Abstract
Background:
Endometrial cancer represents the most prevalent malignant genital tract neoplasm in high-income countries and is the second most common cancer worldwide following cervical cancer. Endometriosis is a benign condition wherein endometrial glands and stroma are found outside the uterine cavity.
Case Presentation:
During a routine care and ultrasound examination of the uterus and adnexa of a 64-year-old woman, an increased endometrial thickness (22 mm) was noted. In 2023, according to ultrasound report, the patient underwent diagnostic curettage with immunohistochemistry, revealing a pathological diagnosis of endometrial cancer (endometrioid adenocarcinoma) with positive staining for p16, estrogen receptor (ER), and vimentin. Subsequently, after one week, she underwent complete surgical staging. Extensive superficial endometriosis disseminated in the pelvis and vulva was noted during surgery and preoperative examinations. Final pathology confirmed a well-differentiated typical endometrioid carcinoma (grade 1) with 40% myometrial invasion and positive lymphovascular invasion. The patient was considered to be at stage 1A.
Conclusion:
Despite some studies suggesting an unclear association between endometriosis and endometrioid or clear-cell ovarian cancers, the correlation between endometriosis and endometrial cancer and its prognosis remains ambiguous. Additionally, although infertility has been linked to both endometrial cancer and endometriosis in various studies, the presented case exhibited no signs of infertility. Extensive pelvic endometriosis with vulvar involvement was present, yet the patient did not exhibit any symptoms. This is in contrast to the typical initial manifestation of endometrial cancer, which is abnormal uterine bleeding. The patient's condition was incidentally detected through routine care due to an abnormal increase in endometrial thickness, prompting this presentation.
Keywords: Endometrial cancer, Endometrioid adenocarcinoma, Endometriosis, Uterine bleeding, Vulva
Introduction
Endometrial cancer represents the most prevalent malignant genital tract neoplasm in high-income countries and is the second most common cancer worldwide following cervical cancer. Hence, it holds particular significance among female cancers. Despite its high prevalence, there lacks a standardized approach to disease diagnosis. Several risk factors for endometrial cancer have been identified, predominantly associated with prolonged exposure to estrogen un-opposed by progesterone in the endometrium. Timely and meticulous endometrial examination is imperative for individuals falling into this category (1–3).
Endometriosis is a benign condition wherein endometrial glands and stroma are found outside the uterine cavity. In most cases, sites of endometriosis include the pelvic peritoneum and abdominal regions, with the ovaries being the most common pelvic site. Other affected areas encompass the anterior and posterior cul-de-sac, posterior broad ligament, uterosacral ligaments, uterus, fallopian tubes, surgical scars, and rare extrapelvic locations such as the appendix, bladder, kidneys, omentum, lymph nodes, lungs, pleura, umbilicus, hernia sacs, and abdominal wall. Involvement of the vulva and vagina is exceedingly rare. The prevalence of pelvic endometriosis ranges from 6 to 10 percent during reproductive age (4, 5).
Despite some studies suggesting an unclear association between endometriosis and endometrioid or clear-cell ovarian cancers, the correlation between endometriosis and endometrial cancer and its prognosis remains ambiguous. Additionally, although infertility has been linked to both endometrial cancer and endometriosis in various studies, the presented case exhibited no signs of infertility. Extensive pelvic endometriosis with vulvar involvement was present, yet the patient did not exhibit any symptoms (Figures 1 and 2). This is in contrast to the typical initial manifestation of endometrial cancer, which is abnormal uterine bleeding. The patient's condition was incidentally detected through routine care due to an abnormal increase in endometrial thickness, prompting this presentation.
Figure 1.
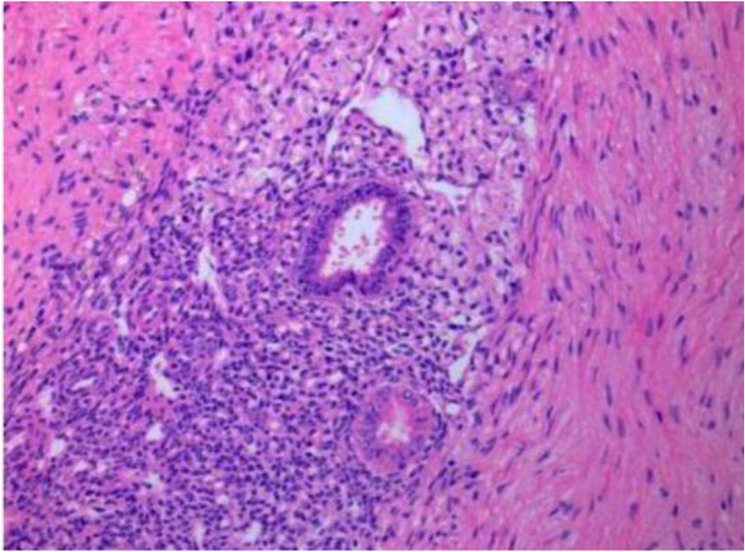
Pathological view of pelvic endometriosis lesions
Figure 2.
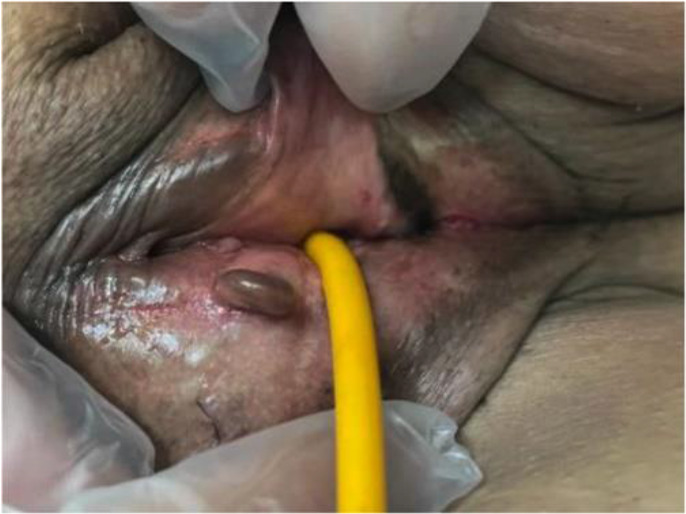
Vulvar endometriosis lesions
Case Presentation
A 64-year-old woman with BMI of 29 kg/m2 (weight=68 kg, height=165 cm) and without any underlying diseases presented to the clinic. During routine care and ultrasound examination of the uterus and adnexa, an increased endometrial thickness of 22 mm was noted. The patient has a history of four pregnancies and four deliveries and has been in menopause for 10 years. In 2023, according to ultrasound report, the patient underwent diagnostic curettage with immunohistochemistry, revealing a pathological diagnosis of endometrial cancer (endometrioid adenocarcinoma) with positive staining for p16, ER, and vimentin. Subsequently, after one week, she underwent complete surgical staging, including hysterectomy, bilateral salpingo-oophorectomy, cytology, and pelvic lymph node dissection. Following a three-day postoperative hospitalization without complications, she was discharged.
Extensive superficial endometriosis disseminated in the pelvis and vulva was noted during surgery and preoperative examinations. Biopsies were taken from the vulvar lesions, confirming the presence of endometriosis. Pre-surgical abdominal and pelvic MRI without contrast exhibited irregular thickening, particularly on the left side of the uterus, indicating irregularities at the endometrial and myometrial margins. They were suggestive of myometrial invasion, along with multiple pelvic endometriotic lesions.
The patient's Pap smear was normal. Tumor markers showed elevated CA19-9 levels (75 U/ml), above the normal range, while CA125 levels were within the normal range (29.15 U/ml). Three months after the surgery, the CA19-9 level was normalized.
The final pathology confirmed a well-differentiated typical endometrioid carcinoma (grade 1 based on FIGO 2023 staging of endometrial cancer) with 40% myometrial invasion and positive lymphovascular invasion. The patient was considered to be at stage 1A. Multiple endometriotic lesions in the pelvis were also confirmed by pathology (Table 1).
Table 1.
FIGO 2023 staging of endometrial cancer
| Stage | Description |
|---|---|
| Stage I | Confined to the uterine corpus and ovary |
| IA | Disease limited to the endometrium or non-aggressive histological type (ie, low-grade endometroid ) with invasion of less than half of myometrium with no or focal lymphovascular space invasion (LVSI) or a good prognosis disease |
| IA1: Non-aggressive histological type limited to an endometrial polyp or confined to the endometrium | |
| IA2: Non-aggressive histological types involving less than half of the myometrium with no or focal LVSI | |
| IA3: Low-grade endometroid carcinomas limited to the uterus and ovary | |
| IB | Non-aggressive histological types with invasion of half or more of the myometrium, with no or focal LVSI |
| IC | Aggressive histological types limited to a polyp or confined to the endometrium |
| Stage II | Invasion of cervical stroma without extrauterine extension or with substantial LVSI or aggressive histological types with myometrial invasion |
| IIA | Invasion of the cervical stroma of non-aggressive histological types |
| IIB | Substantial LVSI of non-aggressive histological types |
| IIC | Aggressive histological types with myometrial involvement |
| Stage III | Local and/or regional spread of the tumor of any histological subtype |
| IIIA | Invasion of uterine serosa, adnexa, or both by direct extension or metastasis |
| IIIA1: Spread to ovary or fallopian tube (except when meeting stage IA3 criteria) | |
| IIIA2: Involvement of uterine subserosa or spread through the uterine serosa | |
| IIB | Metastasis or direct spread to the vagina and/or to the parametria or pelvic peritoneum |
| IIIB1: Metastasis or direct spread to the vagina and/or the parametria | |
| IIIB2: Metastasis to the pelvic peritoneum | |
| IIIC | Metastasis to the pelvic or para-aortic lymph nodes or both |
| IIIC1: Metastasis to the pelvic lymph nodes | |
| IIIC1i: Micrometastasis | |
| IIIC1ii: Macrometastasis | |
| IIIC2: Metastasis to para-aortic lymph nodes up to the renal vessels, with or without metastasis to the pelvic lymph nodes | |
| IIIC2i: Micrometastasis | |
| IIIC2ii: Macrometastasis | |
| Stage IV | Spread to the bladder mucosa and/or intestinal mucosa and/or distant metastasis |
| IVA | Invasion of the bladder mucosa and/or the intestinal/bowel mucosa |
| IVB | Abdominal peritoneal metastasis beyond the pelvis |
| IVC | Distant metastasis, including metastasis to any extra-or intra-abdominal lymph nodes above the renal vessels, lungs, liver, brain, or bone |
Following surgery, due to the patient's age, she received three sessions of brachytherapy to complete the treatment regimen. She underwent thorough follow-up vaginal examinations every three months without any complications. Considering the absence of symptoms related to endometriosis, no further treatment was deemed necessary. Presently, after 12 months post-surgery, the patient remains asymptomatic.
Discussion
Endometrial cancer has emerged as the most common cancer of the reproductive system in women and the fourth most common cancer in women following breast, lung, and colorectal cancer. Therefore, it holds particular significance among female cancers. This cancer often presents noticeable symptoms in its initial stages, with 75–90% of endometrial cancer patients experiencing abnormal uterine bleeding. However, the mentioned patient did not exhibit any symptoms; rather, an increase in endometrial thickness was incidentally detected during routine care through ultrasonography. Several risk factors have been identified for the development of endometrial cancer, most of which are associated with prolonged exposure to estrogen unopposed by progesterone in the endometrium (1–3).
Histologically, 80% of endometrial cancer cases constitute endometrioid adenocarcinoma (Type I), while 20% involve serous carcinoma or clear-cell carcinoma (Type II). This underscores the significance of prolonged exposure to estrogen without progesterone counteraction as a driver for endometrioid adenocarcinoma. Contributing factors to endometrial cancer include obesity, nulliparity, diabetes mellitus, and elevated blood pressure. Additionally, prescribing tamoxifen in post-menopausal women increases the risk of endometrial cancer. Endometrial cancer exhibits an inverse correlation with parity. Chronic anovulation related to infertility might directly associate with or even pave the way for endometrial cancer. Early menarche, late menopause, and estrogen-secreting tumors heighten the susceptibility to this cancer. Individuals carrying the BRCA gene mutation, associated with increased risk for breast and ovarian cancers, face increased endometrial cancer risk if exposed to tamoxifen for breast cancer. A higher incidence of endometrial adenocarcinoma is observed in individuals with Lynch syndrome (which is associated with increased risk of colon and ovarian cancers) and other genetic syndromes like Cowden syndrome (1–3).
Histopathological classification divides endometrial cancer into 80% endometrioid adenocarcinoma (Type I) and 20% serous carcinoma or clear-cell carcinoma (Type II). This classification underscores the significance of prolonged estrogen exposure without progesterone counteraction as a driver for endometrioid adenocarcinoma. Furthermore, a novel staging method for endometrial cancer based on molecular mutations has been proposed by FIGO in 2023 (Table 2) (6).
Table 2.
FIGO 2023 staging of endometrial cancer and molecular classification
| Stage designation | Molecular findings in patients with early endometrial cancer (stages I and II after surgical staging) |
|---|---|
| Stage IAm POLEmut | POLEmut endometrial carcinoma, confined to the uterine corpus or with cervical extension, regardless of the degree of LVSI or histological type |
| Stage IICm p53abn | p53abn endometrial carcinoma, confined to the uterine corpus with any myometrial invasion, with or without cervical invasion, regardless of the degree of LVSI or histological type |
Factors contributing to endometrial cancer include obesity, nulliparity, diabetes mellitus, and hypertension. Additionally, postmenopausal tamoxifen therapy increases the risk of endometrial cancer. There exists an inverse association between endometrial cancer and parity, particularly chronic infertility. Chronic infertility may have a direct correlation with and even be the initiator of endometrial cancer. The patient in question had no history of infertility, experiencing menarche at 15 years old and menopause at 55 years old. Early menarche, late menopause, and estrogen-secreting tumors raise the likelihood of susceptibility to endometrial cancer (2, 7).
Endometriosis is a benign condition wherein endometrial glands and stroma are found outside the uterus, commonly substituting the pelvic peritoneum and abdominal regions. Among pelvic sites, the ovaries exhibit the highest prevalence of involvement. Other affected areas encompass the anterior and posterior cul-de-sac, posterior broad ligament, uterosacral ligaments, uterus, fallopian tubes, sites of surgical scars, and rare extrapelvic locations such as the appendix, bladder, kidneys, omentum, lymph nodes, lungs, pleura, umbilicus, hernia sacs, and abdominal wall. Involvement of the vulva and vagina is exceptionally rare, but extensive vulvar involvement was observed in the mentioned patient (4, 5).
The American Society for Reproductive Medicine recommends staging based on the extent, depth of lesions, and the degree of adhesions for the surgical classification of endometriosis. The patient examined in this study was considered to be at Stage 2 (Table 3, Figure 3) (4).
Table 3.
Criteria for revised classification of endometriosis by the American Society for Reproductive Medicine
| Patient's name | ______________________ | Date _______________________ | ||
|---|---|---|---|---|
| Stage 1 (minimal) | _____________ | 1 to 5 | Laparascopy __________ | |
| Stage 2 (mild) | ______________ | 6 to 15 | Laparatomy ___________ | |
| Stage 3 (moderate) | _____________ | 16 to 40 | Photography __________ | |
| Stage 4 (severe) | _____________ | >40 | Recommended treatment ________ | |
| Total | _________________________ | Prognosis ______________________ | ||
| Peritoneum | Endometriosis | <1 cm | 1 to 3 cm | > 3 cm |
| Superfacial | 1 | 2 | 4 | |
| Deep | 2 | 4 | 6 | |
| Ovary | R superficial | 1 | 2 | 4 |
| Deep | 4 | 16 | 20 | |
| L superficial | 1 | 2 | 4 | |
| Deep | 4 | 16 | 20 | |
| Posterior cul-de-sac obliteration | Partial | Complete | ||
| 4 | 40 | |||
| Ovary | Adhesions | <1/3 enclosure | 1/3 to 2/3 enclosure | >2/3 enclosure |
| R filmy | 1 | 2 | 4 | |
| Dense | 4 | 8 | 16 | |
| L filmy | 1 | 2 | 4 | |
| Dense | 4 | 8 | 16 | |
| Tube | R filmy | 1 | 2 | 4 |
| Dense | 4* | 8* | 16 | |
| L filmy | 1 | 2 | 4 | |
| Dense | 4* | 8* | 16 | |
Figure 3.
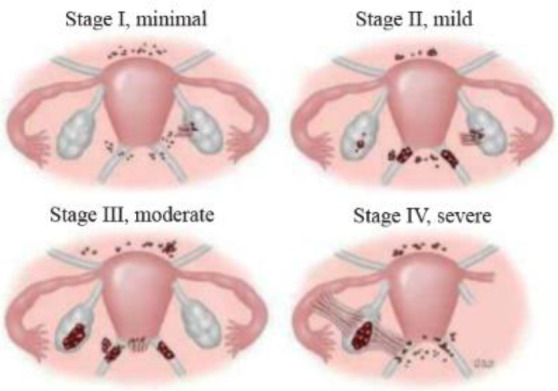
Criteria for revised classification of endometriosis by the American Society for Reproductive Medicine
In the studies conducted by Burghaus et al. as and Janati et al., endometriosis and adenomyosis were introduced as risk factors for endometrial and ovarian cancer in women aged between 40 and 85 years. These findings concide with the presence of extensive pelvic endometriosis in our patient (8, 9). Similarly, another study conducted in China in 2022 confirmed this association (10).
A study conducted in Kazakhstan in 2021 about the molecular basis of endometriosis and endometrial cancer reported inconclusive data regarding the association between endometriosis and risk of endometrial cancer. Further large-scale investigations could help to answer this query (11).
However, other studies did not report a correlation between endometriosis and endometrial cancer, although endometriosis was associated with ovarian cancer, which contracts with our patient's case (12–14). Moreover, another study conducted in Netherlands in 2021 revealed that the prognosis for endometrial cancer in patients with a history of endometriosis was better compared to those without this history. This observation aligns with our case as the endometrial cancer detected was grade 1, indicating a favorable prognosis (15).
In meta-analyses conducted by Painter et al. in 2018, epidemiological, biological, and molecular data suggest links between endometriosis and endometrial cancer, with recent epidemiological studies providing evidence for an association between a previous diagnosis of endometriosis and risk of endometrial cancer (16). In this cross-disease genetic correlation and genome-wide association study, the overlap in genetic risk factors for endometriosis and endometrial cancer was detected. Our genetic correlation analysis supports recent large epidemiological studies indicating an increased risk of endometrial cancer in women previously diagnosed with endometriosis, while the cross-disease meta-analysis has revealed plausible loci that could increase the risk of both diseases which should be further pursued in functional studies (16).
Conclusion
Despite some studies suggesting an unclear association between endometriosis and endometrioid or clear-cell ovarian cancers, the correlation between endometriosis and endometrial cancer and its prognosis remains ambiguous. Additionally, although infertility has been linked to both endometrial cancer and endometriosis in various studies, the presented case exhibited no signs of infertility. Extensive pelvic endometriosis with vulvar involvement was present, yet the patient did not exhibit any symptoms. This is in contrast to the typical initial manifestation of endometrial cancer, which is abnormal uterine bleeding. The patient's condition was incidentally detected through routine care due to an abnormal increase in endometrial thickness, prompting this presentation.
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
References
- 1.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;21(11):1851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American cancer society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230–62. [DOI] [PubMed] [Google Scholar]
- 3.Afrooz N, Nasiri S, Aminimoghaddam S. Comparison of PFS-OS between two treatment methods (surgery and then chemotherapy compared with neoadjuvant and then surgery) in patients with endometrial cancer: a retrospective study. Iran J Obstet Gynecol Infertil. 2022;25(11):fa26–fa33 ref. 22 ref. [Google Scholar]
- 4.Hickey M, Ballard K, Farquhar C. Endometriosis. BMJ. 2014;348:g1752. [DOI] [PubMed] [Google Scholar]
- 5.Rashidi BH, Sarhangi N, Aminimoghaddam S, Haghollahi F, Naji T, Amoli MM, et al. Association of vascular endothelial growth factor (VEGF) gene polymorphisms and expression with the risk of endometriosis: a case–control study. Mol Biol Rep. 2019;46(3):3445–50. [DOI] [PubMed] [Google Scholar]
- 6.Berek JS, Matias-Guiu X, Creutzberg C, Fotopoulou C, Gaffney D, Kehoe S, et al. FIGO staging of endometrial cancer: 2023. Int J Gynecol Obstet. 2023;162(2):383–94. [DOI] [PubMed] [Google Scholar]
- 7.American college of obstetricians and gynecologists . ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106(2):413–25. [DOI] [PubMed] [Google Scholar]
- 8.Burghaus S, Häberle L, Schrauder MG, Heusinger K, Thiel FC, Hein A, et al. Endometriosis as a risk factor for ovarian or endometrial cancer-results of a hospital-based case-control study. BMC Cancer. 2015;15:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnatty SE, Stewart CJ, Smith D, Nguyen A, O’Dwyer J, O’Mara TA, et al. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci Rep. 2020;10(1):3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye J, Peng H, Huang X, Qi X. The association between endometriosis and risk of endometrial cancer and breast cancer: a meta-analysis. BMC Womens Health. 2022;22(1):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terzic M, Aimagambetova G, Kunz J, Bapayeva G, Aitbayeva B, Terzic S, et al. Molecular basis of endometriosis and endometrial cancer: current knowledge and future perspectives. Int J Mol Sci. 2021;22(17):9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of US nurses. Cancer Causes Control. 2017; 28(5):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update. 2021;27(2): 393–420. [DOI] [PubMed] [Google Scholar]
- 14.Guidozzi F. Endometriosis-associated cancer. Climacteric. 2021;24(6):587–92. [DOI] [PubMed] [Google Scholar]
- 15.Hermens M, van Altena AM, Velthuis I, van de Laar DC, Bulten J, van Vliet HA, et al. Endometrial cancer incidence in endometriosis and adenomyosis. Cancers (Basel). 2021;13(18):4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Painter JN, O'mara TA, Morris AP, Cheng TH, Gorman M, Martin L, et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018;7(5):1978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


