Summary
Background
Chronic physical conditions (e.g., heart diseases, diabetes) increase with population ageing, contributing to psychological and cognitive multimorbidities. Yet, little is known about socioeconomic inequalities in this process. We examined the associations between socioeconomic status (SES) and progression to psychological and cognitive multimorbidities after onset of a physical condition.
Methods
We used harmonized individual-level data from five prospective cohort studies across 24 countries in the US, Europe and Asia, with repeated morbidity measurements between 2002 and 2021. Participants with at least one new-onset physical conditions (hypertension, diabetes, heart diseases, stroke, chronic lung diseases, cancer, or arthritis) were followed up for progression to physical-psychological multimorbidity, physical-cognitive multimorbidity, and physical-psychological-cognitive multimorbidity. SES was determined based on educational level and total household wealth at the onset of a physical condition. Time to and incidence rates of progressing psychological and cognitive multimorbidities were estimated in analyses stratified by SES. Fine–Gray subdistribution hazard models and multi-state models were used to estimate the associations between SES and progression to psychological and cognitive multimorbidities.
Findings
Among 20,250 participants aged ≥45 years (mean age at a physical condition onset 65.38 years, standard deviation 8.37) with at least one new-onset physical conditions in the analysis, 7928 (39.2%) progressed to psychological and cognitive multimorbidities during a median follow-up of 8.0 years (168,575 person-years). The mean survival time free from physical-psychological-cognitive multimorbidity was 11.96 years (95% confidence interval 11.57–12.34) in low SES individuals, compared to 15.52 years (15.40–15.63) in high SES individuals, with the corresponding incidence rate of 18.44 (16.32–20.82) and 3.15 (2.48–4.01) per 1000 person-years, respectively. The associations of education, household wealth and SES with multimorbidities followed a dose-dependent relation, with subdistribution hazard ratios per decreasing SES category being 1.24 (1.19–1.29) for physical-psychological multimorbidity, 1.47 (1.40–1.54) for physical-cognitive multimorbidity, and 1.84 (1.72–1.97) for physical-psychological-cognitive multimorbidity. The strongest SES-multimorbidities associations were observed in participants with arthritis, hypertension or diabetes. In multi-state models SES was linked to all five transitions from physical condition to physical-psychological multimorbidity, physical-cognitive multimorbidity and physical-psychological-cognitive multimorbidity.
Interpretation
Socioeconomic inequalities are associated with the progression of a chronic physical condition, with the lower SES groups had both an earlier time to and a higher incidence of psychological and cognitive multimorbidities. These findings underscore the need for more effective equity-oriented policies and healthcare practices to address reduced psychological wellness and cognitive maintenance among individuals with low SES and physical conditions.
Funding
Zhejiang University Hundred Talents Program Research Initiation Fund, Fundamental Research Funds for the Central Universities in China, Wellcome Trust, Medical Research Council, National Institute on Aging, Academy of Finland.
Keywords: Socioeconomic inequalities, Physical conditions, Psychological multimorbidity, Cognitive multimorbidity
Research in context.
Evidence before this study
We searched PubMed, Web of Science and Google Scholar from inception to February 1, 2024, using terms “socioeconomic inequalities in health”, “health inequalities”, “socioeconomic status”, “education”, “wealth”, “onset of physical conditions”, “mental condition”, “psychological condition”, “depression”, “cognitive performance”, “mental and cognitive condition”, “psychological and cognitive condition”, “physical and mental multimorbidity”, “physical and psychological multimorbidity”, “physical and cognitive multimorbidity”. Previous studies reported an ever-increasing epidemic of chronic physical conditions worldwide, and their disproportionate impacts on psychological or cognitive conditions. A powerful predictor of ill health, socioeconomic inequalities exist in the occurrence of physical, psychological and cognitive conditions. However, there is still a lack of evidence on the temporal progression to physical, psychological and cognitive multimorbidities, and the extent to which this progression is socially patterned. This study used data from five prospective cohorts from the US, Europe and Asia, aiming to examine the associations between socioeconomic status (SES) and progression to psychological and cognitive multimorbidities after onset of a physical condition.
Added value of this study
Our analysis represents the first evidence illustrating how socioeconomic factors may influence the transitions from a chronic physical condition to psychological multimorbidity, cognitive multimorbidity, and multimorbidity including physical, psychological, and cognitive disorders. Individuals with lower educational level, lower total household wealth or lower SES had an earlier time to and a higher incidence of progressing psychological and cognitive multimorbidities. The associations between education, total household wealth and SES and progression to psychological and cognitive multimorbidities followed a dose–response relation. When specifying the temporal transitions from a physical condition to psychological and cognitive multimorbidities, the dose-dependent associations of SES were also observable.
Implications of all the available evidence
These findings strengthen the evidence of the far-reaching health effects of social disadvantage and highlight that efforts to address socioeconomic inequalities would not only reduce the occurrence of psychological and cognitive outcomes among patients with a physical condition, but also extend life years free from such outcomes. In terms of public health, it is important to address socioeconomic inequalities in order to improve psychological and cognitive wellness among middle-aged and older adults with a physical condition.
Introduction
With population aging and increased longevity, a greater proportion of the population is living with chronic physical conditions, such as cardiovascular diseases, cancer, diabetes, and chronic lung diseases.1, 2, 3, 4 Additionally, as modern medicine, technology and public health practices have advanced, more people are also surviving long enough to develop multiple long-term health conditions, known as multimorbidity.5 These include both later life psychological problems (e.g., depression) and cognitive impairment.6 For instance, a 13-year follow-up study of 16,080 adults aged ≥50 years in European countries found a dose-dependent relation between chronic physical conditions and the risk of depression,7 a trend supported by a 4-year prospective study in China.8 Another prospective study observed the higher risk of depression and anxiety in those with physical multimorbidity.9 Similarly, research from four Latin American countries identified an inverse association between the number of physical conditions and late-life cognition.10 Furthermore, a 15-year follow-up involving over 23,000 adults in Europe revealed an association between physical multimorbidity—particularly hypercholesterolemia, stroke, diabetes and osteoporosis—and an increased risk of dementia.11
Socioeconomic status (SES) is a powerful predictor of ill health, with many risk factors being socially patterned. These include environmental exposures, lifestyle factors, cardiometabolic risks, life stress, access to health services, safety behaviours (e.g., self-examination, participation in screening and vaccination), and adherence to medical procedures.12,13 Consequently, accumulating evidence suggests a socioeconomic gradient in the prevalence and incidence of chronic conditions and multimorbidity. The longitudinal Twenty-07 cohort study, for instance, found that SES was a risk factor for multimorbidity comprising physical, psychological and cognitive conditions, but failed to differentiate them.12 A recent cross-sectional analysis from our research team reported a higher prevalence of physical, psychological and cognitive multimorbidities among socioeconomically disadvantaged individuals.14 SES was also found to influence downstream health outcomes, such as depression, hospitalization, disability and mortality, in those with pre-existing physical conditions.15,16 In a cross-sectional analysis of women with uncontrolled hypertension, socioeconomic deprivation was an important risk factor for depression.17 Longitudinal research suggests that the risk of depression after the onset of long-term physical conditions is particularly pronounced in individuals from low SES groups,18 while socioeconomic inequalities in subsequent cognitive impairment after physical conditions remain unclear. Given the increasing prevalence of physical conditions globally and their interrelationships with psychological and cognitive conditions,19,20 longitudinal research is essential to determine the temporal progression to physical, psychological and cognitive multimorbidities. Additionally, it is important to assess how this progression is socially patterned.
To facilitate a more comprehensive evaluation of socioeconomic inequalities in progression to physical, psychological and cognitive multimorbidities, we leveraged harmonized data of individuals with at least one new-onset physical conditions from five prospective cohorts across 24 countries. Our aim was to examine whether SES is associated with the progression to psychological and cognitive multimorbidities after onset of a physical condition, such as hypertension, diabetes, cardiovascular diseases, chronic lung diseases, cancer, or arthritis. We hypothesized that: (1) lower SES is associated with both an earlier time to and a higher incidence of psychological and cognitive multimorbidities; and (2) the associations between SES and the risk of progressing psychological and cognitive multimorbidities follow a dose–response relationship.
Methods
Study design and participants
This prospective multicohort study was in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. Individual-level data were pooled across 24 countries (Supplementary Table S1) from five sister cohort studies on ageing in the Global Aging, Health and Policy program21: the US Health and Retirement Study (HRS, 2004–2021); the English Longitudinal Study on Ageing (ELSA, 2002–2019); the Survey of Health, Ageing and Retirement in Europe (SHARE, 2004–2020); the China Health and Retirement Longitudinal Study (CHARLS, 2011–2021); and the Korean Longitudinal Study of Aging (KLoSA, 2006–2021). These studies, collectively known as HRS-family studies, follow similar protocols and administer surveys every two or three years. They cover nationally representative samples of middle-aged and older adults. Additional details for each study have been provided elsewhere.22, 23, 24, 25, 26
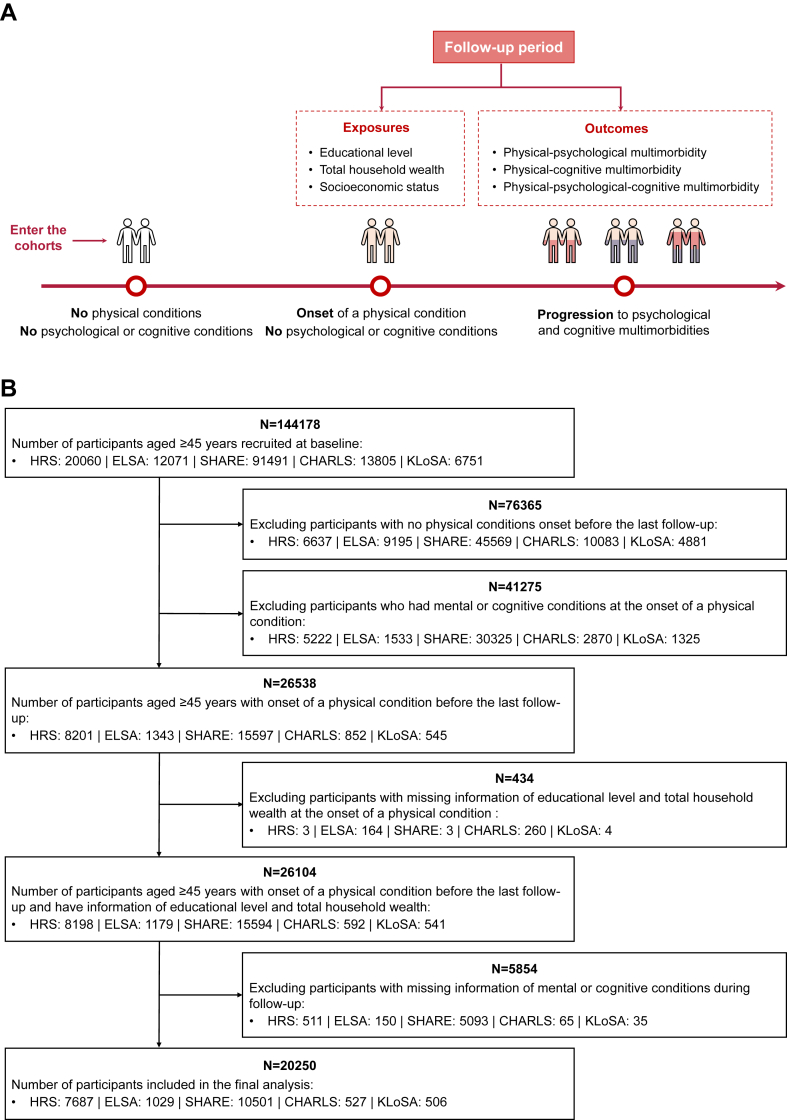
In the present analyses, seven self-reported physical conditions were identified from the abovementioned studies: hypertension, diabetes, cancer, chronic lung diseases, heart diseases, stroke and arthritis (Supplementary Table S2). We included middle-aged and older participants (≥45 years) without abovementioned physical conditions at recruitment; developing at least one of the physical conditions before the last follow-up; and without pre-existing psychological or cognitive conditions at the onset of a physical condition. Individuals with missing information of SES and the psychological or cognitive conditions were excluded. Fig. 1 provides an overview of the study design and the participant selection.
Fig. 1.
The flowchart of the study design. (A) The study design and concept map. (B) The participants selection. HRS, the US Health and Retirement Study; ELSA, the English Longitudinal Study on Ageing; SHARE, the Survey of Health, Ageing and Retirement in Europe; CHARLS, the China Health and Retirement Longitudinal Study; KLoSA, the Korean Longitudinal Study of Aging.
All participating studies were approved by Institutional Review Boards and the respondents provided written informed consent. Ethical approval was not required for the analysis of the anonymized data.
Socioeconomic status
Since most middle-aged and older adults were retirees and had no stable paid work, two indicators, including educational level and total household wealth, were primarily used to measure SES of participants, consistent with our previous research.14,27 Educational level was harmonized into three levels: primary (scored 0), secondary (scored 1) and tertiary (scored 2), according to study-specific categories. Total household wealth was calculated at the onset of a physical condition, referring to the sum of all wealth components (residence, business, vehicles and saving account), excluding other debt or loans. Total household wealth was divided into quartiles in each study to allow cross-cohort harmonization, with scores from 0 to 3 representing the lowest (quartile 1) to the highest (quartile 4). SES was estimated by summing the scores of educational level and total household wealth. We categorized SES into the following four categories: low (scored 0), lower-middle (scored 1 or 2), upper-middle (scored 3 or 4) and high (scored 5). We also constructed SES using educational level and total household income that was also divided into quartiles in additional analyses.
Psychological and cognitive multimorbidities
The presence of psychological and cognitive conditions after onset of a physical condition was ascertained by two approaches: study-specific symptom-based assessments and self-reported diagnosis.
The assessments of psychological conditions included the 8-item version of the Center of Epidemiological Studies Depression Scale (CESD-8) in HRS and ELSA, the 12-item version of the European–Depression Scale (EUROD-12) in SHARE, and the 10-item version of the Center of Epidemiological Studies Depression Scale (CESD-10) In CHARLS and KLoSA.28, 29, 30, 31, 32, 33 In all five studies participants were asked whether they have been diagnosed with affective, emotional, nervous, or psychiatric problems. Detailed descriptions of the ascertainment of psychological conditions in each study are provided in Supplementary Table S2.
In each study, the cognitive performance of participants was assessed through the following domains: episodic memory (immediate and delayed word recall), executive function (serial 7's subtraction test), orientation (recall test of time, place and person), semantic fluency (animal fluency test) and language (only in KLoSA). The mean score of each domain was calculated stratified by eight age groups (group 1: 45–49 years; group 2: 50–54 years; group 3: 55–59 years; group 4: 60–64 years; group 5: 65–69 years; group 6: 70–74 years; group 7: 75–79 years; group 8: ≥80 years) in the initially recruited population.34 The cognitive condition was defined as scoring more than 1.5 standard deviation (SD) below age group-specific means in at least one cognitive domain.35 The self-reported diagnosis of cognitive conditions (e.g., memory-related problems, dementia and Alzheimer's disease) was available in HRS, ELSA, SHARE, and CHARLS, but not in KLoSA. Detailed descriptions of the ascertainment of cognitive conditions in each study are provided in Supplementary Table S2.
The progression to psychological and cognitive multimorbidities was specified into physical-psychological multimorbidity (PP-MM, only occurring the psychological condition), physical-cognitive multimorbidity (PC-MM, only occurring the cognitive condition) and physical-psychological-cognitive multimorbidity (PPC-MM, occurring both psychological and cognitive conditions).
Statistical analysis
The characteristics of participants at the onset of a physical condition were summarized according to SES and progression to psychological and cognitive multimorbidities. Categorical variables were presented as count and percentage, and between-group differences were compared using Chi-squared tests. We also compared the participant characteristics at the onset of a physical condition between the included participants and those excluded due to missing information on SES and outcomes.
Incidence rates of psychological and cognitive multimorbidities by SES category were calculated as the number of new events divided by the time at risk (person-years), with 95% confidence intervals (CIs) calculated according to the Poisson distribution. The time to progressing psychological and cognitive multimorbidities by SES was presented as the multimorbidity-free survival years with 95% CIs, which were generated using Irwin's restricted mean to the longest follow-up years.36,37 The Irwin's restricted mean survival time was defined as the area under the survival curve up to a specific time point and is considered a more reliably estimate than mean or median survival times.38
Competing risk analyses were conducted using the Fine–Gray subdistribution hazard models, wherein death before psychological and cognitive multimorbidities was set as a competing event.39 Participants were censored at the interview year of outcomes, lost to follow-up or the end of follow-up, whichever came first. We constructed the cumulative incidence function (CIF) curves to compare the risks of psychological and cognitive multimorbidities over time across different SES groups. Subdistribution hazard ratios (sHRs) and 95% CIs were used to quantify the strengths of the SES-multimorbidities associations in the whole sample and subsamples with specific physical conditions,40 adjusted for age, sex and study (Supplementary Figure S1A). The interactions between SES and the number of physical conditions were tested by adding a multiplicative interaction term to the Fine–Gray subdistribution hazard models. Considering that educational level and total household wealth might reflect different components of SES, we also estimated the associations between the different combinations of the two SES indicators (3 4) and the multimorbidity outcomes.
Subgroup analyses were performed to explore variations in the effect of SES on progression to psychological and cognitive multimorbidities by age, sex and study. Random-effects meta-analyses were conducted to summarize study-specific sHRs and 95% CIs for outcomes according to SES, with the between-study heterogeneity tested using I2 statistics. Considering the potential influence of new physical conditions on multimorbidity outcomes, we dichotomized the participants into those with and without additional physical conditions occurring before psychological and cognitive multimorbidities and used this variable as a covariate in the analysis of the associations between SES and multimorbidity outcomes. A series of sensitivity analyses were conducted: 1) excluding participants with two or more new-onset physical conditions; 2) excluding outcome events occurring within two years after onset of a physical condition; 3) excluding the outcome events occurring within one follow-up visit after onset of a physical condition; 4) excluding non-educated participants (years of schooling = 0 years); 5) defining SES using educational level and total household income; 6) regarding total household wealth and SES as time-varying variables; 7) defining psychological and cognitive conditions from self-reported diagnoses from HRS, ELSA, SHARE, and CHALRS; 8) repeating the main analyses adjusting for age, sex and country; 9) additionally adjusting for retirement as a time-dependent variable; 10) additionally adjusting for the number of physical conditions as a time-dependent variable; 11) additionally adjusting for additional physical conditions occurred before outcomes; and 12) additionally adjusting for marital status, physical activity, alcohol consumption, smoking status, body mass index, retirement and number of physical conditions. We further applied multiple imputation via chained equations to impute missing values in psychological and cognitive conditions.41 The missingness was assumed at random and was imputed five times, with the model estimates pooled to generate final results.
We further performed multi-state models to assess the associations between SES and temporal transitions from physical conditions to psychological and cognitive multimorbidities. The multi-state model is an extension of the competing risk model, which can explore how the exposures of interest influence different transitions.42 Death was censored as a competing event and not modelled as a direct outcome (Supplementary Figure S1B). We additionally set a direct transition from a physical condition to physical-psychological-cognitive multimorbidity for those reporting psychological and cognitive conditions in the same wave. Therefore, five transitions were identified: 1) from a physical condition to physical-psychological multimorbidity, 2) from a physical condition to physical-cognitive multimorbidity, 3) from a physical condition to physical-psychological-cognitive multimorbidity, 4) from physical-psychological multimorbidity to physical-psychological-cognitive multimorbidity, and 5) from physical-cognitive multimorbidity to physical-psychological-cognitive multimorbidity. The multimorbidity-free years and 95% CIs of these five transitions were calculated and compared across SES. HRs and 95% CIs for the associations between SES and the five transitions were obtained using the R package ‘mstate’ (Version 0.3.2),43 adjusted for age, sex and study.
All tests in this study were two-sided with a significance level of p < 0.05. Statistical analyses were conducted using SAS (Version 9.4) and R (Version 4.2.2).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Of all 144,178 participants without physical conditions at recruitment, we excluded individuals who did not develop physical conditions before the last follow-up (n = 76,365), with pre-existing psychological or cognitive conditions at the onset of a physical condition (n = 41,275), and participants with missing information on exposures (n = 434) or outcomes (n = 5854). This resulted in 20,250 participants for the analysis (Fig. 1). The characteristics of the participants at the onset of a physical condition according to SES are summarized in Table 1. The mean (±SD) age of participants was 65.38 (±8.37) years, and 10,618 (52.4%) were women. There were 7687 individuals from the United States, 527 from China, 506 from South Korea, 1029 from England and 10,501 from other 20 European countries. Participants with higher SES were more likely to be in the age group of 55–74 years, male, from the HRS cohort, and had a higher prevalence of cancer, and a lower prevalence of hypertension, diabetes, chronic lung diseases, heart diseases and stroke. The excluded participants due to missing information on SES and outcomes were more likely to be older, male and from SHARE and CHARLS, and to have diabetes, heart diseases and stroke, while they were less likely to have arthritis (Supplementary Table S3).
Table 1.
Characteristics of participants at the onset of a physical condition by socioeconomic status (n = 20,250).
| Total (N = 20,250) | Socioeconomic status |
p-value | ||||
|---|---|---|---|---|---|---|
| Low (N = 1832) | Lower-middle (N = 8152) | Upper-middle (N = 7788) | High (N = 2478) | |||
| Age groups (years) | <0.001 | |||||
| 45–54 | 1866 (9.2%) | 117 (6.3%) | 831 (44.5%) | 731 (39.2%) | 187 (10.0%) | |
| 55–64 | 7817 (38.6%) | 544 (7.0%) | 3134 (40.1%) | 3084 (39.5%) | 1055 (13.5%) | |
| 65–74 | 7634 (37.7%) | 681 (8.9%) | 2905 (38.1%) | 3049 (39.9%) | 999 (13.1%) | |
| 75∼ | 2933 (14.5%) | 490 (16.7%) | 1282 (43.7%) | 924 (31.5%) | 237 (8.1%) | |
| Sex | <0.001 | |||||
| Male | 9632 (47.6%) | 737 (7.7%) | 3662 (38.0%) | 3883 (40.3%) | 1350 (14.0%) | |
| Female | 10,618 (52.4%) | 1095 (10.3%) | 4490 (42.3%) | 3905 (36.8%) | 1128 (10.6%) | |
| Study | <0.001 | |||||
| HRS | 7687 (38.0%) | 461 (6.0%) | 2686 (34.9%) | 3166 (41.2%) | 1374 (17.9%) | |
| ELSA | 1029 (5.1%) | 104 (10.1%) | 415 (40.3%) | 407 (39.6%) | 103 (10.0%) | |
| SHARE | 10,501 (51.9%) | 1129 (10.8%) | 4557 (43.4%) | 3856 (36.7%) | 959 (9.1%) | |
| CHARLS | 527 (2.6%) | 68 (12.9%) | 257 (48.8%) | 191 (36.2%) | 11 (2.1%) | |
| KLoSA | 506 (2.5%) | 70 (13.8%) | 237 (46.8%) | 168 (33.2%) | 31 (6.1%) | |
| Hypertension | <0.001 | |||||
| No | 8844 (43.7%) | 705 (8.0%) | 3241 (36.6%) | 3584 (40.5%) | 1314 (14.9%) | |
| Yes | 11,401 (56.3%) | 1125 (9.9%) | 4909 (43.1%) | 4203 (36.9%) | 1164 (10.2%) | |
| Diabetes | <0.001 | |||||
| No | 17,997 (88.9%) | 1552 (8.6%) | 7091 (39.4%) | 7057 (39.2%) | 2297 (12.8%) | |
| Yes | 2247 (11.1%) | 280 (12.5%) | 1058 (47.1%) | 728 (32.4%) | 181 (8.1%) | |
| Cancer | <0.001 | |||||
| No | 18,147 (89.6%) | 1698 (9.4%) | 7455 (41.1%) | 6897 (38.0%) | 2097 (11.6%) | |
| Yes | 2101 (10.4%) | 134 (6.4%) | 696 (33.1%) | 890 (42.4%) | 381 (18.1%) | |
| Chronic lung diseases | <0.001 | |||||
| No | 18,857 (93.1%) | 1682 (8.9%) | 7485 (39.7%) | 7315 (38.8%) | 2375 (12.6%) | |
| Yes | 1392 (6.9%) | 150 (10.8%) | 666 (47.8%) | 473 (34.0%) | 103 (7.4%) | |
| Heart diseases | <0.001 | |||||
| No | 16,725 (82.6%) | 1432 (8.6%) | 6676 (39.9%) | 6526 (39.0%) | 2091 (12.5%) | |
| Yes | 3524 (17.4%) | 400 (11.4%) | 1475 (41.9%) | 1262 (35.8%) | 387 (11.0%) | |
| Stroke | <0.001 | |||||
| No | 19,369 (95.6%) | 1718 (8.9%) | 7760 (40.1%) | 7496 (38.7%) | 2395 (12.4%) | |
| Yes | 881 (4.4%) | 114 (12.9%) | 392 (44.5%) | 292 (33.1%) | 83 (9.4%) | |
| Arthritis | 0.131 | |||||
| No | 11,641 (57.5%) | 1044 (9.0%) | 4743 (40.7%) | 4477 (38.5%) | 1377 (11.8%) | |
| Yes | 8606 (42.5%) | 788 (9.2%) | 3406 (39.6%) | 3311 (38.5%) | 1101 (12.8%) | |
HRS, the US Health and Retirement Study; ELSA, the English Longitudinal Study on Ageing; SHARE, the Survey of Health, Ageing and Retirement in Europe; CHARLS, the China Health and Retirement Longitudinal Study; KLoSA, the Korean Longitudinal Study of Aging.
Data are n (%), unless otherwise indicated.
Socioeconomic status is a summed score (ranging 0–5) of educational level (0, 1, or 2) and total household wealth quartile (0, 1, 2, or 3), categorized into four groups: low (0), lower-middle (1–2), upper-middle (3–4) and high socioeconomic status (5).
χ2 test was used to compare differences across groups.
During a median follow-up of 8.0 years [interquartile range 4.0–12.0; person-years at risk 168,575], there were 3810 (18.8%) incident cases of PP-MM, 2618 (12.9%) cases of PC-MM, and 1500 (7.4%) cases of PPC-MM (Table 2, the characteristics of participants according to the progression to psychological and cognitive multimorbidities in Supplementary Table S4). Individuals with lower educational level, lower total household wealth and lower SES had an earlier time to psychological and cognitive multimorbidities after occurring a physical condition. For PPC-MM, for instance, the multimorbidity-free years ranged from 15.52 (95% CI = 15.40–15.63) years for high SES to 11.96 (11.57–12.34) years for low SES. The corresponding incidence rates of progression to psychological and cognitive multimorbidities increased with decreasing educational level, total household wealth and SES category. For example, the incidence rate of PPC-MM ranged from 3.15 (95% CI = 2.48–4.01) per 1000 person-years in high SES to 18.44 (16.32–20.82) per 1000 person-years in low SES. Individuals with lower SES had a higher cumulative incidence of progression to psychological and cognitive multimorbidities than the high-SES group, despite having higher competing risks from death (Supplementary Figure S2). Educational level, total household wealth and SES were dose-dependently associated with the progression to psychological and cognitive multimorbidities (Table 3). The sHRs per decreasing SES category were 1.24 (95% CI = 1.19–1.29) for PP-MM, 1.47 (1.40–1.54) for PC-MM, and 1.84 (1.72–1.97) for PPC-MM. Educational gradient was larger than that of total household wealth both in PC-MM [sHR per category decrease 1.53 (95% CI = 1.45–1.62) for education; 1.13 (1.09–1.17) for household wealth] and PPC-MM [sHR per category decrease 1.79 (1.66–1.94) for education and 1.27 (1.21–1.33) for total household wealth]. The proportional hazard assumption was checked and verified using Schoenfeld's residual methods and no violations were found. Most combinations of educational level and total household wealth also showed significant associations with psychological and cognitive multimorbidities, with the highest sHRs for the combination of primary educational level and lowest wealth quartile (7.15, 95% CI = 5.44–9.40, compared to those with tertiary educational level and highest wealth quartile, Supplementary Table S5).
Table 2.
Events, multimorbidity-free time, and incidence rates of psychological and cognitive multimorbidities in participants with a new-onset physical condition (n = 20,250).
| Total person-years | Physical-psychological multimorbidity (N = 3810) |
Physical-cognitive multimorbidity (N = 2618) |
Physical-psychological-cognitive multimorbidity (N = 1500) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events/N | Mean multimorbidity-free years | Incidence rate per 1000 person-years | Events/N | Mean multimorbidity-free time | Incidence rate per 1000 person-years | Events/N | Mean multimorbidity-free time | Incidence rate per 1000 person-years | ||
| Educational level | ||||||||||
| Primary | 39,524 | 1187/5362 | 11.18 (10.96–11.39) | 30.03 (28.38–31.77) | 812/5362 | 12.23 (12.01–12.45) | 20.54 (19.18–22.00) | 632/5362 | 12.66 (12.44–12.88) | 15.99 (14.79–17.29) |
| Secondary | 67,106 | 1542/8135 | 12.57 (12.43–12.72) | 22.98 (21.86–24.15) | 1118/8135 | 13.27 (13.13–13.41) | 16.66 (15.71–17.66) | 572/8135 | 14.42 (14.30–14.54) | 8.52 (7.85–9.26) |
| Tertiary | 61,945 | 1081/6753 | 13.60 (13.47–13.73) | 17.45 (16.44–18.52) | 688/6753 | 14.32 (14.20–14.44) | 11.11 (10.30–11.97) | 296/6753 | 15.25 (15.16–15.33) | 4.78 (4.26–5.36) |
| Total household wealth | ||||||||||
| Quartile 1 (Lowest) | 42,221 | 1132/5073 | 11.69 (11.49–11.89) | 26.81 (25.30–28.41) | 781/5073 | 12.65 (12.45–12.85) | 18.50 (17.24–19.84) | 559/5073 | 13.38 (13.19–13.57) | 13.24 (12.18–14.39) |
| Quartile 2 | 43,318 | 1040/5054 | 12.44 (12.26–12.63) | 24.01 (22.60–25.50) | 678/5054 | 13.38 (13.20–13.55) | 15.65 (14.51–16.88) | 399/5054 | 14.30 (14.15–14.46) | 9.21 (8.34–10.17) |
| Quartile 3 | 42,258 | 881/5067 | 12.97 (12.80–13.14) | 20.85 (19.52–22.27) | 637/5067 | 13.61 (13.45–13.78) | 15.07 (13.94–16.29) | 325/5067 | 14.65 (14.51–14.79) | 7.69 (6.89–8.58) |
| Quartile 4 (Highest) | 40,778 | 757/5056 | 13.50 (13.34–13.66) | 18.56 (17.29–19.93) | 522/5056 | 14.13 (13.98–14.28) | 12.80 (11.74–13.95) | 217/5056 | 15.12 (15.00–15.23) | 5.32 (4.65–6.09) |
| SES | ||||||||||
| Low | 14,212 | 450/1832 | 10.54 (10.17–10.92) | 31.66 (28.88–34.71) | 301/1832 | 11.71 (11.32–12.11) | 21.18 (18.91–23.72) | 262/1832 | 11.96 (11.57–12.34) | 18.44 (16.32–20.82) |
| Lower-middle | 67,004 | 1691/8152 | 12.11 (11.96–12.26) | 25.24 (24.07–26.46) | 1193/8152 | 12.95 (12.79–13.10) | 17.80 (16.82–18.84) | 759/8152 | 13.84 (13.70–13.98) | 11.33 (10.55–12.17) |
| Upper-middle | 65,152 | 1361/7788 | 13.06 (12.93–13.20) | 20.89 (19.81–22.02) | 900/7788 | 13.85 (13.72–13.98) | 13.81 (12.94–14.75) | 409/7788 | 14.93 (14.82–15.03) | 6.28 (5.69–6.92) |
| High | 22,207 | 308/2478 | 14.18 (13.99–14.38) | 13.87 (12.39–15.52) | 224/2478 | 14.60 (14.42–14.77) | 10.09 (8.83–11.51) | 70/2478 | 15.52 (15.40–15.63) | 3.15 (2.48–4.01) |
N, number; SES, socioeconomic status.
Incidence rate was presented per 1000 person-years; Mean multimorbidity-free years was measured using the median Irwin's restricted mean for multimorbidity-free survival time. Socioeconomic status is a summed score (ranging 0–5) of educational level (0, 1, or 2) and total household wealth quartile (0, 1, 2, or 3), categorized into four groups: low (0), lower-middle (1–2), upper-middle (3–4) and high socioeconomic status (5).
Table 3.
Subdistribution hazard ratios and 95% confidence intervals for the association between socioeconomic status and progression to psychological and cognitive multimorbidities in participants with a new-onset physical condition (n = 20,250).
| sHR (95% CI) |
|||
|---|---|---|---|
| Physical-psychological multimorbidity | Physical-cognitive multimorbidity | Physical-psychological-cognitive multimorbidity | |
| Educational level | |||
| Primary | 1.32 (1.21–1.44) | 2.37 (2.11–2.65) | 3.17 (2.71–3.71) |
| Secondary | 1.16 (1.07–1.26) | 1.60 (1.45–1.77) | 1.70 (1.47–1.97) |
| Tertiary | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Per decreasing category | 1.15 (1.10–1.20) | 1.53 (1.45–1.62) | 1.79 (1.66–1.94) |
| Total household wealth | |||
| Quartile 1 (Lowest) | 1.41 (1.29–1.55) | 1.47 (1.32–1.65) | 2.11 (1.80–2.48) |
| Quartile 2 | 1.28 (1.17–1.40) | 1.20 (1.07–1.34) | 1.51 (1.28–1.78) |
| Quartile 3 | 1.11 (1.01–1.22) | 1.17 (1.04–1.31) | 1.33 (1.12–1.57) |
| Quartile 4 (Highest) | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Per decreasing category | 1.13 (1.09–1.16) | 1.13 (1.09–1.17) | 1.27 (1.21–1.33) |
| SES | |||
| Low | 2.04 (1.77–2.36) | 3.24 (2.72–3.85) | 6.58 (5.01–8.64) |
| Lower-middle | 1.70 (1.50–1.91) | 2.22 (1.92–2.57) | 3.79 (2.95–4.86) |
| Upper-middle | 1.43 (1.27–1.62) | 1.53 (1.32–1.76) | 2.05 (1.59–2.64) |
| High | 1.00 (REF) | 1.00 (REF) | 1.00 (REF) |
| Per decreasing category | 1.24 (1.19–1.29) | 1.47 (1.40–1.54) | 1.84 (1.72–1.97) |
SES, socioeconomic status; sHR, subdistribution hazard ratio; CI, confidence interval.
Socioeconomic status is a summed score (ranging 0–5) of educational level (0, 1, or 2) and total household wealth quartile (0, 1, 2, or 3), categorized into four groups: low (0), lower-middle (1–2), upper-middle (3–4) and high socioeconomic status (5).
Models were adjusted for age, sex, and study.
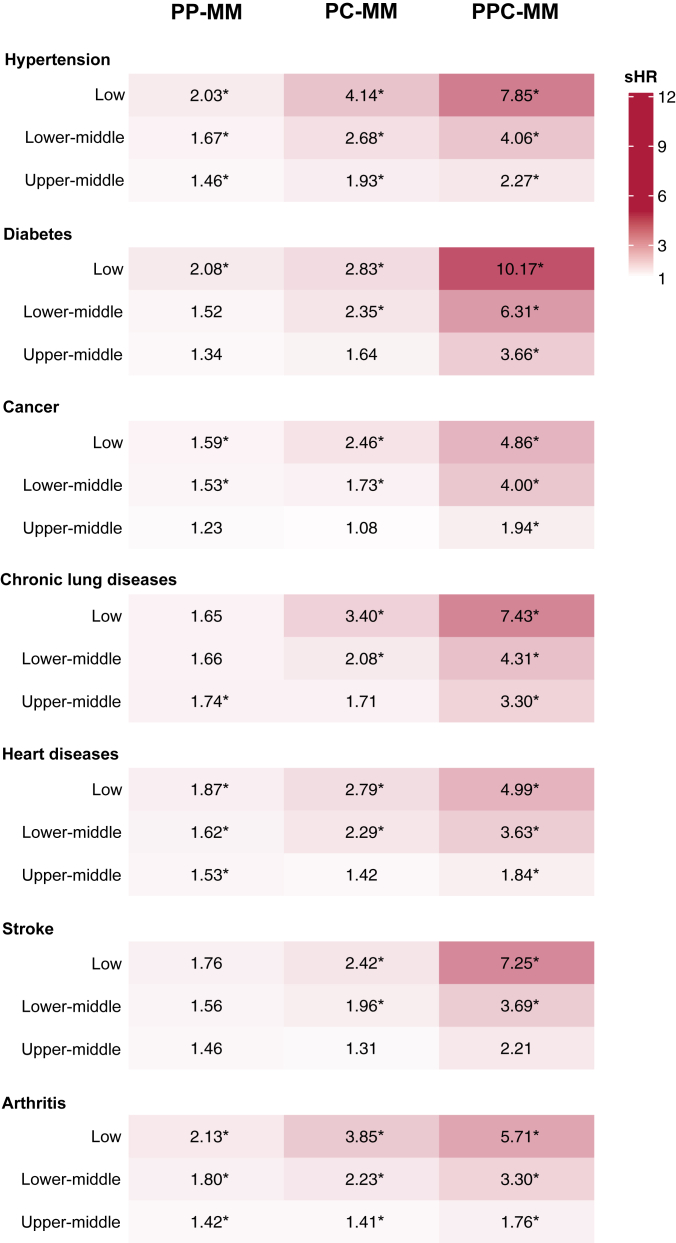
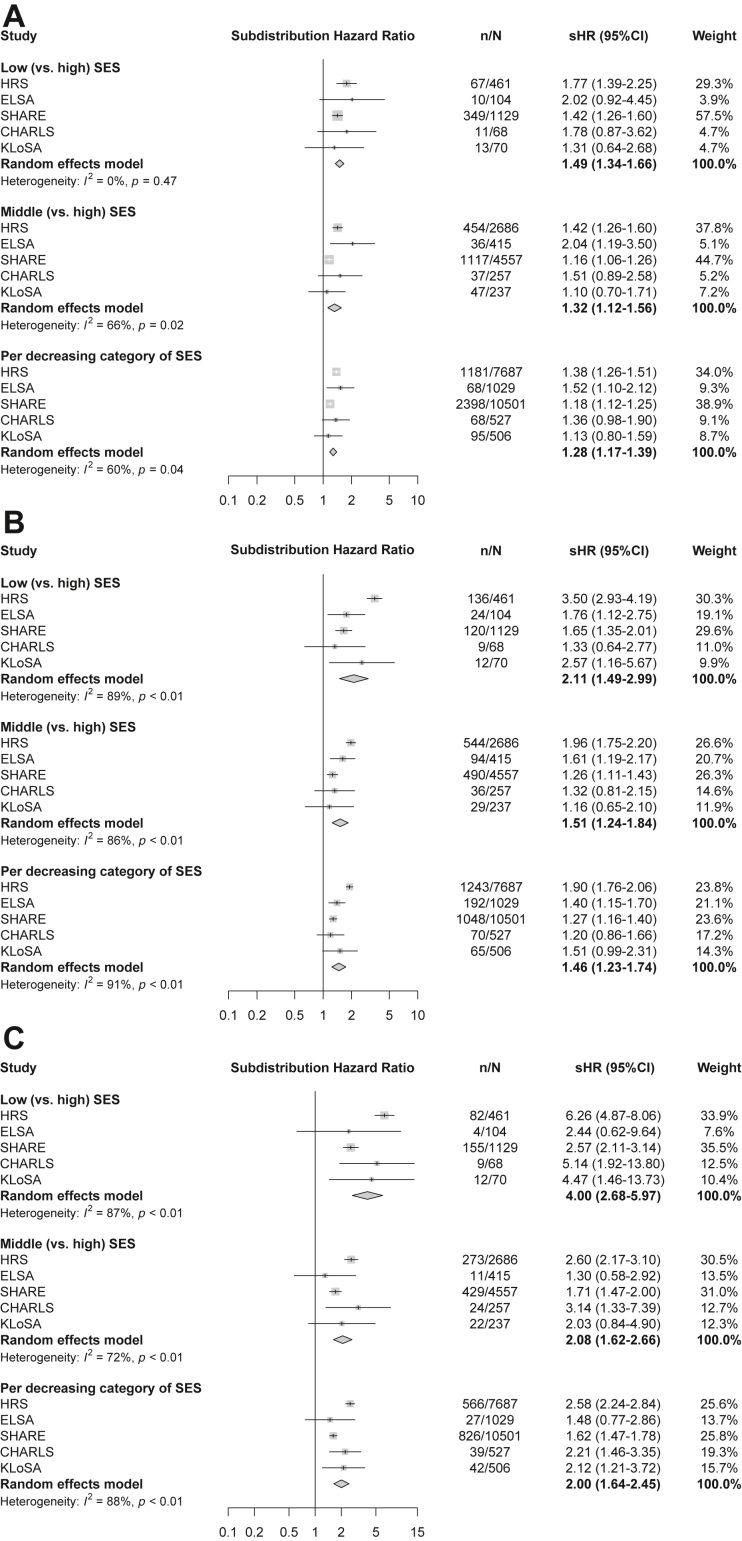
The associations between SES and psychological and cognitive multimorbidities among participants with onset of a specific physical condition are displayed in Fig. 2 and Supplementary Table S6, controlling for age, sex, study and comorbid physical conditions. Compared to those with arthritis and with high SES, individuals with arthritis in lower SES groups experienced a significantly higher risk of PP-MM (e.g., low SES: sHR = 2.13, 95% CI = 1.74–2.61), while those with hypertension and lower SES had markedly increased risk of PC-MM (low SES: sHR = 4.14, 3.23–5.30). For PPC-MM, the associations of lower SES were particularly pronounced in individuals with hypertension (e.g., low SES: sHR = 7.85, 95% CI = 5.30–11.63), and diabetes (e.g., low SES: sHR = 10.17, 3.23–32.06). Educational level, total household wealth and SES significantly interacted with the number of physical conditions (p < 0.001). The SES-multimorbidities associations showed similar patterns by sex, age, study, and additional physical conditions before outcomes, although the effect estimates were imprecise for some groups due to small sample size (Supplementary Tables S7–S10). The results of meta-analyses also showed the marked SES gradient in progression to psychological and cognitive multimorbidities (e.g., for PPC-MM: pooled sHR = 4.00, 95% CI = 2.68–5.97 in the low SES group), despite significant heterogeneity (Fig. 3). We also observed consistent dose–response relationships between SES and psychological and cognitive multimorbidities in sensitivity analyses (Supplementary Tables S11–S22). After imputing missing values in psychological and cognitive conditions, the SES-multimorbidities association was slightly attenuated but still significant in all groups (Supplementary Table S23).
Fig. 2.
Associations between socioeconomic status and progression of psychological and cognitive multimorbidities after onset of a specific physical condition. PP-MM, physical-psychological multimorbidity; PC-MM, physical-cognitive multimorbidity; PPC-MM, physical-psychological-cognitive multimorbidity. Socioeconomic status is a summed score (ranging 0–5) of educational level (0, 1, or 2) and total household wealth quartile (0, 1, 2, or 3), categorized into four groups: low (0), lower-middle (1–2), upper-middle (3–4) and high socioeconomic status (5). The reference group is high socioeconomic status. Numbers are subdistribution hazard ratios for psychological and cognitive multimorbidities after each new-onset condition stratified by socioeconomic status; ∗indicates statistical significance at p < 0.05. Models were adjusted for age, sex, study and physical comorbidity.
Fig. 3.
Meta-analyses of associations between socioeconomic status and progression of psychological and cognitive multimorbidities in participants with a new-onset physical condition including study-specific findings. (A) Progression to physical-psychological multimorbidity; (B) progression to physical-cognitive multimorbidity; (C) progression to physical-psychological-cognitive multimorbidity. The models were adjusted for age and sex. sHR, subdistribution hazard ratio; CI, confidence interval; HRS, US Health and Retirement Study; ELSA, English Longitudinal Study on Ageing; SHARE, Survey of Health, Ageing and Retirement in Europe; CHARLS, China Health and Retirement Longitudinal Study; KLoSA, Korean Longitudinal Study of Aging.
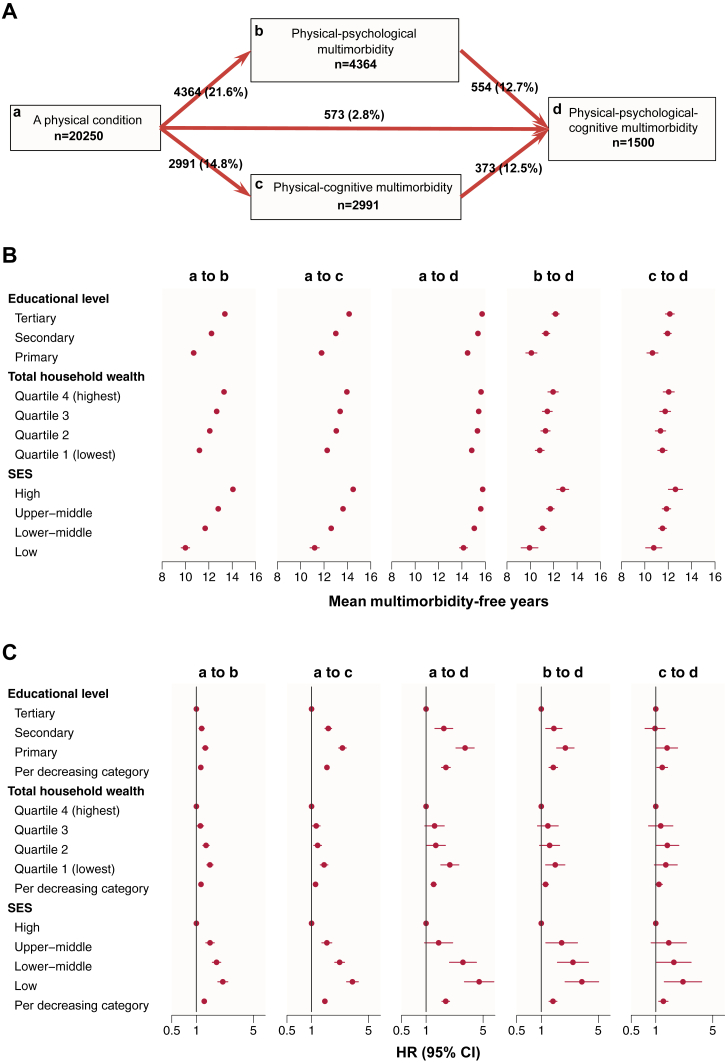
In multi-state models, a total of 4364 (21.6%) individuals transitioned from a physical condition to PP-MM, 2991 (14.8%) from a physical condition to PC-MM, and 573 (2.8%) from a physical condition to PPC-MM. In addition, 544 (12.7%) individuals further transitioned from PP-MM to PPC-MM, and 373 (12.5%) transitioned from PC-MM to PPC-MM (Fig. 4A). Participants with lower educational level, lower total household wealth and lower SES had earlier onsets of the five transitions (Fig. 4B and Supplementary Table S24). In contrast, individuals with high SES had the highest number of years between each health transition: 14.06 (95% CI = 13.86–14.26) for the transition from a physical condition to PP-MM; 14.49 (14.31–14.67) from a physical condition to PC-MM; 15.78 (15.70–15.86) from a physical condition to PPC-MM; 12.77 (12.16–13.28) from PP-MM to PPC-MM; and 12.62 (12.01–13.22) years from PC-MM to PPC-MM.
Fig. 4.
Multi-state models for health transitions by socioeconomic status. (A) Numbers (percentages) of participants in transition from a physical condition to physical, psychological and cognitive multimorbidities. (B) Mean multimorbidity-free time of participants in transition from a physical condition to multimorbidities. (C) Hazard ratios and 95% confidence intervals for associations between socioeconomic status and five transitions from a physical condition to multimorbidities. SES, socioeconomic status; HR, hazard ratio; CI, confidence interval. Socioeconomic status is a summed score (ranging 0–5) of educational level (0, 1, or 2) and total household wealth quartile (0, 1, 2, or 3), categorized into four groups: low (0), lower-middle (1–2), upper-middle (3–4) and high socioeconomic status (5). Models were adjusted for age, sex and study.
The associations between educational level, total household wealth, SES and transitions to psychological and cognitive multimorbidities followed a dose–response pattern (Fig. 4C and Supplementary Table S25). The sHRs per decreasing SES category were 1.25 (95% CI = 1.20–1.30) for the transition to PP-MM, 1.46 (1.39–1.52) for the transition to PC-MM, and 1.74 (1.56–1.94) for the transition to PPC-MM. Individuals with lower SES also had an increased risk of progressing from PP-MM to PPC-MM (sHR = 1.39, 1.25–1.56) and from PC-MM to PPC-MM (sHR = 1.24, 1.09–1.41), although sHRs did not reach statistical significance for some SES groups.
Discussion
In this prospective multicohort study of 20,250 participants with at least one new-onset physical conditions from five ageing cohorts across 24 countries, we present three key findings. First, individuals with lower educational level, lower total household wealth or lower SES had an earlier time to and a higher incidence of progressing psychological and cognitive multimorbidities. Second, the associations between educational level, total household wealth and SES and progression to psychological and cognitive multimorbidities followed a dose–response relation, with the educational gradient in progression to PC-MM and PPC-MM being more pronounced than that of total household wealth. Third, the dose-dependent associations of SES were observable also in an analysis specifying each of the five temporal transitions from a physical condition to psychological and cognitive multimorbidities.
Chronic physical conditions are most prevalent in later life and are often accompanied with an increased risk of psychological and cognitive disorders.44 Our findings on social inequalities in this process add to previous research, which often regarded multimorbidity as a unified entity, without differentiating psychological or cognitive components.45, 46, 47 The current study also complements a recent study which reported a cross-sectional dose–response association between SES and physical, psychological and cognitive multimorbidities in middle-aged and older adults.14 While extensive research has shown that socioeconomic disadvantage heightens the risk of incident diseases,48, 49, 50 our analysis focused on individuals already diagnosed with a physical condition, examining how socioeconomic factors might influence the transition from a chronic physical condition to psychological multimorbidity, cognitive multimorbidity, and multimorbidity including physical, psychological, and cognitive disorders in middle-aged and older adults. Taken together, these findings reveal a social gradient from single physical conditions to complex multimorbidities, through the development of psychological and cognitive conditions. These findings have implications for optimising secondary prevention. Thus, SES emerges as a significant risk factor with a pervasive impact on all stages of health deterioration, from disease etiology to disease course and prognosis.
Considering the analysis was conducted in a limited time duration, we used the Irwin's restricted mean as an index for comparing multimorbidity-free survival time in different SES groups to accommodate censoring and truncation. We found that individuals with lower SES have an earlier time to and a higher incidence of progressing psychological and cognitive multimorbidities, suggesting that efforts to address socioeconomic inequalities would not only reduce the occurrence of psychological and cognitive outcomes among patients with a physical condition, but also extend life years free from such outcomes. With death as a competing risk event, the dose–response relationships of educational level, total household wealth and SES with psychological and cognitive multimorbidities were observed. Of these, education inequalities were larger than adulthood wealth inequalities, particularly in cognitive multimorbidities. Our results combining education and wealth into 12 subgroups (3 4) also supported the prominence of education over wealth. This finding is consistent with other studies. A prospective study of 13,699 participants from the HRS suggested education attainment but not wealth was associated with disease accumulation.51 A case–control study found higher formal education reduced the risk of oesophageal cancer, while the effects of wealth score did not reach significance.52 Several factors may explain these findings. First, education is attained in childhood, and therefore influence the subsequent SES in adulthood, including occupation, income and wealth46; education is more likely to influence behavioral and psychological factors, like lifestyles, social support and knowledge acquisition which all affect later-life mental wellness and cognitive function,53 while adulthood wealth tends to influence material factors, including financial position, living arrangements and access to healthcare.54 These findings support equality-oriented policies and strategies, especially those promoting educational attainment in early life, in preventing the accumulation of physical, psychological and cognitive conditions in late life. Our meta-analyses showed that SES inequalities existed across studies, although there was between-study heterogeneity suggesting that the gradients may be influenced by contextual factors, such as the general level of elementary education.55
Our study is also one of the first large-scale investigation to evaluate the associations between SES and psychological and cognitive multimorbidities among patients with a specific physical condition. We found that individuals with arthritis presented a larger SES gradient in progressing physical-psychological multimorbidity. It is possible that the long-lasting pain and psychological distress caused by arthritis56 are particularly under-treated among the socioeconomic disadvantaged groups.57 For cognitive multimorbidities, the higher SES disparities occurred among patients with hypertension and diabetes, which could be due to factors, such as poorer blood pressure management in those with socially disadvantage, lower brain and cognitive reserve in this group as well as a higher prevalence of risk behaviours adversely affecting brain perfusion, neuroinflammation and cerebral microvasculature.58, 59, 60
In the process of the paradigm shift in multimorbidity management towards person-centered care, health authorities should consider the interplay between specific chronic conditions when narrowing the health inequalities. Since the development of psychological and cognitive conditions has specific temporal sequences, we applied the multi-state models to explore the effects of SES on the timings of transitions from a physical condition to psychological and cognitive multimorbidities. In addition to the transitions from a physical condition, SES gradient was also evident in the transitions to physical-psychological-cognitive multimorbidity from physical-psychological multimorbidity and physical-cognitive multimorbidity, although some associations did not achieve statistical significance at conventional levels due to limited sample size. These findings strengthen the evidence of the far-reaching health effects of social disadvantage and highlight the need for strategies targeting social determinants at advanced stages of the disease process. In terms of public health, it is important to prioritise the socioeconomically disadvantaged, as well as those already with multimorbidity, in health care.
The strengths of this study include the use of prospective and harmonized data from five nationally representative cohorts covering 24 countries and resulting in a large sample size, which allowed us to explore socioeconomic inequalities in the progression to psychological and cognitive multimorbidities after onset of a physical condition. In addition, a series of sensitivity and subgroup analyses confirmed the robustness of our results. However, this study also has several limitations. First, the information on physical conditions, educational level and household wealth were self-reported by the participants, such data are subject to recall and social desirability biases.61 Second, harmonized data were only available for seven common chronic physical conditions. Consequently, we may have missed many conditions with a socioeconomic gradient in prevalence and incidence. This may have reduced our ability to observe pathways to psychological and cognitive multimorbidities. Third, the follow-ups were conducted every two to four years, therefore not necessarily capturing all the temporal transitions between physical, psychological and cognitive conditions. Fourth, due to predefined cohort-specific cut-offs for psychological and cognitive measurements and the limited proportion of identical data items across the studies, we relied on study-specific instruments to measure psychological and cognitive multimorbidities. This approach may have contributed to increased heterogeneity in the cohort-specific results. Despite the availability of harmonization methodologies such as that by the CLOSER group,62 these were not used because of the heterogeneity in our pooled cohorts and the instruments they used. Ideally, future research should utilize similar protocols and optimized harmonization methods across the aging cohorts to confirm the validity of the findings. Five, our study included 20,250 participants, but only 527 were from CHARLS and 506 from KLoSA, limiting the possibilities to conduct cohort-specific analyses and evaluate the generalisability of the findings to Asian countries. Further multi-cohort studies are warranted to elucidate socioeconomic inequalities in the progression to psychological and cognitive multimorbidities progression across varied cultures and settings. Six, although we conducted sensitivity analyses by excluding outcome events occurring within two years or one follow-up visit after onset of a physical condition, this cannot exclude the possibility of reverse causality bias. This is because cognitive decline is a long-term process, and psychological problems can fluctuate over time. In addition, the limited follow-up period precluded us from exploring the role of SES across all health transitions—from no conditions to incident disease, and the development of physical, psychological and cognitive multimorbidities—in a single study design. Therefore future studies with larger sample sizes and longer follow-ups are warranted. Seven, despite the multivariable-adjusted models in the sensitivity analysis, the potential for residual confounding due to unmeasured or imprecisely measured factors could not been excluded. Eight, no sample weights were used in our analysis, due to the multi-cohort design and inconsistent methods of the construction of weights across the studies. Nine, the complicated interactions between physical, psychological and cognitive conditions may contribute to immortal survival bias in the limited follow-up time. Although we use the Irwin's restricted mean, the Fine–Gray subdistribution hazard model and the multi-state models to reduce this bias, our findings should be still interpreted with caution. Lastly, we did not have information on the symptoms, severity and subtypes of the studied physical, psychological and cognitive conditions. Future studies should collect more detailed data to deepen our understanding of the socioeconomic gradients in physical, psychological and cognitive multimorbidities.
In conclusion, in this large, prospective and multi-national cohort study, we found marked socioeconomic inequalities in time, incidence and risk of progressing from a physical condition to psychological and cognitive multimorbidities. Continuous and reinforced efforts to address socioeconomic inequalities are needed to improve psychological and cognitive wellness among middle-aged and older adults with a physical condition.
Contributors
XX contributed the study conceptualization and supervised the whole project. YaZ made the analysis plan and conducted the statistical analyses. YaZ and XX validated data and statistical analyses. YaZ contributed to visualization and wrote the initial draft of the manuscript. MK, LLY, RMCL, YuZ, YC, HW, MZ and XX provided valuable revision advice on writing and statistical methods. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. XX and YaZ have accessed and verified the data, and XX were responsible for the decision to submit the manuscript.
Data sharing statement
The original data for this study are available on their respective websites: The Health and Retirement Study-HRS (https://hrs.isr.umich.edu/), the English Longitudinal Study of Ageing-ELSA (https://www.elsa-project.ac.uk), the Survey of Health, Ageing and Retirement in Europe-SHARE (http://www.share-project.org/home0.html), the Korean Longitudinal Study of Aging-KLoSA(https://survey.keis.or.kr/eng/klosa/klosa01.jsp), and the China Health and Retirement Longitudinal Study-CHARLS (http://charls.pku.edu.cn/index/en.html).
Declaration of interests
Authors declare that they have no competing interests.
Acknowledgements
XX was supported by the Hundred Talents Program Research Initiation Fund from Zhejiang University, and the Fundamental Research Funds for the Central Universities. MK was supported by Wellcome Trust (221854/Z/20/Z), UK Medical Research Council (S011676, Y014154), National Institute on Aging (NIH), US (R01AG056477, R01AG062553), and Academy of Finland (350426).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102739.
Appendix A. Supplementary data
References
- 1.Alanazi J., Unnisa A., Patel R.D., et al. Prevalence of cardiovascular disease and osteoarthritis in obese population of Hail region, Saudi Arabia. Eur Rev Med Pharmacol Sci. 2022;26(19):7161–7168. doi: 10.26355/eurrev_202210_29903. [DOI] [PubMed] [Google Scholar]
- 2.Mistry S.K., Ali A.R.M.M., Yadav U.N., et al. Older adults with non-communicable chronic conditions and their health care access amid COVID-19 pandemic in Bangladesh: findings from a cross-sectional study. PLoS One. 2021;16(7):e0255534. doi: 10.1371/journal.pone.0255534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansah J.P., Chiu C.T. Projecting the chronic disease burden among the adult population in the United States using a multi-state population model. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.1082183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajat C., Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Rep. 2018;12:284–293. doi: 10.1016/j.pmedr.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maresova P., Javanmardi E., Barakovic S., et al. Consequences of chronic diseases and other limitations associated with old age – a scoping review. BMC Public Health. 2019;19(1):1431. doi: 10.1186/s12889-019-7762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y., Xiang Q., Yan C., Liao H., Wang J. Relationship between chronic diseases and depression: the mediating effect of pain. BMC Psychiatry. 2021;21(1):436. doi: 10.1186/s12888-021-03428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng M.Y., Bi Y.H., Wang H.X., Pei J.J. Influence of chronic diseases on the occurrence of depression: a 13-year follow-up study from the survey of health, ageing and retirement in Europe. Psychiatry Res. 2023;326 doi: 10.1016/j.psychres.2023.115268. [DOI] [PubMed] [Google Scholar]
- 8.Bi Y.H., Pei J.J., Hao C., Yao W., Wang H.X. The relationship between chronic diseases and depression in middle-aged and older adults: a 4-year follow-up study from the China health and retirement longitudinal study. J Affect Disord. 2021;289:160–166. doi: 10.1016/j.jad.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 9.Ronaldson A., Arias de la Torre J., Prina M., et al. Associations between physical multimorbidity patterns and common mental health disorders in middle-aged adults: a prospective analysis using data from the UK Biobank. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socal M.P., Trujillo A.J. Links between chronic illness and late-life cognition: evidence from four Latin American countries. J Aging Health. 2018;30(2):262–304. doi: 10.1177/0898264316674557. [DOI] [PubMed] [Google Scholar]
- 11.Veronese N., Koyanagi A., Dominguez L.J., et al. Multimorbidity increases the risk of dementia: a 15 year follow-up of the SHARE study. Age Ageing. 2023;52(4) doi: 10.1093/ageing/afad052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katikireddi S.V., Skivington K., Leyland A.H., Hunt K., Mercer S.W. The contribution of risk factors to socioeconomic inequalities in multimorbidity across the lifecourse: a longitudinal analysis of the twenty-07 cohort. BMC Med. 2017;15(1):152. doi: 10.1186/s12916-017-0913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivimäki M., Bartolomucci A., Kawachi I. The multiple roles of life stress in metabolic disorders. Nature Rev Endocrinol. 2023;19(1):10–27. doi: 10.1038/s41574-022-00746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Y., Zhou Y., Kivimäki M., et al. Socioeconomic inequalities in physical, psychological, and cognitive multimorbidity in middle-aged and older adults in 33 countries: a cross-sectional study. Lancet Healthy Longev. 2023;4(11):e618–e628. doi: 10.1016/S2666-7568(23)00195-2. [DOI] [PubMed] [Google Scholar]
- 15.Calocer F., Dejardin O., Kwiatkowski A., et al. Socioeconomic deprivation increases the risk of disability in multiple sclerosis patients. Mult Scler Relat Disord. 2020;40 doi: 10.1016/j.msard.2020.101930. [DOI] [PubMed] [Google Scholar]
- 16.Witte K.K., Patel P.A., Walker A.M.N., et al. Socioeconomic deprivation and mode-specific outcomes in patients with chronic heart failure. Heart. 2018;104(12):993–998. doi: 10.1136/heartjnl-2017-312539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahon S., Lahmek P., Durance C., et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18(11):2086–2091. doi: 10.1002/ibd.22888. [DOI] [PubMed] [Google Scholar]
- 18.Elwell-Sutton T., Folb N., Clark A., Fairall L.R., Lund C., Bachmann M.O., et al. Socioeconomic position and depression in South African adults with long-term health conditions: a longitudinal study of causal pathways. Epidemiol Psychiatr Sci. 2019;28(2):199–209. doi: 10.1017/S2045796017000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng C., Du Y., Bai J. Physical multimorbidity and psychological distress among Chinese older adults: findings from Chinese longitudinal healthy longevity survey. Asian J Psychiatr. 2022;70 doi: 10.1016/j.ajp.2022.103022. [DOI] [PubMed] [Google Scholar]
- 20.Chen S., Nagel C.L., Liu R., et al. Mental-somatic multimorbidity in trajectories of cognitive function for middle-aged and older adults. PLoS One. 2024;19(5):e0303599. doi: 10.1371/journal.pone.0303599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Phillips D., Wilkens J. Gateway to global aging data: resources for cross-national comparisons of family, social environment, and healthy aging. J Gerontol B Psychol Sci Soc Sci. 2021;76(Suppl 1):S5–S16. doi: 10.1093/geronb/gbab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonnega A., Faul J.D., Ofstedal M.B., et al. Cohort profile: the health and retirement study (HRS) Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steptoe A., Breeze E., Banks J., Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42(6):1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y., Hu Y., Smith J.P., Strauss J., Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS) Int J Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Börsch-Supan A., Brandt M., Hunkler C., et al. Data resource profile: the survey of health, ageing and retirement in Europe (SHARE) Int J Epidemiol. 2013;42(4):992–1001. doi: 10.1093/ije/dyt088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin C. In: Encyclopedia of gerontology and population aging. Gu D., Dupre M.E., editors. Springer International Publishing; Cham: 2019. Korean longitudinal study of ageing; pp. 1–4. [Google Scholar]
- 27.Wang D., Dai X., Mishra S.R., et al. Association between socioeconomic status and health behaviour change before and after non-communicable disease diagnoses: a multicohort study. Lancet Public Health. 2022;7(8):e670–e682. doi: 10.1016/S2468-2667(22)00157-8. [DOI] [PubMed] [Google Scholar]
- 28.Wingo T.S., Gerasimov E.S., Canon S.M., Lah J.J., Levey A.I., Wingo A.P. Alzheimer's disease genetic burden is associated with mid-life depression among persons with normal cognition. Alzheimers Dementia. 2022 doi: 10.1002/alz.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank P., Kaushal A., Poole L., Poole L., Chalder T., Cadar D. Systemic low-grade inflammation and subsequent depressive symptoms: is there a mediating role of physical activity? Brain Behavior Immun. 2019;80:688–696. doi: 10.1016/j.bbi.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Hamer M., Batty G.D., Kivimaki M. Risk of future depression in people who are obese but metabolically healthy: the English longitudinal study of ageing. Mol Psychiatry. 2012;17(9):940–945. doi: 10.1038/mp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.López-Bueno R., Calatayud J., Andersen L.L., et al. Dose-response association of handgrip strength and risk of depression: a longitudinal study of 115 601 older adults from 24 countries. Br J Psychiatry. 2023;222(3):135–142. doi: 10.1192/bjp.2022.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prince M.J., Reischies F., Beekman A.T., et al. Development of the EURO-D scale--a European, Union initiative to compare symptoms of depression in 14 European centres. Br J Psychiatry. 1999;174:330–338. doi: 10.1192/bjp.174.4.330. [DOI] [PubMed] [Google Scholar]
- 33.Han C.H., Chung J.H., Lee S. Depression, chronic obstructive pulmonary disease, and healthcare utilization: results from the Korean Longitudinal Study of Aging (KLoSA) Clin Respir J. 2021;15(9):937–943. doi: 10.1111/crj.13384. [DOI] [PubMed] [Google Scholar]
- 34.Dove A., Marseglia A., Shang Y., et al. Cardiometabolic multimorbidity accelerates cognitive decline and dementia progression. Alzheimers Dementia. 2023;19(3):821–830. doi: 10.1002/alz.12708. [DOI] [PubMed] [Google Scholar]
- 35.Caracciolo B., Palmer K., Monastero R., Winblad B., Bäckman L., Fratiglioni L., et al. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70(19 Pt 2):1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 36.Cronin A., Tian L., Uno H. Strmst2 and Strmst2pw: new commands to compare survival curves using the restricted mean survival time. STATA J. 2016;16(3):702–716. [Google Scholar]
- 37.Muchira J.M., Gona P.N., Mogos M.F., et al. Parental cardiovascular health predicts time to onset of cardiovascular disease in offspring. Eur J Prev Cardiol. 2022;29(6):883–891. doi: 10.1093/eurjpc/zwaa072. [DOI] [PubMed] [Google Scholar]
- 38.Han K., Jung I. Restricted mean survival time for survival analysis: a quick guide for clinical researchers. Korean J Radiol. 2022;23(5):495–499. doi: 10.3348/kjr.2022.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 40.Grunkemeier G.L., Jin R., Eijkemans M.J., et al. Actual and actuarial probabilities of competing risks: apples and lemons. Ann Thorac Surg. 2007;83(5):1586–1592. doi: 10.1016/j.athoracsur.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 41.Schafer J.L. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 42.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 43.de Wreede L.C., Fiocco M., Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. doi: 10.1016/j.cmpb.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 44.WHO . World Health Organazation; 2021. Non communicable diseases.https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases [Google Scholar]
- 45.Dugravot A., Fayosse A., Dumurgier J., et al. Social inequalities in multimorbidity, frailty, disability, and transitions to mortality: a 24-year follow-up of the Whitehall II cohort study. Lancet Public Health. 2020;5(1):e42–e50. doi: 10.1016/S2468-2667(19)30226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pathirana T.I., Jackson C.A. Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health. 2018;42(2):186–194. doi: 10.1111/1753-6405.12762. [DOI] [PubMed] [Google Scholar]
- 47.Wagner C., Carmeli C., Chiolero A., Cullati S. Life course socioeconomic conditions and multimorbidity in old age - a scoping review. Ageing Res Rev. 2022;78 doi: 10.1016/j.arr.2022.101630. [DOI] [PubMed] [Google Scholar]
- 48.Kivimäki M., Batty G.D., Pentti J., et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140–e149. doi: 10.1016/S2468-2667(19)30248-8. [DOI] [PubMed] [Google Scholar]
- 49.Mackenbach J.P., Bakker M.J. Tackling socioeconomic inequalities in health: analysis of European experiences. Lancet. 2003;362(9393):1409–1414. doi: 10.1016/S0140-6736(03)14639-9. [DOI] [PubMed] [Google Scholar]
- 50.Marmot M., Friel S., Bell R., Houweling T.A., Taylor S., Commission on Social Determinants of Health Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669. doi: 10.1016/S0140-6736(08)61690-6. [DOI] [PubMed] [Google Scholar]
- 51.O'Neill A.S., Newsom J.T., Trubits E.F., et al. Racial, ethnic, and socioeconomic disparities in trajectories of morbidity accumulation among older Americans. SSM Popul Health. 2023;22 doi: 10.1016/j.ssmph.2023.101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islami F., Kamangar F., Nasrollahzadeh D., et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38(4):978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw M. Health inequality: an introduction to theories, concepts and methods. Mel bartley. Cambridge: Polity Press, 2004, pp. 224, £16.99 (PB), ISBN: 0745627803. Int J Epidemiol. 2005;34(2):500–502. [Google Scholar]
- 54.Skalická V., van Lenthe F., Bambra C., Krokstad S., Mackenbach J. Material, psychosocial, behavioural and biomedical factors in the explanation of relative socio-economic inequalities in mortality: evidence from the HUNT study. Int J Epidemiol. 2009;38(5):1272–1284. doi: 10.1093/ije/dyp262. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y.T., Daskalopoulou C., Muniz Terrera G., et al. Education and wealth inequalities in healthy ageing in eight harmonised cohorts in the ATHLOS consortium: a population-based study. Lancet Public Health. 2020;5(7):e386–e394. doi: 10.1016/S2468-2667(20)30077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harth M., Nielson W.R. Pain and affective distress in arthritis: relationship to immunity and inflammation. Expert Rev Clin Immunol. 2019;15(5):541–552. doi: 10.1080/1744666X.2019.1573675. [DOI] [PubMed] [Google Scholar]
- 57.Badley E.M., Canizares M., Gunz A.C., Davis A.M. Visits to rheumatologists for arthritis: the role of access to primary care physicians, geographic availability of rheumatologists, and socioeconomic status. Arthritis Care Res (Hoboken) 2015;67(2):230–239. doi: 10.1002/acr.22413. [DOI] [PubMed] [Google Scholar]
- 58.Novak V., Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7(12):686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sörös P., Whitehead S., Spence J.D., Hachinski V. Antihypertensive treatment can prevent stroke and cognitive decline. Nat Rev Neurol. 2013;9(3):174–178. doi: 10.1038/nrneurol.2012.255. [DOI] [PubMed] [Google Scholar]
- 60.Ehtewish H., Arredouani A., El-Agnaf O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int J Mol Sci. 2022;23(11):6144. doi: 10.3390/ijms23116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paulhus D.L. American Psychological Association; US: 1984. Two-component models of socially desirable responding; pp. 598–609. [Google Scholar]
- 62.McElroy E., Villadsen A., Patalay P., et al. CLOSER: London; UK: 2020. Harmonisation and measurement properties of mental health measures in six British cohorts. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.