Abstract
Objective
Dietary medium-chain fatty acids (MCFAs), characterized by chain lengths of 8–12 carbon atoms, have been proposed to have beneficial effects on glucose and lipid metabolism, yet the underlying mechanisms remain elusive. We hypothesized that MCFA intake benefits metabolic health by inducing the release of hormone-like factors.
Methods
The effects of chow diet, high-fat diet rich in long-chain fatty acids (LCFA HFD) fed ad libitum or pair-fed to a high-fat diet rich in MCFA (MCFA HFD) on glycemia, hepatic gene expression, circulating fibroblast growth factor 21 (FGF21), and liver fat content in both wildtype and Fgf21 knockout mice were investigated. The impact of a single oral dose of an MCFA-rich oil on circulating FGF21 and hepatic Fgf21 mRNA expression was assessed. In flag-tagged Crebh knockin mice and liver-specific Crebh knockout mice, fed LCFA HFD or MCFA HFD, active hepatic CREBH and hepatic Fgf21 mRNA abundance were determined, respectively.
Results
MCFA HFD improves glucose tolerance, enhances glucose clearance into brown adipose tissue, and prevents high-fat diet-induced hepatic steatosis in wildtype mice. These benefits are associated with increased liver expression of CREBH target genes (Apoa4 and Apoc2), including Fgf21. Both acute and chronic intake of dietary MCFAs elevate circulating FGF21. Augmented hepatic Fgf21 mRNA following MCFA HFD intake is accompanied by higher levels of active hepatic CREBH; and MCFA-induced hepatic Fgf21 expression is blocked in mice lacking Crebh. Notably, while feeding male and female Fgf21 wildtype mice MCFA HFD results in reduced liver triacylglycerol (TG) levels, this liver TG-lowering effect is blunted in Fgf21 knockout mice fed MCFA HFD. The reduction in liver TG levels observed with MCFA HFD was independent of weight loss.
Conclusions
Dietary MCFAs reduce liver fat accumulation via activation of a CREBH-FGF21 signaling axis.
Keywords: Medium-chain fatty acids, Insulin resistance, Liver metabolism, Hepatokines, FGF21
Highlights
-
•
Medium-chain fatty acids (MCFAs) enhance glucose clearance into brown adipose tissue.
-
•
MCFA consumption boosts Fgf21 expression, dependent on CREBH.
-
•
FGF21 is essential for MCFAs to prevent excessive liver fat accumulation.
1. Introduction
For decades, saturated fatty acids (FAs) have been considered detrimental to metabolic health, based on reports indicating that individuals with higher blood levels of saturated FAs have an increased risk of developing metabolic syndrome [1]. However, this connection overlooks the fact that blood levels of saturated FAs are unrelated to dietary intake of saturated fats. Studies have shown that doubling or tripling the consumption of saturated fats either has no effect or results in decreased blood levels of saturated FAs in humans [[2], [3], [4], [5]]. Furthermore, a study involving men with obesity consuming a eucaloric 64 energy % (E%) high-fat diet (HFD) enriched in saturated FAs for six weeks showed maintained peripheral insulin sensitivity and lower hepatic glucose production and de novo lipogenesis [6]. This suggests that saturated FAs per se are not detrimental to health unless consumed in excess of caloric requirements. Recent research also emphasizes the importance of considering the specific type of saturated FAs [7], as they comprise a diverse group categorized by their carbon chain length: short-chain (4–6 carbon atoms), medium-chain (8–12 carbon atoms), long-chain (14–20 carbon atoms), and very long-chain (22 or more carbon atoms) FAs.
Most saturated fats in the western diet and the human body consist of triacylglycerols (TGs) with long-chain FAs (LCFAs) (i.e., long-chain triacylglycerols, LCTs). In contrast, TGs with medium-chain FAs (MCFAs) (i.e., medium-chain TGs, MCTs) are far less abundant. Due to a shorter carbon tail and smaller size, MCFAs are highly ionized at physiological pH and thus more soluble in aqueous fluids than LCFAs [8]. These properties affect their digestion, absorption, and cellular metabolism. For example, unlike LCFAs, which enter the systemic circulation as LCTs primarily via the lymphatic route, MCFAs largely remain non-esterified after digestion and are absorbed from the intestine into the portal vein, which feeds into the liver. This alternative absorption route is what initially rendered MCFAs an attractive dietary strategy to treat patients with intestinal malabsorption.
In humans, MCT intake has been associated with increased satiety [9], lowered food intake [10], and increased postprandial metabolic rate [11]. MCT intake has been associated with weight loss in both rodents [12] and humans [13]. Moreover, positive effects of MCT intake on insulin sensitivity have been reported in healthy individuals [14] and patients with type 2 diabetes [15]. In rodents, MCT intake has been associated with protection against hepatic lipid accumulation [16,17]. Despite these reported benefits on energy balance, glycemia, and liver fat, the underlying mechanisms for these effects are unknown.
We herein used moderate high-fat diets containing either MCFAs or LCFAs to investigate the effects of dietary MCFA on endpoints related to glycemia and liver fat in mice. To identify potential mediators underpinning MCFA-rich diet associated metabolic benefits, we investigated the liver transcriptome.
2. Materials and methods
2.1. Animals
Female and male C57BL/6J mice (Janvier, France), female and male fibroblast growth factor 21 (Fgf21) wildtype (WT) and knockout (KO) were used. Generation of Fgf21 KO mice has been described in detail in [18] and comprised replacement of most of exon 1 and all of exons 2 and 3 of Fgf21 with an IRES-LacZ-polyA/PGK-neo cassette and a diphtheria toxin A cassette, creating a null mutant allele. Male Crebh WT (Crebhloxp/loxp; Alb-Cre−/−) or liver-specific KO (LiKO) (Crebhloxp/loxp; Alb-Cre+/−) (described in [19]) were used.
Male 3× Flag Crebh knockin (KI) mice, expressing the endogenous CREBH protein tagged with a 3× Flag at the N-terminus, were generated by inserting the 3× Flag sequence in front of the start methionine of the Crebh gene using the CRISPR/Cas9 system. Mice were group-housed when possible, kept on a 12:12-h light–dark cycle, and had free access to standard rodent chow diet (Altromin no. 1324; Brogaarden, Denmark) and water before the start of any experiments. Animal experiments on C57BL/6J mice and Fgf21 KO mice were approved by the Danish Animal Experiments Inspectorate and complied with the European convention for the protection of vertebrate animals used for scientific purposes. Animal experiments on flag-tagged Crebh KI and Crebh LiKO mice were performed in accordance with the Regulation of Animal Experiments of the University of Tsukuba and were approved by the Animal Experiment Committee, University of Tsukuba (Crebh KO experiments).
All experiments were performed at 22 °C with a 12:12 h light–dark cycle with light from 6 am to 6 pm.
2.2. Diets
Two moderate high-fat diets containing either LCFA (LCFA HFD) or MCFA (MCFA HFD) were used in the feeding studies. The LCFA diet comprised 45 kcal% fat (lard and soy bean oil at 88% and 12%, respectively) with 35 and 20 kcal% carbohydrate and protein, respectively (D01060502G; Research Diets). The MCFA HFD was matched in macronutrient composition (Supplemental Table 1) but the 45 kcal% fat was solely comprised of MCT oil (caprylic and capric TG at 60% and 40%, respectively) (D18010905; Research Diets). Two commercially available TG-oils were used in oral gavage studies: 99% pure C8:0 MCT oil (MCT oil; Fitnessguru, Sweden or glyceryl trioctanoate, #538-23-8, Sigma, Denmark, respectively, as indicated below) and corn oil (LCT oil; Coop, Denmark).
2.3. Bomb calorimetry
LCFA HFD and MCFA HFD were weighed and energy content was determined in triplicates by bomb calorimetric combustion (IKA C5003, IKA Werke, Germany).
2.4. Food intake measurements
Food in the feeding racks was weighed on the specified days shown in the graphs, and the difference in weight was recorded as food intake. Visible food pieces in the cages were included in the weighing process. Data were excluded if mice exhibited visible food shredding. From food intake, energy intake (as kJ) was calculated, using the information for energy content provided by the manufacturer for the chow diet and the energy content determined by bomb calorimetry for the LCFA HFD and MCFA HFD diets.
2.5. Diet interventions
2.5.1. Chow, LCFA HFD, or MCFA HFD diet in male C57BL/6J mice
12–16 weeks old male C57BL/6J mice were single-housed and subjected to ad libitum access to either a chow, LCFA HFD, or an MCFA HFD diet for 16 days. During the intervention, food intake was recorded biweekly. On day 13, an i.p. glucose tolerance test (GTT) was performed. On day 15, body weight was measured and fat-free mass and fat mass were determined by MRI-scan (EchoMRI 4-in-1; EchoMRI). On day 16, glucose-stimulated glucose clearance was investigated by use of radiolabeled 2-deoxyglucose. This study relates to Figures 1A–H and 2D.
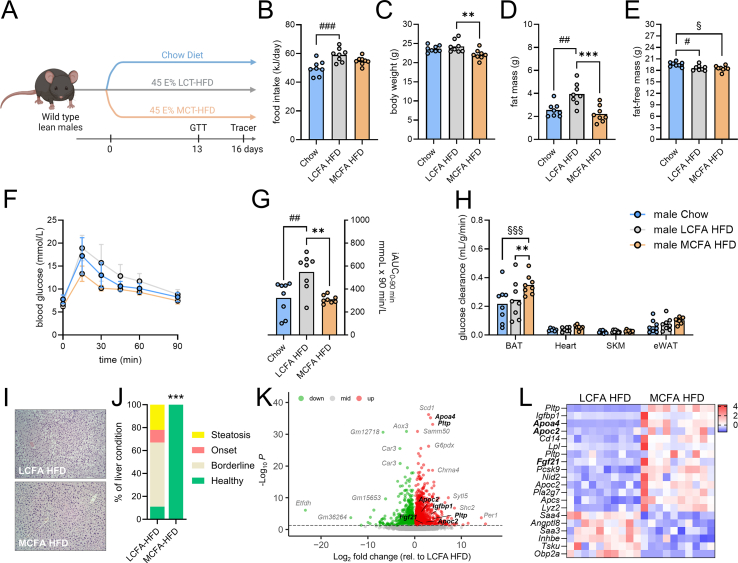
Figure 1.
Dietary MCFAs improve glycemia, prevent high-fat diet-associated steatosis and regulate hepatic genes associated with systemic lipid homeostasis in male mice. Study schematic (A) related to results presented in (B–H). Daily energy intake (B), body weight (C), fat mass (D), and fat-free mass (E) determined 16 days after putting mice on the indicated diets; glucose excursion curves (F) and the incremental area under the curve (iAUC) (G) based on an i.p. glucose tolerance test (2 g of glucose per kg body weight) conducted 13 days after putting mice on the indicated diets (B–G; n = 8). Glucose clearance (H) in indicated tissues determined 16 days after putting mice on the indicated diets (n = 6–8). H&E staining (I, representative of n = 9) and associated scoring of steatosis status (J, n = 9) in livers collected after 4 h of fasting from male C57BL/6J mice fed indicated diets for five weeks. Whole-genome sequencing of the same livers (as in I) with a volcano plot (K) depicting detected liver transcripts over the threshold of one fragment per kilobase million (FPKM) comparing LCFA HFD vs. MCFA HFD. Regulated (adjP < 0.05 and log2 FC > 1 or <−1) transcripts by MCFA HFD relative to LCFA HFD (L) of genes encoding for secreted factors (K, L; n = 10). Data (B–H) were analyzed by one-way ANOVA (B–E, G), or two-way ANOVA (H). Tukey (for B–H) post-hoc tests were conducted. Data in (j) were analyzed by a Chi-Square and Fisher's exact test. ∗∗p < 0.01, ∗∗∗p < 0.001 for differences between MCFA HFD and LCFA HFD; #p < 0.05, ##p < 0.01, ###p < 0.001 for differences between chow and LCFA HFD; §p < 0.05, §§§p < 0.001 for differences between chow and MCFA HFD.
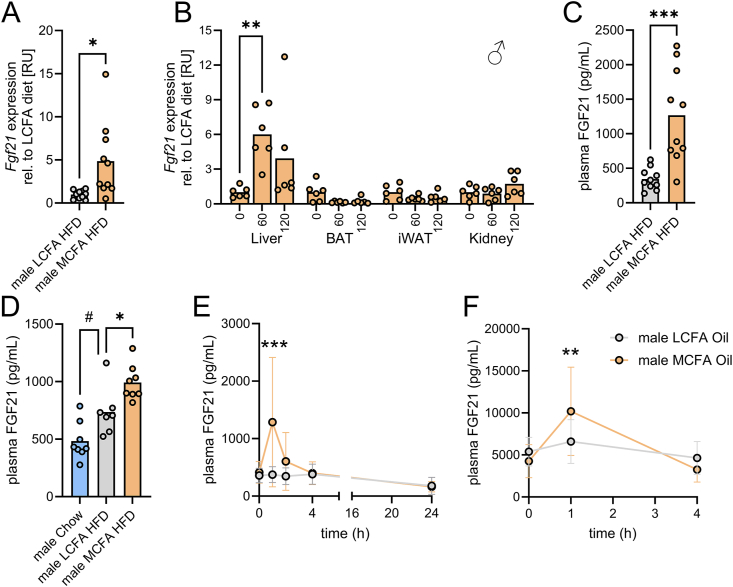
Figure 2.
Acute and chronic intake of MCFAs increase hepatic Fgf21 mRNA and circulating FGF21 in male mice. mRNA abundance of Fgf21 in liver from male mice fed indicated diets for five weeks (A; n = 10), or from mice in indicated organs that were orally gavaged at 0 min with 200 μL of C8:0 MCFA-rich oil (B; n = 6). Plasma FGF21 concentrations in mice fed indicated diets for five weeks (C; n = 10); fed indicated diets for 16 days (D; n = 7–8); in lean (E; n = 10) or in diet-induced obese mice (F; n = 10–14) orally gavaged at 0 min with 200 μL of C8:0 MCFA-rich oil or LCFA-rich oil (corn oil) as control. Relative expression of each sample was normalized to the housekeeping gene (Tbp in (A); Rpl13a in (B)), then relative units (RU) were calculated by normalizing each relative expression values to the mean of LCFA HFD (A) or 0 min (B). Data were analyzed by unpaired t test (A, C), one-way AVONA (D), or two-way ANOVA (B, E, F). Tukey (for B, D) and Bonferroni (for E–F) post-hoc tests were conducted. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 compared to LCFA HFD (or compared to 0 min (b)); #p < 0.05 for difference between chow and LCFA HFD.
2.5.2. LCFA HFD and MCFA HFD in WT and Fgf21 KO mice
2.5.2.1. Male mice
Age-matched male Fgf21 KO mice (10–20 weeks old) and WT littermates (10–20 weeks old) were single-housed and had ad libitum access to LCFA HFD or MCFA HFD for 37 days. Body weight and food intake were measured biweekly. Food intake measurement was discontinued after 30 days because of excessive food shredding. After four weeks, glucose tolerance was determined by an i.p. GTT initiated at 12 pm. At day 37, mice were fasted for 4 h from 8 am to 12 pm, before blood glucose was measured in mixed tail-blood at 12 pm. Then, mice were sacrificed by cervical decapitation, trunk blood was collected, and livers were quickly excised, snap-frozen in liquid nitrogen and then stored at −80 °C until further processing (liver TG measurement). This study relates to Figures 1I–L and 4.
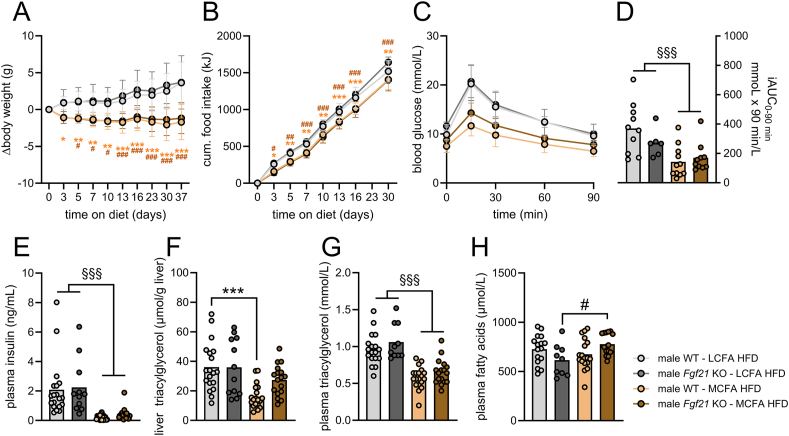
Figure 4.
FGF21 is required for the liver fat-lowering effects of dietary MCFAs in male mice. Male age-matched Fgf21 KO mice (10–20 weeks old) and WT littermates (10–20 weeks old) were fed indicated diets for five weeks and body weight (A) and food intake (B) were recorded at designated time points; glucose excursion curves (C) and the incremental area under these curves (iAUC) (D) determined during an i.p. glucose tolerance test (2 g of glucose per kg body weight) conducted four weeks after placing mice on the indicated diets (A–D; n = 6–11). Plasma insulin (E), liver triacylglycerol (F), plasma triacylglycerol (G), and plasma fatty acids (h) determined after a 4 h fast, five weeks after putting mice on indicated diets (E–H; n = 9–19). Data in (A, B) were analyzed by two-way repeated-measured ANOVA. Data in (D–H) were log 2-transformed before analysis by two-way ANOVA. Tukey (for A, B, F, H) post hoc tests were conducted. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 for differences between LCFA HFD and MCFA HFD within Fgf21 wildtype mice. #p < 0.05, ##p < 0.01, ###p < 0.001 for differences between LCFA HFD and MCFA HFD within Fgf21 KO mice. §§§p < 0.001 for main effect of diet.
2.5.2.2. Female mice
Age-matched female Fgf21 KO (15–25 weeks old) mice and WT littermates (15–25 weeks old) were group-housed (4–6 mice per cage) and had ad libitum access to LCFA HFD or MCFA HFD for 37 days. At day 37, body weight was measured. Then, mice were fasted for 4 h from 8 am to 12 pm, before blood glucose was measured in mixed tail-blood at 12 pm. Then, mice were sacrificed by cervical decapitation, trunk blood was collected, and livers were quickly excised, snap-frozen in liquid nitrogen and then stored at −80 °C until further processing (liver TG measurement). This study relates to Figure S3.
2.5.3. LCFA HFD and MCFA HFD in male WT and Crebh LiKO, and male flag-tagged Crebh KI mice
10 weeks old male Crebh LiKO mice and WT littermates as well as Crebh-FLAG Tag mice and WT littermates had ad libitum access to LCFA HFD or MCFA HFD for 5 weeks. After 5 weeks, mice were fasted for 4 h, sacrificed by cervical decapitation, and livers were quickly excised, snap-frozen in liquid nitrogen and then stored at −80 °C until further processing. All samples were collected at 10 am during the light period. This study relates to Figure 3.
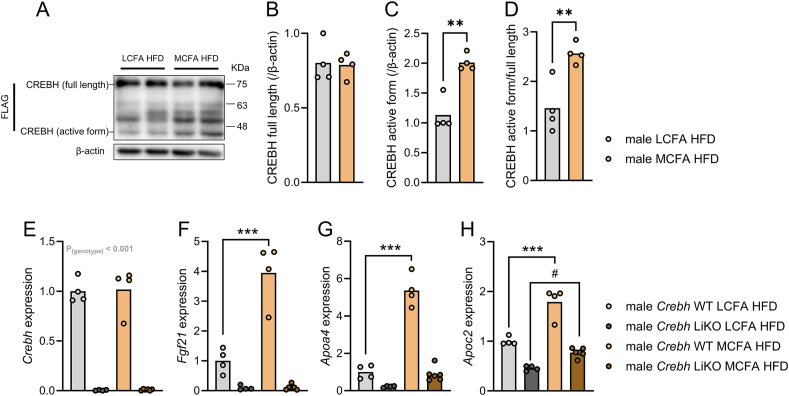
Figure 3.
MCFA-induced increase in hepatic Fgf21 mRNA requires CREBH in male mice. Representative image (A) and quantification of full length (B) and active CREBH (C) in livers from Crebh knockin male mice expressing flag-tagged CREBH fed indicated diets for five weeks (B–D; n = 4). mRNA abundance of the indicated genes (E–H) from liver of Crebh wildtype (WT) or liver-specific Crebh knockout (LiKO) mice fed indicated diets for five weeks (E–H; n = 4–6). Data were analyzed by unpaired t test (C, D); and two-way ANOVA (E–H). Tukey (for F–H) post hoc tests were conducted. ∗∗p < 0.01, ∗∗∗p < 0.001 for differences between LCFA HFD and MCFA HFD within Crebh knockin and Crebh WT mice. #p < 0.05 for differences between LCFA HFD and MCFA HFD within Crebh LiKO mice.
2.6. Oral gavage studies
2.6.1. 2-h time course in male chow-fed mice
Single-housed, 2-h fasted chow-fed male C57BL/6J mice (12 weeks of age) were randomized by body weight into different time point groups for tissue collection (0, 60, 120 min). All mice, except those sacrificed at time point 0, were orally administered with C8:0 MCFA-rich oil (glyceryl trioctanoate, #538-23-8, Sigma, Denmark) (10 μL/g body weight) at 10 am. Mice were decapitated and liver, interscapular brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), and kidney were harvested between 11 am and 1 pm, snap-frozen in liquid nitrogen and stored at −80 °C until Fgf21 mRNA analyses. This study relates to Figure 2B.
2.6.2. 24-h time course in male chow-fed mice
4-hour fasted male C57BL/6J mice (12–16 weeks) were orally gavaged with 200 μL of MCFA-rich or LCFA-rich oil at 12 pm. Blood was sampled from the tail-vein before and 1, 2, 4, and 24 h after administration. The animals were fasted during the first 4 h of the measurement, and were allowed access to chow food thereafter. This study relates to Figure 2E.
2.6.3. 4-h time course in male diet-induced obese mice
4-hour fasted male diet-induced obese C57BL/6J mice (aged 25–30 weeks) were orally gavaged with 300 μL of MCFA-rich or LCFA-rich oil at 12 pm. Blood was sampled from the tail-vein before and 1, 2, 4, and 24 h after administration. The mice were fasted during the first 4 h, and allowed access to chow food thereafter. This study relates to Figure 2F.
2.9. Pair feeding study
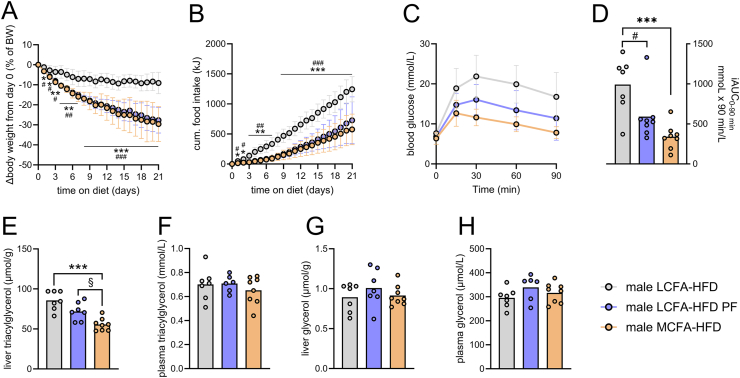
Diet-induced obese C57BL/6J mice (Taconic, DK) (35 weeks old) were single-housed and had either ad libitum access to LCFA HFD or MCFA HFD or were pair-fed LCFA HFD to the MCFA HFD-fed mice for 21 days. Body weight and food intake were measured daily. At day 16, glucose tolerance was determined by an i.p. GTT initiated at 12 pm. At day 21, mice were fasted for 4 h from 8 am to 12 pm, before blood glucose was measured in mixed tail-blood at 12 pm. Then, mice were sacrificed by cervical decapitation, trunk blood was collected, and livers were quickly excised, snap-frozen in liquid nitrogen and then stored at −80 °C until further processing (liver TG and glycerol measurement). This study relates to Figure 5.
Figure 5.
Improved glycemia and liver fat lowering by dietary MCFA are partly independent of body weight loss in male mice. Diet-induced obese C57BL/6j mice were fed indicated diets for 21 days with one group being fed pair-fed (PF) LCFA HFD (LCFA HFD PF) to the MCFA HFD group. Daily body weight (A) and food intake (B) were recorded; glucose excursion curves (C) and the incremental area under these curves (iAUC) (D) determined during an i.p. glucose tolerance test (1.5 g of glucose per kg body weight) conducted on day 16 (A–D; n = 7–8). Liver triacylglycerol (E), plasma triacylglycerol (F), liver glycerol (G) and plasma glycerol (H) determined after a 4 h fast, 21 days after putting mice on indicated diets (E–H; n = 6–8). Data in (A, B) were analyzed by two-way repeated-measured ANOVA. Data in (D–H) were analyzed by one-way ANOVA. Tukey (for A, B, D, E) post hoc tests were conducted. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 for differences between LCFA HFD and MCFA HFD. #p < 0.05, ##p < 0.01, and ###p < 0.001 for difference between LCFA HFD and LCFA HFD pair-fed. §p < 0.05 for difference between MCFA HFD and LCFA HFD pair-fed.
2.10. Glucose tolerance test (GTT)
GTT was commenced after a 4-h morning fast from 8 am to 12 pm with an i.p. injection of 1.5 or 2 g of d-glucose per kilogram body weight. Blood glucose was determined in mixed tail blood at 0, 15, 30, 60, and 90 or 120 min with a glucometer (Contour XT, Bayer, CH) from 12 pm to 2 pm.
2.11. Glucose-induced glucose clearance
Glucose-induced glucose clearance was evaluated as previously [20]. After 4 h of fasting from 8 am to 12 pm, 2 g/kg body weight d-glucose also containing 3H-2-DG (0.6 μCi/g body weight) were intraperitoneally injected. At 0, 10, 20, 30, and 40 min, glucose concentration was determined in mixed tail blood and 10 μL additional blood was collected into capillary tubes for blood [3H]-2DG appearance determination by scintillation counting. At 40 min, mice were sacrificed by cervical decapitation, and brown adipose tissue, heart, quadriceps muscle, and epididymal white adipose tissue were collected and snap-frozen for [3H]-2DG tissue uptake analysis. For this, 10–40 mg of each tissue was used to determine the accumulation of phosphorylated 3H-2-DG (3H-2-DG-6-P) by the precipitation method [21]. Glucose clearance was calculated by dividing the tissue 3H-2-DG-6-P DPM, accumulated in tissues over 40 min from tracer injection, by the average 3H-2-DG DPM in blood sampled during this period [21].
2.12. Tissue analyses
2.12.1. Liver TG
Liver TG content was measured as previously described [6]. Lipids were extracted from 25 mg liver tissue in 0.15 M sodium acetate with 25% Triton-X in ethanol, then homogenized for 1 min at a frequency of 30 Hz and subsequently heated for 3 min in a 97 °C water bath. After centrifugation at 9000g for 10 min, TG (triacylglycerol GPO-PAP kit, Roche Diagnostics, Mannheim, DE) and glycerol (Randox, Crumlin, UK) were measured colorimetrically on an autoanalyzer (Pentra C400 analyzer, Horiba, Japan) according to manufacturer's instructions.
2.13. Histology
Tissue samples were fixed in paraformaldehyde and embedded in paraffin. Five-micron sections were mounted on glass slides. The slides were subsequently dewaxed and stained with H&E. Samples were then re-dehydrated and mounted on coverslips with Pertex. The slides were examined in a Leitz Orthoplan microscope (Leitz Wetzlar, Germany), and pictures were obtained using DeltaPix camera with associated software (DeltaPix InSight) and 2.5x or 10x objectives. Liver steatosis was scored by 4 grades- 0: normal or mild; 1: moderate, little ballooning and proliferation; 2: steatosis, obvious ballooning and proliferation; and 3: severe steatosis, ongoing ballooning and proliferation.
2.14. RNA sequencing
The RNA sequencing was carried out by BGI Genomics laboratory in Hong Kong via nanoball sequencing technology. Raw 150 bp paired reads have been adapter trimmed and quality controlled. The filtered sequences resulted in ∼ 9 GB reads per sample. Subsequently, an additional quality control was assessed on the 9 GB filtered reads using FASTQC V0.12.1 and aligned to the mouse reference GRCm39.110 using STAR (V2.7.11a). The gene abundance was calculated by Stringtie (V2.2.1) and expressed by fragment per kilobase of exon per million (FPKM). Differentially gene expression was calculated using DESeq2 (V1.34.0). The heatmap of the genes encoding for secreted factors was based on z-scores.
2.15. Gene expression
RNA from tissues was isolated by Trizol-Chloroform (peqGOLD TriFastTM, 30-2010P, VWR) extraction from homogenized tissues and precipitation with Isopropanol. Genomic DNA was removed (DNAse I, EN0521, Fisher Scientific; RiboLock RNAse Inhibitor, EO0382, Fisher Scientific) and 1 μg RNA was transcribed into cDNA using LunaScript RT Super Mix (E3010L, New England Biolabs). Afterwards gene expression was quantified by qPCR using SYBR Green (Luna Universal qPCR Master Mix, M3003E, New England Biolabs) on a VIA7 Real Time PCR System (4453534, Thermo Fischer Scientific). Primer efficiency was determined with an experiment-specific standard curve and target gene expression was normalized to the expression levels of appropriate housekeeping genes glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The Primer sequences were as follows: Gapdh: forward 5′-AGTTCAACGGCACAGTCAAG-3′, reverse 5′- TACTCAGCACCAGCATCACC-3′, Crebh: forward 5′-CCTGTTTGATCGGCAGGAC-3′, reverse 5′-CGGGGGACGATAATGGAGA-3′, Fgf21: forward 5′-AGATCAGGGAGGATGGAACA-3′, reverse 5′-TCAAAGTGAGGCGATCCATA-3′, Apoa4: forward 5′-TTACCCAGCTAAGCAACAATGC-3′, reverse 5′-GAGGGTACTGAGCTGCTGAGTGA-3′, Apoc2: forward 5′-CCAAGGAGCTTGCCAAAGAC-3′, reverse 5′-TGCCTGCGTAAGTGCTCATG-3′, Tbp: forward 5′-AGAACAATCCAGACTAGCAGCA-3′, reverse 5′-GGGAACTTCACATCACAGCTC-3′.
Rpl13a: forward 5′- GGAGGGGCAGGTTCTGGTAT-3′, reverse 5′-TGTTGATGCCTTCACAGCGT-3′. Hprt: forward 5′-CTCATGGACTGATTATGGACAGGAC-3′, reverse 5′-GCAGGTCAGCAAAGAACTTATAGCC-3′.
2.16. Western blotting
Proteins were extracted from mice liver using lysis buffer (10 mM Tris–HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4PO7, 2 mM Na3VO4, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 10% glycerol) supplemented with protease inhibitors (leupeptin, aprotinin, PMSF). Aliquots of the proteins were separated by SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were incubated with the following antibodies diluted in 5% skim milk: FLAG antibodies (Sigma Aldrich, F3165, 1:1,000) and β-actin antibodies (Santa Cruz Biotechnology, sc-47778, 1:500) as control. Bound antibodies were visualized with peroxidase-conjugated anti-mouse IgG (Cell Signaling Technology, 7076, 1:2,000) and HRP luminescent substrate (FJIFILM Wako, 291-72401), then detected using a ChemiDoc XRS+ system (Bio-Rad). The intensity of immunoreactive bands was quantified using Image Lab software (Bio-Rad).
2.17. Blood analyses
Plasma insulin concentrations were measured by ELISA (Cat. 80-INSMSU-E10, Alpco, US). The concentrations of plasma FA (NEFA C kit; Wako Chemicals, Denmark), glycerol (Randox, Crumlin, UK), and TG (GPO-PAP kit; Roche Diagnostics, Denmark) were measured colorimetrically on an autoanalyzer (Pentra C400 analyzer, Horiba, Japan). Plasma cholesterol was determined using the Cholesterol liquicolor Kit (Human Gesellschaft für Biochemica und Diagnostica mbH, catalog no. 10017) following the manufacturer's instructions. Plasma FGF21 concentration was measured by Quantikine ELISA Mouse FGF21 immunoassay (Catalog # MF2100, R&D Systems, Denmark) following the manufacturer's instruction. Plasma β-Hydroxybutyrate (β-HB) measurement was performed using β-Hydroxybutyrate (Ketone body) Colorimetric Assay Kit (Cayman Chemical 700190) following the manufacturer's protocol.
2.18. Statistics
All time course data are present as means ± SD, and all bar graphs are present as means with individual data points. The statistical analyses performed are described in each figure legend. Post-hoc testing was conducted when one-way ANOVA revealed an effect (p < 0.05) and when two-way ANOVA revealed a trend (p < 0.1); statistical significance was defined as p < 0.05. Statistical analyses were performed in GraphPad PRISM 10 (GraphPad, CA, US).
3. Results
3.1. Dietary MCFAs prevent high-fat diet-associated steatosis and regulate genes associated with systemic lipid homeostasis
To explore the metabolic effects of dietary MCFA intake and compare it with standard “healthy” and high fat diets, we fed mice a chow diet, a 45 E% LCFA-rich high-fat diet (HFD), or a 45 E% MCFA-rich HFD (hereafter referred to as LCFA HFD or MCFA HFD) for 16 days (Figure 1A). The MCFA HFD had the same energy content (kJ per gram) as the LCFA HFD (Supplemental Table 2). Compared to LCFA HFD, mice fed MCFA HFD had similar energy intake but lower body weight due to a lower amount of fat mass, while the amount of fat-free mass was similar between LCFA HFD and MCFA HFD (Figure 1B–E). Compared to chow-fed mice, mice fed the MCFA HFD had similar energy intake, similar body weight, less fat-free mass and similar fat mass (Figure 1B–E). Following injection with glucose, MCFA HFD mice exhibited the lowest glucose excursion and overall glucose handling of MCFA HFD mice was similar to chow-fed mice but superior to LCFA HFD-fed mice (Figure 1F–G). MCFA HFD mice had increased glucose clearance into brown adipose tissue compared to both chow and LCFA HFD mice (Figure 1H). When we fed mice MCFA HFD or LCFA HFD for five weeks, we observed no detrimental effects of augmented MCFA intake on liver morphology (Figure 1I). Instead, MCFA HFD prevented the onset of steatosis that was observed in mice fed LCFA HFD (Figure 1J).
Since MCFAs are known to be absorbed from the intestine into the portal vein and first pass through the liver, we investigated the effects of dietary MCFAs on hepatic gene expression, using whole genome transcriptome analysis. We found 2363 transcripts being differentially expressed (adjP < 0.05) between the MCFA HFD and LCFA HFD groups (Figure 1K). We identified 20 transcripts regulated by MCFA HFD (adjP < 0.05 and log2 FC > 1 or <−1) mapping to 18 genes that encode for secretory factors (Figure 1L). These included Apoc2, Apoa4, and Fgf21, which are key regulators of liver and systemic lipid homeostasis [22].
3.2. Acute and chronic intake of MCFAs increase hepatic Fgf21 mRNA and circulating FGF21
Recent evidence indicates that FGF21 holds clinical promise to become a therapeutic option to treat excessive fat accumulation in the liver [23]. By targeted qPCR we could confirm that five weeks of MCFA HFD increased hepatic Fgf21 mRNA (Figure 2A). A single bolus of MCFA-rich oil augmented Fgf21 mRNA in liver 6-fold, without effects in brown and white adipose tissues and in kidney (Figure 2B). Consistent with the effects on mRNA, circulating FGF21 was 4-fold higher in mice fed MCFA HFD compared to LCFA HFD (Figure 2C). In mice subjected to either a standard carbohydrate-rich chow diet, an MCFA HFD, or an LCFA HFD for 16 days, circulating FGF21 was highest in MCFA HFD mice (Figure 2D). A single oral administration of MCFA-rich oil increased circulating FGF21 by 3-fold after 1 h and FGF21 levels returned to baseline levels by 4 h (Figure 2E). In contrast, circulating FGF21 was unaffected over 24 h after oral administration of LCFA-rich oil (Figure 2E). In diet-induced obese (DIO) mice, oral administration of MCFA-rich oil increased FGF21 levels by 2.5-fold to peak levels of 10 ng/mL after 1 h (Figure 2F), with levels back to baseline after 4 h. As in lean chow-fed mice, LCFA-rich oil had no effect on circulating FGF21 in DIO mice. Overall, MCFA intake was associated with increased circulating FGF21 levels, both acutely and following longer-term exposure.
3.3. MCFA-induced increases in hepatic Fgf21 mRNA require CREBH
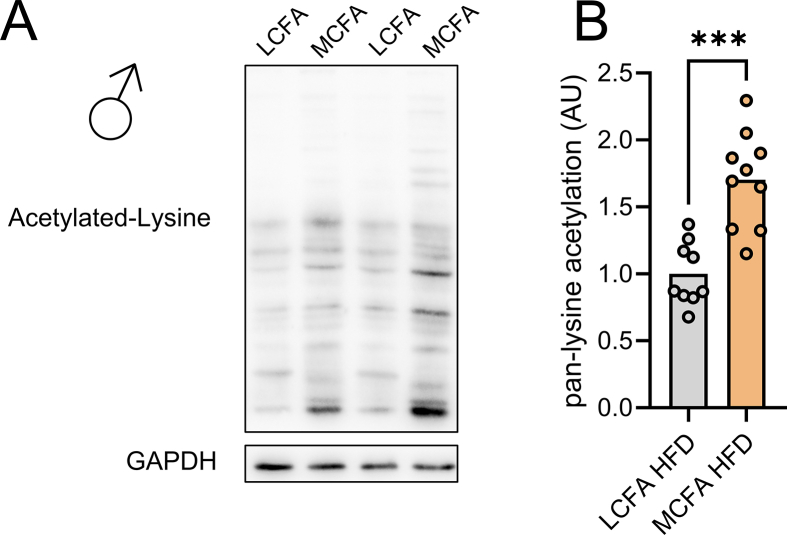
The simultaneous increase of Apoc2, Apoa4, and Fgf21 mRNA in livers from mice fed MCFA HFD (Figure 1L) indicated increased activity of cyclic adenosine monophosphate (cAMP)-responsive element-binding protein H (CREBH), a liver-enriched transcription factor that interacts with peroxisome proliferator-activated receptor α (PPARα) to regulate FGF21 levels [24,25]. Consistent with this notion, we found a higher abundance of the active form of CREBH in livers of MCFA HFD-fed mice (Figure 3A–D). This was accompanied by increased MCFA-associated global protein acetylation in liver (Figure S1). Moreover, while MCFA HFD increased hepatic Fgf21 mRNA in WT mice, MCFA HFD failed to induce Fgf21 expression in mice lacking hepatic Crebh (Figure 3E,F). Apoa4 mRNA levels also increased with MCFA HFD in WT, but not in Crebh LiKO mice (Figure 3G). Hepatic Apoc2 mRNA levels were overall lower in the absence of Crebh, and increased with MCFA HFD both in WT and Crebh LiKO mice, however, to a lesser extent in mice lacking Crebh (Figure 3H).
3.4. FGF21 is required for the lowering of hepatic fat content by dietary MCFAs
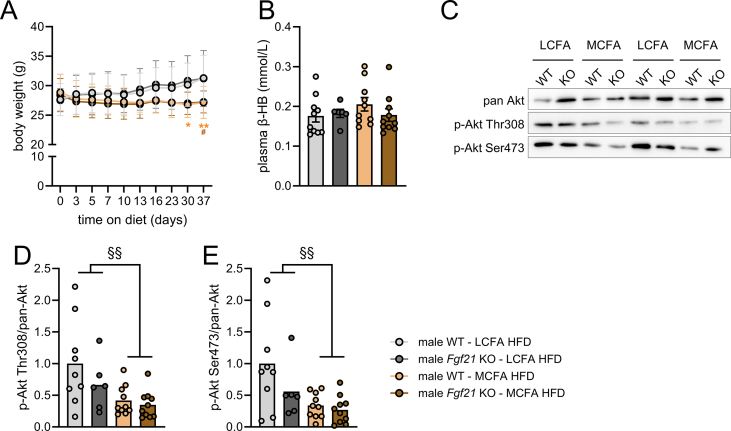
FGF21 and its analogs have been shown to lower hepatic and circulating lipid levels across species [26,27]. Therefore, we investigated whether FGF21 is required for MCFA-associated metabolic benefits in liver while also assessing endpoints related to energy balance and glycemia. We fed Fgf21 wildtype (WT) and Fgf21 knockout (KO) mice MCFA HFD or LCFA HFD for 5 weeks. MCFA HFD did not result in weight gain that was observed in mice fed LCFA HFD (Figures 4A, S2A). Energy intake was lower in mice during the first days in MCFA HFD, but then progressed at a similar rate (Figure 4B). The divergence in body weight development and the transient lowering of energy intake with MCFA HFD occurred to the same extent in Fgf21 WT and Fgf21 KO mice. MCFA HFD diet improved glucose tolerance to largely similar extents in male Fgf21 WT and Fgf21 KO mice (Figure 4C–D). Fasting plasma insulin levels (Figure 4E) were ∼80% lower in MCFA HFD-fed Fgf21 WT and Fgf21 KO mice. Hepatic Akt phosphorylation at Thr308 and Ser473 was lower in MCFA HFD mice with no effect of genotype (Figure S2C–E). Overall, dietary intake of MCFA lowers energy intake, body weight and improves glycemic control in male mice, but FGF21 is not required for these effects.
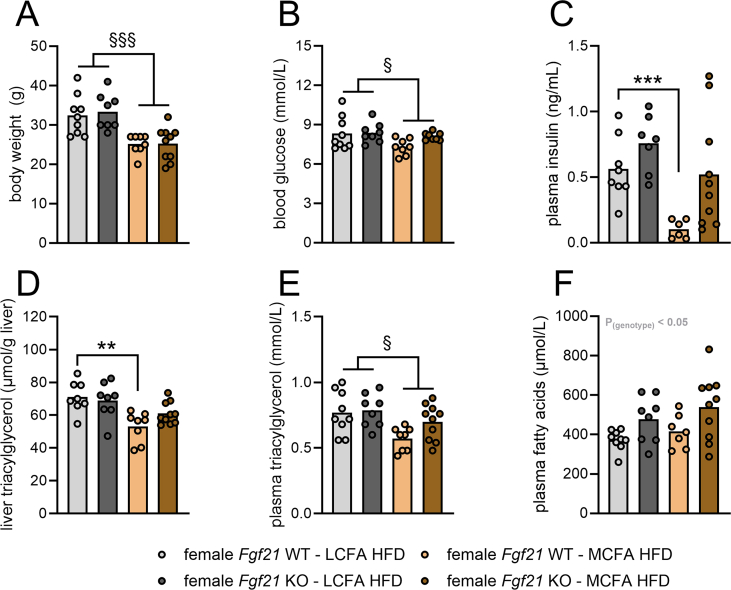
In male Fgf21 WT mice, liver TG content was approximately 55% lower with MCFA HFD compared to LCFA HFD (Figure 4F). In contrast, in Fgf21 KO mice, liver TG content was largely similar between MCFA HFD- and LCFA HFD-fed Fgf21 KO mice (Figure 4F). Plasma TG levels were also lower with MCFA feeding, but this reduction occurred in both Fgf21 WT and Fgf21 KO mice (Figure 4G). Plasma FA concentrations were similar between LCFA-HFD and MCFA-HFD in Fgf21 WT mice, but in Fgf21 KO mice, MCFA HFD increased circulating FAs by approximately 25% (Figure 4H). Plasma levels of the ketone body, beta-hydroxybutyrate, were overall unchanged (Figure S2B). Regarding the effects on liver TG content, a similar result was observed in female mice: MCFA HFD lowered liver TG content in female Fgf21 WT mice but failed to do so statistically in female mice lacking Fgf21 (Figure S3). Interestingly, while MCFA HFD attenuated plasma insulin levels in female WT mice, it failed to do so in female Fgf21 KO mice (Figure S3), indicating that FGF21 is required for dietary MCFAs to lower insulin in female mice. Overall, these data demonstrate that FGF21 is required for dietary MCFAs to lower liver lipid content in both male and female mice.
3.5. Dietary MCFAs reduce liver TG independent of weight loss
MCFA HFD results in lower energy intake and decreased body weight compared to LCFA HFD and this likely contributes to some of the metabolic benefits. To investigate food intake/body weight-dependent effects on liver TG we performed a pair-feeding study in diet-induced obese mice fed either LCFA HFD, MCFA HFD, or LCFA HFD that was pair-fed to the MCFA HFD mice (LCFA HFD PF). Compared to LCFA HFD mice, both MCFA HFD and LCFA HFD PF resulted in 25% weight loss over 22 days (Figure 5A), and this was accompanied by lower energy intake (Figure 5B). Glucose tolerance was to similar extent superior after MCFA HFD and LCFA HFD PF compared to LCFA HFD (Figure 5C,D). MCFA HFD-fed mice had lower liver TG compared to LCFA HFD but also compared to LCFA HFD PF-fed mice (Figure 5E). Liver TG tended to be lower in LCFA-HFD PF group (p = 0.058) compared to the LCFA HFD group (Figure 5E). Liver glycerol, plasma TG, and plasma glycerol levels were similar among the diet groups (Figure 5F–H). These data indicate that the reduction in liver TG by dietary MCFAs is not solely due to weight loss.
4. Discussion
We demonstrated that dietary MCFAs enhance glycemic control and improve glucose clearance into brown adipose tissue. We observed a beneficial effect on liver lipid accumulation that is largely independent of changes in body weight. We identified that the CREBH-FGF21 axis likely facilitates MCFA-induced prevention of hepatic fat accumulation.
Our whole transcriptome analysis of livers from mice fed a diet rich in MCFAs versus a macronutrient-matched typical moderate high-fat diet primarily composed of LCFAs, serves as a resource for identifying MCFA-specific effects. Although our study investigates the role of FGF21 in the metabolic benefits of MCFAs, other secreted factors might be of interest. Phospholipid transfer protein (PLTP), for example, exchanges phospholipids among lipoproteins in the bloodstream [28,29], but has also been shown to improve glucose tolerance, insulin sensitivity, and energy expenditure in mice [30], indicating its role in metabolic processes beyond lipoprotein metabolism. Apolipoprotein A4 (ApoA4), a key player in lipid metabolism through promoting HDL formation and cholesterol excretion [31], has also been associated with satiety [32] and reduced hepatic triacylglycerols [33] in mice. In humans, circulating Apoa4 correlates with improved metabolic health [34,35]. In contrast, ApoA4 deficiency in mice results in glucose intolerance [36] and insulin resistance [37], underscoring its importance in metabolic regulation and disease prevention. Given the significant induction of both Pltp and Apoa4 by MCFA intake, exploring their roles in the diverse metabolic benefits associated with MCFAs seems relevant.
We showed that dietary intake of MCFAs lowered liver TG content in a manner dependent on FGF21. This is consistent with previous research demonstrating that FGF21 can reduce hepatic lipid levels [26,38]. Furthermore, FGF21 has been shown to decrease hepatic steatosis by increasing FA oxidation and decreasing lipogenesis in rodents [39,40], and to improve hepatic health in obese mice [27,41]. Interestingly, we observed that while the MCFA HFD-induced lowering of liver lipid levels depended on FGF21, the reduction in circulating TG levels did not. Dietary FGF21-mediated lowering of hepatic lipid content has been suggested to be due to reduced liver de novo lipogenesis and increased VLDL-TG secretion [42], whereas pharmacological doses of recombinant FGF21 also lower circulating TG levels with an additional effect on white and brown adipose tissue TG clearance [43]. Together, these findings suggest that different mechanisms may be at play in the regulation of FGF21-mediated lowering of circulating and liver lipid accumulation under physiological versus supra-physiological doses of recombinant FGF21 protein or its analogs. Notably, MCFA-induced plasma insulin attenuation was only prevented by deleting Fgf21 in female mice, highlighting sex-specific differences in the regulation underpinning the glycemic benefit of MCFA intake.
The present findings highlight the ability of MCFAs to reduce liver TG content, consistent with observations in obese rats with metabolic associated fatty liver disease, where MCFAs prevented liver pathology and decreased lipid accumulation [44]. However, contrasting studies suggest that including MCFAs in diet may actually increase liver fat [45,46]. Clarification is needed regarding whether these discrepancies relate to the amount relative to other FA or type(s) of MCFA(s) included in the diet. Importantly, clinical studies in humans have shown that fat-rich diets effectively reduce liver TG accumulation in patients with type 2 diabetes [[47], [48], [49]] and markers of de novo lipogenesis and liver health in healthy individuals [6]. Future studies investigating whether the inclusion of MCFAs in high-fat diets could help treat fatty liver disease are warranted.
Our study found that MCFA intake upregulates Fgf21 expression and other CREBH targets. We demonstrated the dependence of Fgf21 induction on CREBH. The mechanism by which MCFAs activate the CREBH-FGF21 axis remains unclear. Previous research suggests that CREBH can induce Fgf21 expression through synergistic interaction with PPARα [[50], [51], [52]], and CREBH acetylation is required for CREBH-PPARα interaction in order to induce Fgf21 [53]. Our result indicates that dietary MCFAs enhance global protein acetylation, likely due to excess acetyl-CoA production as evident from the well-known ketogenic potential of MCFAs [12]; and it should be investigated whether MCFA-mediated induction of Fgf21 requires acetylation of CREBH.
Our study has several limitations. We used a diet with relatively high enrichment of MCFA, and it remains to be determined whether less MCFA will still produce hepatic TG-lowering effects and if these effects are FGF21-dependent. Interestingly, both our previous research and that of others have shown that small amounts of MCFAs in the diet can improve insulin sensitivity in humans [14] and reduce body weight in rodents [54]. Another limitation is the overreliance on male mice in our experiments and we cannot completely rule out that all shown effects are evident in both sexes. However, key experiments investigating the influence of FGF21 on liver TG levels were conducted in both male and female mice.
In conclusion, our data suggest that dietary MCFAs have chronic metabolic benefits, including improved glycemic control and lipid homeostasis, particularly in preventing liver fat accumulation associated with high-fat feeding. We provide evidence that these effects are mediated through a CREBH-FGF21 axis.
CRediT authorship contribution statement
Ye Cao: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Masaya Araki: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Yoshimi Nakagawa: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Luisa Deisen: Investigation, Formal analysis. Annemarie Lundsgaard: Writing – review & editing, Investigation. Josephine M. Kanta: Writing – review & editing, Investigation. Stephanie Holm: Investigation. Kornelia Johann: Investigation. Jens Christian Brings Jacobsen: Investigation. Markus Jähnert: Formal analysis, Data curation. Annette Schürmann: Writing – review & editing, Supervision, Resources. Bente Kiens: Writing – review & editing, Supervision, Resources, Conceptualization. Christoffer Clemmensen: Writing – review & editing, Supervision, Resources, Funding acquisition. Hitoshi Shimano: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization. Andreas M. Fritzen: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition, Formal analysis, Conceptualization. Maximilian Kleinert: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: C.C. is co-founder of Ousia Pharma ApS, a biotech company developing therapeutics for obesity. C.C. is also on the editorial board of Molecular Metabolism. The remaining authors declare no competing interests.
Acknowledgment
We thank Irene Bech Nielsen, Betina Bolmgren, Charlotte Sashi Aier Svendsen, Annemette Overgaard Brethvad, Finnja Koppel, and Nele Jeschinowski for skilled technical assistance. We thank Kerstin Stemmer and Nobuyuki Itoh for providing the Fgf21 knockout mice. This study was supported by the Novo Nordisk Foundation (grant# NNF20OC0063744), Arla Food for Health, the Danish Dairy Research Foundation. Y.N. was supported by Kobayashi Foundation and Firstbank of Toyama Scholarship Foundation Program. J.M.K. was supported by a research grant from the Danish Cardiovascular Academy, which is funded by the Novo Nordisk Foundation, grant # NNF20SA0067242, and the Danish Heart Foundation. S.H., A-M.L. and A.M.F. were funded by the Danish Diabetes Academy, funded by the Novo Nordisk Foundation (grant# NNF17SA0031406). A.M.F. was also funded directly by the Novo Nordisk Foundation (grant# NNF22OC0074110). B.K. was funded by Mejeribrugets Forskningsfond Denmark. M.J. and A.S. were supported by the German Ministry of Education and Research (BMBF: DZD grant 82DZD03D03) and the Brandenburg State. C.C. is supported by the Novo Nordisk Foundation (Grant number NNF22OC0073778). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center, based at the University of Copenhagen, Denmark, and partially funded by an unconditional donation from the Novo Nordisk Foundation (www.cbmr.ku.dk) (Grant numbers NNF18CC0034900 and NNF23SA0084103). M.K. was supported by the Deutsche Forschungsgemeinschaft (DFG; KL 3285/5-1), the German Center for Diabetes Research (DZD; 82DZD03D03 and 82DZD03D1Y), the Novo Nordisk Foundation (NNF; NNF19OC0055192) and the Deutsche Diabetes Gesellschaft (DDG).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2024.101991.
Contributor Information
Hitoshi Shimano, Email: hshimano@md.tsukuba.ac.jp.
Andreas M. Fritzen, Email: amfritzen@sund.ku.dk.
Maximilian Kleinert, Email: maximilian.kleinert@dife.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Figure S1.
Dietary MCFAs stimulate lysine acetylation in the liver. Representative western blot (A) and quantification (B) of pan-lysine acetylation in livers from mice fed indicated diets for five weeks. Data were analyzed by unpaired t test. ∗∗∗p < 0.001 for differences between LCFA HFD and MCFA HFD.
Figure S2.
Dietary MCFAs in male wildtype or Fgf21 knockout mice. See Figure 4 for more details. Body weights of mice at indicated time points (A; n = 6–11). Plasma β-Hydroxybutyrate (B) from blood collected after a 4 h fast, five weeks after putting mice on indicated diets (n = 6–10). Representative western blot (C) and quantification (D, E) of indicated phosphorylation sites on Akt in livers from mice collected after a 4 h fast, five weeks after putting mice on indicated diets (n = 6–10). Data were analyzed by repeated measures (A) or regular (B, D, E) two-way ANOVA, and a Tukey post-hoc test was performed for (A). ∗p < 0.05 and ∗∗p < 0.01 for differences between LCFA HFD and MCFA HFD within Fgf21 wildtype mice. #p < 0.05 for differences between LCFA HFD and MCFA HFD within Fgf21 KO mice. §§p < 0.01 for main effect of diet.
Figure S3.
FGF21 is required for the full liver fat-lowering effects of dietary MCFAs in female mice. Female Fgf21 wildtype or Fgf21 knockout (KO) mice were fed indicated diets for five weeks. Body weight (A; n = 8–10) after five weeks is shown, as well as blood glucose (B; n = 8–9), plasma insulin (C; n = 6–10), liver triacylglycerol (D; n = 8–10), plasma triacylglycerol (E; n = 8–10), and plasma fatty acids (F; n = 7–10) determined after a 4 h fast at the end of the study five weeks after putting mice on the indicated diets. Data were log 2-transformed before analysis by two-way ANOVA. Tukey (for C, D) post hoc tests were conducted. ∗∗p < 0.01, ∗∗∗p < 0.001 for differences between LCFA HFD and MCFA HFD within Fgf21 wildtype mice. §p < 0.05, §§§p < 0.001 for main effects of diet.
Data availability
Data will be made available on request.
References
- 1.Warensjo E., Riserus U., Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48(10):1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 2.Forsythe C.E., Phinney S.D., Fernandez M.L., Quann E.E., Wood R.J., Bibus D.M., et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43(1):65–77. doi: 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- 3.Hyde P.N., Sapper T.N., Crabtree C.D., LaFountain R.A., Bowling M.L., Buga A., et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King I.B., Lemaitre R.N., Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. Am J Clin Nutr. 2006;83(2):227–236. doi: 10.1093/ajcn/83.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Volk B.M., Kunces L.J., Freidenreich D.J., Kupchak B.R., Saenz C., Artistizabal J.C., et al. Effects of step-wise increases in dietary carbohydrate on circulating saturated Fatty acids and palmitoleic acid in adults with metabolic syndrome. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundsgaard A.M., Holm J.B., Sjoberg K.A., Bojsen-Moller K.N., Myrmel L.S., Fjaere E., et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019;29(1):50–63 e54. doi: 10.1016/j.cmet.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Astrup A., Bertram H.C., Bonjour J.P., de Groot L.C., de Oliveira Otto M.C., Feeney E.L., et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ. 2019;366:l4137. doi: 10.1136/bmj.l4137. [DOI] [PubMed] [Google Scholar]
- 8.Schonfeld P., Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57(6):943–954. doi: 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norgren J., Sindi S., Sandebring-Matton A., Kareholt I., Daniilidou M., Akenine U., et al. Ketosis after intake of coconut oil and caprylic acid-with and without glucose: a cross-over study in healthy older adults. Front Nutr. 2020;7:40. doi: 10.3389/fnut.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Wymelbeke V., Himaya A., Louis-Sylvestre J., Fantino M. Influence of medium-chain and long-chain triacylglycerols on the control of food intake in men. Am J Clin Nutr. 1998;68(2):226–234. doi: 10.1093/ajcn/68.2.226. [DOI] [PubMed] [Google Scholar]
- 11.Scalfi L., Coltorti A., Contaldo F. Postprandial thermogenesis in lean and obese subjects after meals supplemented with medium-chain and long-chain triglycerides. Am J Clin Nutr. 1991;53(5):1130–1133. doi: 10.1093/ajcn/53.5.1130. [DOI] [PubMed] [Google Scholar]
- 12.Kanta J.M., Deisen L., Johann K., Holm S., Lundsgaard A., Lund J., et al. Dietary medium-chain fatty acids reduce food intake via the GDF15-GFRAL axis in mice. Mol Metab. 2023;74 doi: 10.1016/j.molmet.2023.101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumme K., Stonehouse W. Effects of medium-chain triglycerides on weight loss and body composition: a meta-analysis of randomized controlled trials. J Acad Nutr Diet. 2015;115(2):249–263. doi: 10.1016/j.jand.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Lundsgaard A.M., Fritzen A.M., Sjoberg K.A., Kleinert M., Richter E.A., Kiens B. Small amounts of dietary medium-chain fatty acids protect against insulin resistance during caloric excess in humans. Diabetes. 2021;70(1):91–98. doi: 10.2337/db20-0582. [DOI] [PubMed] [Google Scholar]
- 15.Eckel R.H., Hanson A.S., Chen A.Y., Berman J.N., Yost T.J., Brass E.P. Dietary substitution of medium-chain triglycerides improves insulin-mediated glucose metabolism in NIDDM subjects. Diabetes. 1992;41(5):641–647. [PubMed] [Google Scholar]
- 16.Baba N., Bracco E.F., Hashim S.A. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am J Clin Nutr. 1982;35(4):678–682. doi: 10.1093/ajcn/35.4.678. [DOI] [PubMed] [Google Scholar]
- 17.Leveille G.A., Pardini R.S., Tillotson J.A. Influence of medium-chain triglycerides on lipid metabolism in the rat. Lipids. 1967;2(4):287–294. doi: 10.1007/BF02532113. [DOI] [PubMed] [Google Scholar]
- 18.Hotta Y., Nakamura H., Konishi M., Murata Y., Takagi H., Matsumura S., et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa Y., Oikawa F., Mizuno S., Ohno H., Yagishita Y., Satoh A., et al. Hyperlipidemia and hepatitis in liver-specific CREB3L3 knockout mice generated using a one-step CRISPR/Cas9 system. Sci Rep. 2016;6 doi: 10.1038/srep27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundsgaard A.M., Fritzen A.M., Nicolaisen T.S., Carl C.S., Sjoberg K.A., Raun S.H., et al. Glucometabolic consequences of acute and prolonged inhibition of fatty acid oxidation. J Lipid Res. 2020;61(1):10–19. doi: 10.1194/jlr.RA119000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller L.L.V., Raun S.H., Fritzen A.M., Sylow L. Measurement of skeletal muscle glucose uptake in mice in response to acute treadmill running. J Biol Methods. 2022;9(3) doi: 10.14440/jbm.2022.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa Y., Shimano H. CREBH regulates systemic glucose and lipid metabolism. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chui Z.S.W., Shen Q., Xu A. Current status and future perspectives of FGF21 analogues in clinical trials. Trends Endocrinol Metab. 2024;35(5):371–384. doi: 10.1016/j.tem.2024.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Satoh A., Han S.I., Araki M., Nakagawa Y., Ohno H., Mizunoe Y., et al. CREBH improves diet-induced obesity, insulin resistance, and metabolic disturbances by FGF21-dependent and FGF21-independent mechanisms. iScience. 2020;23(3) doi: 10.1016/j.isci.2020.100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Mendez R., Zheng Z., Chang L., Cai J., Zhang R., et al. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology. 2014;155(3):769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flippo K.H., Potthoff M.J. Metabolic messengers: FGF21. Nat Metab. 2021;3(3):309–317. doi: 10.1038/s42255-021-00354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaffin A.T., Larson K.R., Huang K.P., Wu C.T., Godoroja N., Fang Y., et al. FGF21 controls hepatic lipid metabolism via sex-dependent interorgan crosstalk. JCI Insight. 2022;7(19) doi: 10.1172/jci.insight.155848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albers J.J., Vuletic S., Cheung M.C. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim Biophys Acta. 2012;1821(3):345–357. doi: 10.1016/j.bbalip.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huuskonen J., Olkkonen V.M., Jauhiainen M., Ehnholm C. The impact of phospholipid transfer protein (PLTP) on HDL metabolism. Atherosclerosis. 2001;155(2):269–281. doi: 10.1016/s0021-9150(01)00447-6. [DOI] [PubMed] [Google Scholar]
- 30.Sponton C.H., Hosono T., Taura J., Jedrychowski M.P., Yoneshiro T., Wang Q., et al. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT-liver communication. EMBO Rep. 2020;21(9) doi: 10.15252/embr.201949828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng J., Li X.P. Apolipoprotein A-IV: a potential therapeutic target for atherosclerosis. Prostaglandins Other Lipid Mediat. 2018;139:87–92. doi: 10.1016/j.prostaglandins.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto K., Cardelli J.A., Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262(6 Pt 1):G1002–G1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 33.VerHague M.A., Cheng D., Weinberg R.B., Shelness G.S. Apolipoprotein A-IV expression in mouse liver enhances triglyceride secretion and reduces hepatic lipid content by promoting very low density lipoprotein particle expansion. Arterioscler Thromb Vasc Biol. 2013;33(11):2501–2508. doi: 10.1161/ATVBAHA.113.301948. [DOI] [PubMed] [Google Scholar]
- 34.Doumatey A.P., Zhou J., Zhou M., Prieto D., Rotimi C.N., Adeyemo A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: a proteomics study. Obesity. 2016;24(6):1257–1265. doi: 10.1002/oby.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Toerne C., Huth C., de Las Heras Gala T., Kronenberg F., Herder C., Koenig W., et al. MASP1, THBS1, GPLD1 and ApoA-IV are novel biomarkers associated with prediabetes: the KORA F4 study. Diabetologia. 2016;59(9):1882–1892. doi: 10.1007/s00125-016-4024-2. [DOI] [PubMed] [Google Scholar]
- 36.Wang F., Kohan A.B., Kindel T.L., Corbin K.L., Nunemaker C.S., Obici S., et al. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc Natl Acad Sci U S A. 2012;109(24):9641–9646. doi: 10.1073/pnas.1201433109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Xu M., Wang F., Kohan A.B., Haas M.K., Yang Q., et al. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. J Biol Chem. 2014;289(4):2396–2404. doi: 10.1074/jbc.M113.511766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mottillo E.P., Desjardins E.M., Fritzen A.M., Zou V.Z., Crane J.D., Yabut J.M., et al. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol Metab. 2017;6(6):471–481. doi: 10.1016/j.molmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C., Chen Y., et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149(12):6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 40.Lee J.H., Kang Y.E., Chang J.Y., Park K.C., Kim H.W., Kim J.T., et al. An engineered FGF21 variant, LY2405319, can prevent non-alcoholic steatohepatitis by enhancing hepatic mitochondrial function. Am J Transl Res. 2016;8(11):4750–4763. [PMC free article] [PubMed] [Google Scholar]
- 41.Keinicke H., Sun G., Mentzel C.M.J., Fredholm M., John L.M., Andersen B., et al. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr Connect. 2020;9(8):755–768. doi: 10.1530/EC-20-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher F.M., Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M., et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab. 2017;6(1):14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlein C., Talukdar S., Heine M., Fischer A.W., Krott L.M., Nilsson S.K., et al. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and Brown adipose tissues. Cell Metab. 2016;23(3):441–453. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Ronis M.J., Baumgardner J.N., Sharma N., Vantrease J., Ferguson M., Tong Y., et al. Medium chain triglycerides dose-dependently prevent liver pathology in a rat model of non-alcoholic fatty liver disease. Exp Biol Med. 2013;238(2):151–162. doi: 10.1258/ebm.2012.012303. [DOI] [PubMed] [Google Scholar]
- 45.Turner N., Hariharan K., TidAng J., Frangioudakis G., Beale S.M., Wright L.E., et al. Enhancement of muscle mitochondrial oxidative capacity and alterations in insulin action are lipid species dependent: potent tissue-specific effects of medium-chain fatty acids. Diabetes. 2009;58(11):2547–2554. doi: 10.2337/db09-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucci S., Primassin S., Ter Veld F., Spiekerkoetter U. Medium-chain triglycerides impair lipid metabolism and induce hepatic steatosis in very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. Mol Genet Metab. 2010;101(1):40–47. doi: 10.1016/j.ymgme.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Hansen C.D., Gram-Kampmann E.M., Hansen J.K., Hugger M.B., Madsen B.S., Jensen J.M., et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease : a randomized controlled trial. Ann Intern Med. 2023;176(1):10–21. doi: 10.7326/M22-1787. [DOI] [PubMed] [Google Scholar]
- 48.Skytte M.J., Samkani A., Petersen A.D., Thomsen M.N., Astrup A., Chabanova E., et al. A carbohydrate-reduced high-protein diet improves HbA(1c) and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62(11):2066–2078. doi: 10.1007/s00125-019-4956-4. [DOI] [PubMed] [Google Scholar]
- 49.Thomsen M.N., Skytte M.J., Samkani A., Carl M.H., Weber P., Astrup A., et al. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia. 2022;65(3):506–517. doi: 10.1007/s00125-021-05628-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa Y., Satoh A., Yabe S., Furusawa M., Tokushige N., Tezuka H., et al. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology. 2014;155(12):4706–4719. doi: 10.1210/en.2014-1113. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa Y., Satoh A., Tezuka H., Han S.I., Takei K., Iwasaki H., et al. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARalpha. Sci Rep. 2016;6 doi: 10.1038/srep39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruppert P.M.M., Park J.G., Xu X., Hur K.Y., Lee A.H., Kersten S. Transcriptional profiling of PPARalpha-/- and CREB3L3-/- livers reveals disparate regulation of hepatoproliferative and metabolic functions of PPARalpha. BMC Genom. 2019;20(1):199. doi: 10.1186/s12864-019-5563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H., Mendez R., Chen X., Fang D., Zhang K. Lysine acetylation of CREBH regulates fasting-induced hepatic lipid metabolism. Mol Cell Biol. 2015;35(24):4121–4134. doi: 10.1128/MCB.00665-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou S., Wang Y., Jacoby J.J., Jiang Y., Zhang Y., Yu L.L. Effects of medium- and long-chain triacylglycerols on lipid metabolism and gut microbiota composition in C57BL/6J mice. J Agric Food Chem. 2017;65(31):6599–6607. doi: 10.1021/acs.jafc.7b01803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.