597–605. 10.1016/j.athoracsur.2004.08.061 [DOI] [PubMed] [Google Scholar]
- 39.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304: 1787–94. 10.1001/jama.2010.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett AD, Alam SS, Owens SL, Parker DM, Goodrich C, Likosky DS, et al. The association between cytokines and 365-day readmission or mortality in adult cardiac surgery. J Extra Corpor Technol. 2019;51:201–9. 10.1182/JECT-1900014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramlawi B, Rudolph JL, Mieno S, Feng J, Boodhwani M, Khabbaz K, et al. C-reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery. 2006;140:221–6. 10.1016/j.surg.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 42.Sabe AA, Dalal RS, Chu LM, Elmadhun NY, Ramlawi B, Bianchi C, et al. Preoperative gene expression may be associated with neurocognitive decline after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2015;149:613–23. 10.1016/j.jtcvs.2014.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abrahamov D, Levran O, Naparstek S, Refaeli Y, Kaptson S, Abu Salah M, et al. Blood-brain barrier disruption after cardiopulmonary bypass: diagnosis and correlation to cognition. Ann Thorac Surg. 2017;104:161–9. 10.1016/j.athoracsur.2016.10.043 [DOI] [PubMed] [Google Scholar]
- 44.Reinsfelt B, Ricksten SE, Zetterberg H, Blennow K, Fredén-Lindqvist J, Westerlind A. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann Thorac Surg. 2012;94:549–55. 10.1016/j.athoracsur.2012.04.044 [DOI] [PubMed] [Google Scholar]
- 45.Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid β. Acta Anaesthesiol Scand. 2013;57:82–8. 10.1111/j.1399-6576.2012.02769.x [DOI] [PubMed] [Google Scholar]
- 46.Greaves D, Psaltis PJ, Davis DHJ, Ross TJ, Ghezzi ES, Lampit A, et al. Risk factors for delirium and cognitive decline following coronary artery bypass grafting surgery: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e017275. 10.1161/JAHA.120.017275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebert AD, Walzer TA, Huth C, Herrmann M. Early neurobehavioral disorders after cardiac surgery: a comparative analysis of coronary artery bypass graft surgery and valve replacement. J Cardiothorac Vasc Anesth. 2001;15:15–9. 10.1053/jcan.2001.20211 [DOI] [PubMed] [Google Scholar]
- 48.Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, Pagel PS. Postoperative delirium and short-term cognitive dysfunction occur more frequently in patients undergoing valve surgery with or without coronary artery bypass graft surgery compared with coronary artery bypass graft surgery alone: results of a pilot study. J Cardiothorac Vasc Anesth. 2011;25:811–6. 10.1053/j.jvca.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 49.Shayan S, Okocha O, Srdanovic N, Balmert L, Grafman J, Madhan AS, et al. Preoperative anemia and risk for perioperative neurocognitive dysfunction in cardiac surgery patients: a retrospective analysis. J Cardiothorac Vasc Anesth. 2022;36:1056–63. 10.1053/j.jvca.2021.09.035 [DOI] [PubMed] [Google Scholar]
- 50.Hammon JW, Stump DA, Butterworth JF, Moody DM, Rorie K, Deal DD, et al. Coronary artery bypass grafting with single cross-clamp results in fewer persistent neuropsychological deficits than multiple clamp or off-pump coronary artery bypass grafting. Ann Thorac Surg. 2007;84:1174–9. 10.1016/j.athoracsur.2007.04.100 [DOI] [PubMed] [Google Scholar]
- 51.Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ, et al. The cardiotomy trial: a randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116(11 Suppl):I89–97. 10.1161/CIRCULATIONAHA.106.678987 [DOI] [PubMed] [Google Scholar]
- 52.Djaiani G, Fedorko L, Borger MA, Green R, Carroll J, Marcon M, et al. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–95. 10.1161/CIRCULATIONAHA.107.698001 [DOI] [PubMed] [Google Scholar]
- 53.Mathew JP, Mackensen GB, Phillips-Bute B, Stafford-Smith M, Podgoreanu MV, Grocott HP, et al. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107:577–84. 10.1097/01.anes.0000281896.07256.71 [DOI] [PubMed] [Google Scholar]
- 54.Abdaljawad MN, Taha S. Nadir oxygen delivery to the brain as a risk factor for postoperative neurocognitive impairment in patients undergoing coronary artery bypass grafting: a myth or fact. QJM. 2020;113(Suppl 1):hcaa042.014. 10.1093/qjmed/hcaa042.014 [DOI] [Google Scholar]
- 55.Mathew JP, Shernan SK, White WD, Fitch JCK, Chen JC, Bell L, et al. Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery. Stroke. 2004;35:2335–9. 10.1161/01.STR.0000141938.00524.83 [DOI] [PubMed] [Google Scholar]
- 56.Mora CT, Henson MB, Weintraub WS, Murkin JM, Martin TD, Craver JM, et al. The effect of temperature management during cardiopulmonary bypass on neurologic and neuropsychologic outcomes in patients undergoing coronary revascularization. J Thorac Cardiovasc Surg. 1996;112:514–22. 10.1016/S0022-5223(96)70280-5 [DOI] [PubMed] [Google Scholar]
- 57.Grigore AM, Mathew J, Grocott HP, Reves JG, Blumenthal JA, White WD, et al. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95:1110–9. 10.1097/00000542-200111000-00014 [DOI] [PubMed] [Google Scholar]
- 58.Hori D, Everett AD, Lee JK, Ono M, Brown CH, Shah AS, et al. Rewarming rate during cardiopulmonary bypass is associated with release of glial fibrillary acidic protein. Ann Thorac Surg. 2015;100:1353–8. 10.1016/j.athorac-sur.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uysal S, Lin HM, Trinh M, Park CH, Reich DL. Optimizing cerebral oxygenation in cardiac surgery: a randomized controlled trial examining neurocognitive and perioperative outcomes. J Thorac Cardiovasc Surg. 2020;159:943–53.e3. 10.1016/j.jtcvs.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 60.Shaefi S, Shankar P, Mueller AL, O’Gara BP, Spear K, Khabbaz KR, et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery: a randomized clinical trial. Anesthesiology. 2021;134:189–201. 10.1097/ALN.0000000000003650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selnes OA, Grega MA, Borowicz LM, Barry S, Zeger S, Baumgartner WA, et al. Cognitive outcomes three years after coronary artery bypass surgery: a comparison of on-pump coronary artery bypass graft surgery and nonsurgical controls. Ann Thorac Surg. 2005;79:1201–9. 10.1016/j.athoracsur.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 62.Marasco SF, Sharwood LN, Abramson MJ. No improvement in neurocognitive outcomes after off-pump versus on-pump coronary revascularisation: a meta-analysis. Eur J Cardiothorac Surg. 2008;33:961–70. 10.1016/j.ejcts.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 63.Sabe SA,Feng J, Liu Y, Scrimgeour LA, Ehsan A, Sellke FW. Decreased contractile response of peripheral arterioles to serotonin after CPB in patients with diabetes. Surgery. 2018;164:288–93. 10.1016/j.surg.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sellke FW, Shafique T, Schoen FJ, Weintraub RM. Impaired endothelium-dependent coronary microvascular relaxation after cold potassium cardioplegia and reperfusion. J Thorac Cardiovasc Surg. 1993;105:52–8. [PubMed] [Google Scholar]
- 65.Sellke FW, Wang SY, Stamler A, Johnson RG, Cohn WE, Weintraub RM. Changes in autonomic response of the cerebral circulation after normothermic extracorporeal circulation. J Thorac Cardiovasc Surg. 1996;112:450–61. 10.1016/s0022-5223(96)70273-8 [DOI] [PubMed] [Google Scholar]
- 66.Stamler A, Wang SY, Li J, Thurer RL, Schoen FJ, Sellke FW. Moderate hypothermia reduces cardiopulmonary bypass-induced impairment of cerebrovascular responses to platelet products. Ann Thorac Surg. 1996;62:191–8. 10.1016/0003-4975(96)00240-8 [DOI] [PubMed] [Google Scholar]
- 67.Mirman B, Ikeda I, Zhang Z, Liu Y, Yu L, Ehsan A, et al. Effects of neuropeptide Y on the microvasculature of human skeletal muscle. Surgery. 2020;168:155–9. 10.1016/j.surg.2020.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiratzka LF, Eastham CL, Carter JG, Moyers JR, Elliott DR, Doty DB, et al. The effects of cardiopulmonary bypass and cold cardioplegia on coronary flow velocity and the reactive hyperemic response in patients and dogs. Ann Thorac Surg. 1988;45:474–81. 10.1016/s0003-4975(10)64518-3 [DOI] [PubMed] [Google Scholar]
- 69.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. The cardiac microvasculature in hypertension, cardiac hypertrophy and diastolic heart failure. Curr Vasc Pharmacol. 2008;6:292–300. 10.2174/157016108785909779 [DOI] [PubMed] [Google Scholar]
- 70.Linassi F, Maran E, De Laurenzis A, Tellaroli P, Kreuzer M, Schneider G, et al. Targeted temperature management in cardiac surgery: a systematic review and meta-analysis on postoperative cognitive outcomes. Br J Anaesth. 2022;128:11–25. 10.1016/j.bja.2021.09.042 [DOI] [PubMed] [Google Scholar]
- 71.Hogue CW, Brown CH, Hori D, Ono M, Nomura Y, Balmert LC, et al. Personalized blood pressure management during cardiac surgery with cerebral autoregulation monitoring: a randomized trial. Semin Thorac Cardiovasc Surg. 2021;33:429–38. 10.1053/j.semtcvs.2020.09.032 [DOI] [PubMed] [Google Scholar]
- 72.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–70. 10.1681/ASN.2007080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai HH, Yen RF, Lin CL, Kao CH. Increased risk of dementia in patients hospitalized with acute kidney injury: a nationwide population-based cohort study. PLoS One. 2017;12:e0171671. 10.1371/journal.pone.0171671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Messé SR, Overbey JR, Thourani VH, Moskowitz AJ, Gelijns AC, Groh MA, et al. The impact of perioperative stroke and delirium on outcomes after surgical aortic valve replacement. J Thorac Cardiovasc Surg. March 18, 2022. [Epub ahead of print]. 10.1016/j.jtcvs.2022.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Selnes OA, Grega MA,Bailey MM, Pham LD, Zeger SL, Baumgartner WA, et al. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445–54. 10.1016/j.athoracsur.2009.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Selnes OA, Grega MA, Bailey MM, Pham L, Zeger S, Baumgartner WA, et al. Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: a controlled study. Ann Thorac Surg. 2007;84:1885–96. 10.1016/j.athoracsur.2007.06.054 [DOI] [PubMed] [Google Scholar]
- 77.Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. 10.1056/NEJMoa1202753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med. 2008;8:754–67. 10.2174/156652408786733685 [DOI] [PubMed] [Google Scholar]
- 79.Giang KW, Jeppsson A, Karlsson M, Hansson EC, Pivodic A, Skoog I, et al. The risk of dementia after coronary artery bypass grafting in relation to age and sex. Alzheimers Dement. 2021;17:1042–50. 10.1002/alz.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson K, Ziegler O, Shi G, Sodha N, Ikeda I, Feng J, et al. Younger age is associated with greater early neurocognitive decline postcardiopulmonary bypass. J Thorac Cardiovasc Surg Open. 2020;1:1–9. 10.1016/j.xjon.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


