Abstract
The effects of separately coinoculating Lactiplantibacillus plantarum S8 (LP) with Staphylococcus carnosus L8 (LP + SC), Pichia kudriavzevii M6 (LP + PK), and S. carnosus L8 and P. kudriavzevii M6 (LP + SC + PK) on the flavor characteristics and biogenic amines (BAs) production in Harbin dry sausages were investigated. The coinoculated sausages exhibited higher free amino acids (FAAs) content than the noninoculated and LP sausages. Moreover, inoculated dry sausages exhibited lower BA contents (174.45, 239.43, 190.24, and 206.7 mg/kg for the LP, LP + SC, LP + PK, and LP + PK + SC sausages, respectively) than the noninoculated sausage (339.73 mg/kg). Meanwhile, the LP + PK and LP + SC + PK sausages had the highest contents of esters (996.70 μg/kg) and alcohols (603.46 μg/kg), respectively. A sensory evaluation demonstrated that the LP + SC + PK sausage had the highest fermented odor and the lowest fatty odor. Pearson correlation analysis revealed that FAAs were correlated with most key volatile compounds and BAs. This study provides new insights into flavor development and BA inhibition in dry sausages through coinoculation.
Keywords: Harbin dry sausage, Free amino acid, Volatile compound, Biogenic amine, Sensory quality, Correlation analysis
Highlights
-
•
Coinoculation of autochthonous starters promoted flavor and inhibited biogenic amine.
-
•
Inoculation of L. plantarum reduced the histamine content.
-
•
Coinoculation of L. plantarum and P. kudriavzevii promoted alcohol formation.
-
•
L. plantarum, S. carnosus and P. kudriavzevii promoted ester formation.
-
•
L. plantarum, S. carnosus and P. kudriavzevii improved sensory characteristics.
1. Introduction
Harbin dry sausage, a traditional fermented meat product, is famous in Northeast China because of its unique flavor and texture. Dry sausages are typically prepared by mixing pork lean meat, pork backfat, salt, Chinese liquor, mixed spices, and nitrite, with the small intestine of pigs being used as casings; this is then fermented for 9–15 days under natural conditions (Chen, Kong, Han, Xia, & Xu, 2017). During spontaneous fermentation and ripening, carbohydrates, proteins, and lipids are decomposed into small molecular peptides, free amino acids (FAAs), and free fatty acids under metabolism by endogenous and microbial enzymes and microorganisms, which directly or indirectly affect the flavor formation of dry sausages (Hu et al., 2022). Particularly, volatile compounds based on proteolysis and amino acid metabolism are crucial in developing the characteristic flavor of fermented sausages. Certain microorganisms in fermented meat products can hydrolyze the proteins therein, generating various volatile flavor substances and improving the flavor of the product. However, the microbial diversity in dry sausages fermented under natural conditions cannot guarantee uniformity in product quality. In addition, some microorganisms with high decarboxylase activity can decarboxylate the FAAs of protein hydrolysate to produce biogenic amines (BAs) (Wang et al., 2022). BAs are biologically active and low-molecular-weight basic nitrogen-containing compounds (Wang et al., 2022). Although a few BAs improve the biological functions of humans and organisms, excessive intake can cause amine poisoning (Ye et al., 2021). The most toxic of the eight biogenic amines is histamine, and studies have found that healthy people should not consume >50 mg of histamine per person per meal (EFSA Panel on Biological Hazards (BIOHAZ), 2011).
Inoculating products with suitable starter cultures can promote color and flavor formation, prevent oxidative denaturation, inhibit the growth of harmful microorganisms, and reduce the BA accumulation, thereby improving their quality and safety (Ren, Deng, & Wang, 2022). Lactic acid bacteria (LAB) are commonly used as starter cultures. Lactiplantibacillus plantarum, for instance, can promote protein degradation to produce flavor substances (Chen, Liu, Sun, Kong, & Xiong, 2015). Additionally, they can produce bacteriocins to inhibit pathogens and prevent the formation of off-flavors and rancidity in dry fermented sausages during fermentation (Xiao, Liu, Chen, Xie, & Li, 2020). Lactobacillus sakei can promote protein degradation and decrease the tyramine content of dry sausages (Dong et al., 2022). Staphylococcus, such as Staphylococcus xylosus and Staphylococcus carnosus, are conducive to color improvement, unique flavor formation, and delayed rancidity in fermented sausages during fermentation (Hu et al., 2019; Zhao, Hu, & Chen, 2022). Yeasts, such as Debaryomyces hansenii and Pichia kudriavzevii, can promote protein and lipid degradation to produce flavor substances in dry sausage (Wen et al., 2023). In recent years, extensive research has focused on the role of functional starters in flavor development, but the concomitant accumulation of BAs has not received sufficient attention. Multi-strain coinoculation has become an effective approach for improving the uniformity and quality of fermented foods. Multi-strain coinoculation can make up for the monotony of single-strain fermentation, increase the diversity of products, enrich the flavor substances richer, and improve the safety of the product (Giello, Storia, Filippis, Ercolini, & Villani, 2018). Although the functions of these starter cultures are well-known, their synergetic contributions to the flavor characteristics and BA reduction in Harbin dry sausages have not been fully described.
Therefore, the effects of the coinoculation of autochthonous starter cultures, including L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6, isolated from Harbin dry sausages on the formation of volatile compounds and BAs were investigated in this study. Furthermore, correlations between FAAs and volatile compounds, as well as FAAs and BAs, were established. This study will provide valuable information for improving the safety and flavor of dry sausages.
2. Materials and methods
2.1. Preparation of starter cultures
L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 isolated from Harbin dry sausages were identified in previous studies as having good protein degradation ability and volatile flavor-producing capacity based on the dry sausages simulation system (data unpublished). In this study, these strains were used as autochthonous starter cultures, and L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 were cultured according to the methods of Hu, Tian, et al. (2022), Hu et al. (2019) and Wen et al. (2023), respectively. Briefly, the L. plantarum S8 was cultured twice in de Man Rogosa and Sharpe (MRS, Haibo Biotechnology Co., Qingdao, China) broth at 37 °C for 18 h; S. carnosus L8 was cultured twice in mannitol medium (Haibo Biotechnology Co., Qingdao, China) at 37 °C for 18 h; and P. kudriavzevii M6 was cultured twice in yeast extract peptone dextrose (YPD, Haibo Biotechnology Co., Qingdao, China) broth at 30 °C for 18 h. Subsequently, cells in the logarithmic phase were separately centrifuged at 6000 ×g for 15 min at 4 °C. Finally, the resuspended cell pellets were washed twice with sterile water. Before use, the cells were suspended in sterile water.
2.2. Preparation of dry sausages
Dry sausages were prepared according to our previous method (Li et al., 2024). Briefly, 7200 g pork lean meat and 800 g pork back fat were ground in a sieve plate with a diameter of 1.5 cm with the following ingredients: 200 g NaCl, 80 g dextrose, 0.72 g sodium nitrite, 24 g monosodium glutamate, 64 g mixed spices (mainly including Foeniculum vulgare Mill., Citrus reticulata Blanco, Amomum cardamon, Eugenia caryophyllata Thunb., Cinnamomum cassia Presl, Amomum villosum, and Angelica dahurica), and 80 g Daqu (traditional Chinese wine). Each strain concentration was adjusted by an OD600/stain count standard curve, ultimately ensuring that the stains concentration after inoculated suspension into raw meat was 7 log CFU/g.
Three independent batches of dry sausages were prepared using the same raw meat, and five treatments were prepared for each batch. A control treatment was noninoculated with the starter cultures, and the other four treatments were inoculated with single or mixed strains: (i) a noninoculated treatment (Control); (ii) single inoculation of L. plantarum S8 (LP); (iii) coinoculation of L. plantarum S8 and S. carnosus L8 (LP + SC); (iv) coinoculation of L. plantarum S8 and P. kudriavzevii M6 (LP + PK); (v) coinoculation of L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 (LP + SC + PK). Subsequently, meat batter was thoroughly mixed in a filling pot (Yiwu Zhanzhi daily necessities Co., Zhejiang, China) and stuffed into the small intestine of the porcine casings using a sausage stuffer (Yongkang Ayton plastic products Co., Zhejiang, Chian). Each sausage was approximately 2.5 cm in diameter and 20 cm in length. All dry sausages were placed in a well-ventilated chamber (25 ± 2 °C) for 24 h then transferred to an incubator (Climatic Chamber FITOCLIMA 600PH, Baiguan Scientific Instrument Co., Shanghai, Chian) at 25 °C (65–70% humidity) for fermentation for nine days. The physicochemical properties and microbial counts, were determined on days 0, 3, 6, and 9. The contents of FAA, BA, and volatile compounds as well as sensory attributes were measured on day 9.
2.3. Analysis of physical properties and microbial counts
The moisture content of dry sausages was determined according to AOAC (AOAC, 1995). The water activity (aw) of dry sausages was measured using a water activity meter (AquaLab 4TE Duo, Decagon Devices, Pullman, Washington, USA) following the method described by Hu et al. (2019). The pH of dry sausages was measured using a pH meter (2018C132–1, Sardonis Scientific Instruments Co., Shanghai, China) following the method described by Wen et al. (2023). The LAB, Staphylococcus, and yeast counts were determined using the total plate count method (Wen et al., 2023).
2.4. Free amino acid analysis
The FAA content was determined using the method described by Wen et al. (2023). Dry sausages (10 g) were homogenized with 0.1 mol/L HCl (Merck Life Sciences Ltd., Nantong, China) 1:5 (w/v, 50 mL) for 30 min and centrifuged at 10000 ×g at 0 °C for 20 min. The supernatant was then filtered through a 0.22 μm filter (Sterile Syringe Filter; Chengdu Belanmijin Biotechnology Co., Chengdu, China). Then, an aliquot of 1.0 mL was transferred to glass tubes and derivatized using phenyl isothiocyanate (PITC, Merck Life Sciences Ltd., Nantong, China) reagent. FAAs were analyzed on a high-performance liquid analyzer (Agilent, 1100 LC, Agilent Technologies Co., California, USA) equipped with an Accucore C18 column (3 × 150 mm, 2.6 μm, Thermo Fisher, Massachusetts, USA). Norleucine (Merck Life Sciences Ltd., Nantong, China) was added as an internal standard, and the wavelength of the detector was 254 nm. Each FAA was identified and quantified by comparing its retention time and peak area against FAA standards. Notably, the FAA content was expressed in milligrams per gram of dry sausage.
2.5. BAs analysis
The procedure of the extraction, derivatization, purification, and determination of BAs in dry sausages was conducted according to the method described by China National Standard, 2016. BAs were analyzed using the COSMOSIL-5C18-AR-II column (4.6 mm × 250 mm, 5 μm Diamonsil, Dikma Technologies, Beijing, China). The gradient elution program included the following: 0–26 min, 75% A, 25% B; 27–30 min, 85% A, 15% B; and 31–40 min, 100% A. mobile phase A consisted of 90% acetonitrile (Merck Life Sciences Ltd., Nantong, China)/10% (0.01 mol/L ammonium acetate solution containing 0.1% acetic acid; Merck Life Sciences Ltd., Nantong, China), and mobile phase B consisted of 10% acetonitrile/90% (0.01 mol/L ammonium acetate solution containing 0.1% acetic acid). The samples and standard solutions (20 μL, Shanghai Amperexperiment Technology Co., Shanghai, China) were automatically injected at a flow rate of 0.8 mL/min. The UV detection wavelength was 254 nm, and the column temperature was 35 °C. The limits of detection and quantification of eight biogenic amines in meat were 20 mg/kg and 50 mg/kg, respectively. Each chromatographic peak was detected at 254 nm, and BAs content was expressed in milligrams per kilogram of dry sausage.
2.6. Volatile compound analysis
The volatile compounds of dry sausages were extracted and detected using a solid-phase microextraction (SPME) and gas chromatography–mass spectrometry, as specified in Chen et al. (2017)’s method (GCMS-QP2020 NX, Shimadzu Co., Kyoto, Japan). A total of 3.0 g of minced sausage sample and 3 μL (100 mg/L) o-dichlorobenzene (internal standard, Shanghai Amperexperiment Technology Co., Shanghai, China) were added sequentially to a 20 mL headspace vial (CNW Technologies, Shanghai, China). The headspace vial was sealed with a butyl rubber diaphragm. After equilibration at 45 °C for 25 min, the extraction was performed using SPME fiber coated with a 50/30 μm polydimethylsilocane/divinylbenzene/carboxen (PDMS/DVB/CAR) for 30 min at 45 °C. Thereafter, the fiber was injected into an InertCap WaX inert capillary column (0.25 mm × 60 m × 0.25 μm, Qingdao Hongpu Biotechnology Co., Qingdao, China) with high-purity nitrogen (99.99%) to analyze the volatile compounds. The volatile compounds were identified by comparing their mass spectra with the NIST 17 mass spectra library. Volatile compounds were semi-quantified using the internal standard method, and the results were expressed in micrograms per kilogram of dry sausage.
2.7. Sensory evaluation
The sensory attributes of dry sausages after a nine-day fermentation were evaluated using the method of Hu et al. (2022). The sensory evaluation took place in a specially prepared room in accordance with ISO (2012). A sensory panel of 15 panelists (seven males and eight females, aged 20–30 years) was trained to distinguish color, texture, odor, and taste in cooked dry sausages. Dry sausages were steamed for 20 min at 100 °C and cut into 0.5 cm thick pieces. Each treatment was coded with a 2-digit number at random. Qualitative descriptive analyses were used to assess the appearance (color), texture (hardness), odor attributes (fermented and fatty odors), and taste attributes (sour and salty tastes). All attributes were scored on a scale of 1–7, with 1 indicating no perception and 7 indicating maximum perception.
2.8. Statistical analysis
Three independent batches of sausages (replicates) were prepared, and the measurements were conducted in triplicate (triplicate observations) for each batch. Statistical analysis of the data was performed using the general linear model with Statistix 8.1 software (Analytical Software, St Paul, MN, USA). The results were expressed as mean ± standard deviation (SD). Significant differences (p < 0.05) were compared using the Tukey HSD test. A box plot of sensory evaluation was drawn using Origin 2022 software (Origin-Lab, MA, USA). Spearman correlation analysis was performed using IBM SPSS 23 (SPSS Software Development (Xi'an) Co., Ltd., Chicago, USA), and the correlations between FAAs, BAs, and volatile flavor compounds were visualized using Cystoscope software (University of California, San Diego, USA).
3. Results and discussion
3.1. Analysis of physicochemical properties and microbial counts
As shown in Fig. 1, the LAB, Staphylococcus, and yeast counts in all dry sausages first increased and then decreased (p < 0.05). The LAB counts increased rapidly from an initial level of 4.26 to 7.17 log CFU/g (control), 6.89 to 8.75 log CFU/g (LP), 6.98 to 8.63 log CFU/g (LP + SC), 6.95 to 8.48 log CFU/g (LP + PK), and 7.00 to 8.41 log CFU/g (LP + SC + PK) during the first 6 days of fermentation. Afterward, the LAB counts of all dry sausages decreased until the end of fermentation (day 9). Hu, Tian, et al. (2022) also observed a rapid increase in lactic acid bacteria during the first 6 days of fermentation in dry sausage, followed by a subsequent decline after this period. The lowest LAB count was observed in the control treatment (p < 0.05) at the end of fermentation. In addition, the LAB counts of the coinoculated treatments revealed that Staphylococcus and yeast inoculations had little effect on the growth of LAB. At the end of fermentation, the counts of Staphylococcus and yeast in the control and inoculated treatments (control, LP, LP + SC, LP + PK, and LP + SC + PK) were 6.94, 4.77, 7.72, 4.75, and 7.62 and 6.87, 4.65, 4.51, 7.93, and 7.70 log CFU/g, respectively. The counts of Staphylococcus and yeast in the coinoculated treatments (LP + SC, LP + PK, and LP + SC + PK) were lower (p < 0.05) than the LAB counts, which may be due to the higher competitive ability of L. plantarum and the large amount of lactic acid produced by its proliferation in the former stage of fermentation inhibiting the growth of other microorganisms (Wang, Zhao, Su, & Jin, 2019).
Fig. 1.
Effect of coinoculation of different autochthonous starter cultures on the counts of total lactic acid bacteria (A), staphylococci (B), yeast (C), and pH (D), moisture content (E), aw (F) of dry sausages during ripening. Different lowercase letters (a–c) among the different treatments at the same time indicate significant differences (p < 0.05), and different uppercase letters (A–D) among the different times for the same treatment indicate significant differences (p < 0.05). Control: non-inoculation; LP: Lactiplantibacillus plantarum S8; LP + SC: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + SC + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8 + Pichia kudriavzevii M6.
At the beginning of fermentation (day 0), the difference in pH values between the control and inoculated treatments was not significant (Fig. 1D). On day 9, the lowest pH value was observed in the LP, LP + PK, and LP + SC + PK treatments (p < 0.05). The lower pH in the inoculated dry sausages may be attributed to acid production by L. plantarum S8. The decrease in pH promotes the microbiological safety of dry sausages (Essid & Hassouna, 2013). After 6 days, the pH values of dry sausages remained stable, which might be due to the decomposition of organic acids metabolized by yeast and mold growing in the dry sausages and the formation of peptides, amino acids, and non-protein nitrogen compounds produced from proteolysis (Hu et al., 2021).
The moisture content and aw of all dry sausages decreased significantly as fermentation progressed (Fig. 1E and Fig. 1F; p < 0.05). At the end of fermentation, the highest moisture content was observed in the LP + SC treatment (27.37%), followed by the LP + SC + PK (26.40%), LP + PK (24.75%), control (24.38%), and LP (22.49%) treatments. Similarly, the LP + SC + PK treatment had the highest aw value (0.723; p < 0.05), and the LP treatment had the lowest aw value (0.698, p < 0.05) at the end of fermentation. The decreases in the moisture content and aw may be due to water evaporation during fermentation (Wang et al., 2022). Among all treatments, the LP treatment had the lowest moisture content and aw values. On the one hand, the proliferation of LAB leads to the generation of significant amounts of acid and a decrease in pH, resulting in muscle protein denaturation, muscle bundle shrinkage, and water loss from protein networks in the LP treatment (Hu et al., 2019). On the other hand, higher moisture content and aw values in the coinoculated treatments may have been due to the consumption of organic acids by Staphylococcus and yeast (Cavalheiro, Ruiz-Capillas, Herrero, & Pintado, 2020). In addition, the LP + PK and LP + SC + PK treatments demonstrated higher aw than the control, LP, and LP + SC treatments, which may be attributable to yeast consuming organic acids and regulating the release of water during fermentation (Wen et al., 2023).
3.2. Free amino acid analysis
The FAA contents largely exhibited significant differences among the different sausages (p < 0.05; Table 1). A total of 17 FAAs were detected, and the main FAAs were aspartic acid, glutamic acid, threonine, arginine, and cysteine, which accounted for 64.48–67.91% of the total FAAs in dry sausages. In general, FAAs originate from the degradation of muscle proteins by meat endogenous enzymes and microbial proteolytic enzymes and play a significant role in the formation of flavor in the fermented meat products (Zhao et al., 2022). Meanwhile, FAAs can be decarboxylated by an endogenous or bacterial decarboxylase to form BAs (Wang et al., 2022). High total FAAs contents were observed in the LP + SC, LP + PK, and LP + PK + SC treatments (19.23, 21.62, and 20.27 mg/g, respectively) compared with the LP and control treatments (17.01 and 17.66 mg/g). This confirms the participation of microbial peptidases in FAA formation during fermentation (Wen et al., 2023). Previous studies also reported that coinoculation accelerates protein hydrolysis and produces more FAAs (Hua, Sun, Xu, Gao, & Xia, 2022; Zang et al., 2020).
Table 1.
Free amino acid contents (mg/g) of the dry sausages coinoculated with different autochthonous starter cultures after a nine-day ripening.
| Free amino acid | Control | LP | LP + SC | LP + PK | LP + PK + SC |
|---|---|---|---|---|---|
| Aspartic acid | 1.35 ± 0.06c | 0.90 ± 0.04d | 0.52 ± 0.01e | 2.75 ± 0.05a | 1.97 ± 0.02b |
| Glutamic acid | 4.08 ± 0.14d | 4.42 ± 0.15c | 4.93 ± 0.28b | 4.97 ± 0.64b | 5.51 ± 0.21a |
| Serine | 0.48 ± 0.04a | 0.26 ± 0.02b | 0.43 ± 0.04ab | 0.31 ± 0.03ab | 0.37 ± 0.02ab |
| Glycine | 0.35 ± 0.07ab | 0.36 ± 0.06ab | 0.28 ± 0.04b | 0.43 ± 0.08a | 0.42 ± 0.07a |
| Histidine | 0.94 ± 0.22c | 1.72 ± 0.27ab | 1.41 ± 0.20bc | 2.15 ± 0.16a | 2.01 ± 0.27a |
| Threonine | 1.21 ± 0.38b | 0.70 ± 0.12c | 1.87 ± 0.34a | 1.91 ± 0.14a | 1.78 ± 0.10a |
| Alanine | 1.18 ± 0.09a | 0.89 ± 0.07b | 0.95 ± 0.22b | 1.10 ± 0.21a | 0.92 ± 0.15b |
| Arginine | 3.46 ± 0.41a | 3.50 ± 0.35a | 3.79 ± 0.17a | 2.56 ± 0.29b | 2.37 ± 0.12b |
| Proline | 0.34 ± 0.06b | 0.03 ± 0.02c | 0.90 ± 0.05a | 0.07 ± 0.03c | 0.04 ± 0.02c |
| Tyrosine | 0.23 ± 0.06a | 0.19 ± 0.05ab | 0.18 ± 0.05ab | 0.22 ± 0.06a | 0.13 ± 0.04b |
| Valine | 0.15 ± 0.02c | 0.24 ± 0.03b | 0.22 ± 0.02b | 0.30 ± 0.02a | 0.30 ± 0.01a |
| Methionine | 0.15 ± 0.02a | 0.09 ± 0.03ab | 0.06 ± 0.04b | 0.17 ± 0.03a | 0.14 ± 0.04ab |
| Cysteine | 1.45 ± 0.32a | 1.63 ± 0.28a | 1.95 ± 0.28a | 1.75 ± 0.30a | 1.62 ± 0.21a |
| Isoleucine | 0.30 ± 0.05a | 0.28 ± 0.05a | 0.31 ± 0.06a | 0.31 ± 0.05a | 0.27 ± 0.04a |
| Leucine | 0.38 ± 0.01c | 0.41 ± 0.03bc | 0.38 ± 0.03c | 0.53 ± 0.02a | 0.44 ± 0.06b |
| Phenylalanine | 0.16 ± 0.01a | 0.07 ± 0.01b | 0.08 ± 0.02b | 0.08 ± 0.01b | 0.07 ± 0.01b |
| Lysine | 1.13 ± 0.19a | 1.09 ± 0.11a | 1.03 ± 0.20a | 0.99 ± 0.10a | 1.00 ± 0.17a |
| Total | 17.7 ± 0.64d | 17.0 ± 0.71d | 19.2 ± 0.54c | 21.6 ± 0.81a | 20.3 ± 0.66b |
a-d Means within the same row with different lowercase letters differ significantly (p < 0.05).
Control: noninoculation; LP: Lactiplantibacillus plantarum S8; LP + SC: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + SC + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8 + Pichia kudriavzevii M6.
Aspartic acid and glutamic acid have an umami taste, which could contribute to the overall taste of dry sausages (Hu, Wang, et al., 2022). The aspartic acid contents in the LP + PK (2.57 mg/g) and LP + SC + PK (1.97 mg/g) treatments were significantly higher than those in the control, LP, and LP + SC treatments (1.35, 0.90, and 0.52 mg/g, respectively), which suggests that P. kudriavzevii may play a crucial role in intensifying the umami taste of dry sausages (Wen et al., 2023). The content of glutamic acid in the coinoculated treatments (4.93–5.51 mg/g) was higher than that in the control and LP treatments (4.08 and 4.42 mg/g, p < 0.05). This may be due to the fact that coinoculation promotes the activity of endogenous enzymes and microbial proteolysis enzymes in the system, increases the proteolysis capacity, and thus increases the content of glutamate (Zang et al., 2020). Among the coinoculated treatments, the LP + SC + PK treatment had the highest glutamic acid content, indicating that coinoculation promoted the formation of umami in dry sausages.
Threonine has sweet characteristics, and it was found in the coinoculated treatments (1.87, 1.97, and 1.78 mg/g for the LP + SC, LP + PK, and LP + PK + SC treatments, respectively) in higher amounts than in the control and LP treatments (1.21 and 0.70 mg/g, p < 0.05). Arginine, a bitter FAA, was found in the LP + PK (2.56 mg/g) and LP + PK + SC (2.37 mg/g) treatments (p < 0.05) at low contents. Wen et al. (2023) also found that the P. kudriavzevii inoculation could reduce the content of bitter FAAs. The difference in cysteine content in all dry sausages was not significant (p > 0.05). Compared with the control and LP treatments, the coinoculated treatments had higher contents of sweet and umami FAAs (p < 0.05).
Additionally, some FAAs, such as leucine, isoleucine, and valine, can be transformed into methyl-branched alcohols, aldehydes, and acids that contribute to the distinctive flavor of dry sausages (Hu et al., 2019). The contents of valine and leucine were found to be higher in the inoculated treatments than the control treatment (p < 0.05). This phenomenon may be related to the differences between strains and strain interactions.
3.3. Biogenic amine analysis
Eight BAs were found in all dry sausages, including tryptamine, 2-phenylethylamine, cadaverine, putrescine, histamine, tyramine, spermidine, and spermine. The total contents of BAs were lower in the inoculated treatments (175, 239, 190, and 207 mg/kg for the LP, LP + SC, LP + PK, and LP + PK + SC treatments, respectively) than in the control treatment (340 mg/kg, p < 0.05). These results indicated that the inoculation of these starter cultures could inhibit the production and accumulation of BAs. Similarly, Sun, Chen, Li, Zheng, and Kong (2016) observed that the BA content of the coinoculation with L. plantarum was significantly lower than that of the noninoculated treatment (p < 0.05).
Tryptamine, formed by the decarboxylation of tryptophan, was significantly higher in the LP treatment than the other treatments (Table 2). This may be attributable to the single inoculation of L. plantarum S8 producing a large amount of acid during fermentation, as evinced by it having lowest pH in the LP treatment. Some microorganisms with tryptophan decarboxylase in the sausage system produced basic tryptamine to resist the acid stress (Wang et al., 2022). However, no differences in tryptamine contents were observed between the control and coinoculated treatments (except for the LP + PK treatment). 2-Phenethylamine is a type of aromatic amine that can be oxidized by monoamine oxidase (Sun et al., 2022). Inoculation of these starter cultures reduced the content of 2-phenylethylamine after a nine-day fermentation (24.00–39.11%; p < 0.05), which was similar to the finding of Sun et al. (2022). Cadaverine and putrescine often serve as important indicators of food hygiene and are mainly associated with the microorganisms attached to the surface of dry sausages, such as Enterobacter and Pseudomonas (Lorenzo et al., 2010). In this study, the inoculation of L. plantarum S8 effectively reduced the putrescine and cadaverine contents, which may be due to the large amount of bacteriocins produced by L. plantarum inhibiting the growth of amine-producing bacteria from the previously mentioned genera of Enterobacter and Pseudomonas (Zhang, Qin, Wang, & Li, 2020). Recently, Ye et al. (2021) revealed that L. plantarum could compete with other microorganisms possessing amino acid decarboxylase to inhibit the growth of amine-producing microorganisms and the activity of their decarboxylase.
Table 2.
Biogenic amine contents (mg/kg) of the dry sausages coinoculated with different autochthonous starter cultures after a nine-day ripening.
| Biogenic amine | Control | LP | LP + SC | LP + PK | LP + PK + SC |
|---|---|---|---|---|---|
| Tryptamine | 8.91 ± 0.27b | 11.5 ± 0.25a | 9.06 ± 0.30b | 7.63 ± 0.16c | 9.36 ± 0.32b |
| 2-Phenylethylamine | 2.25 ± 0.06a | 1.71 ± 0.05b | 1.37 ± 0.66b | 1.50 ± 0.06b | 1.59 ± 0.22b |
| Putrescine | 2.76 ± 0.20a | 1.48 ± 0.10b | 0.65 ± 0.11c | 1.17 ± 0.07bc | 2.28 ± 0.18a |
| Cadaverine | 2.08 ± 0.10a | 0.75 ± 0.08c | 1.33 ± 0.16b | 1.38 ± 0.13b | 0.72 ± 0.08c |
| Histamine | 188 ± 5.79a | 0.55 ± 0.03c | 63.3 ± 2.04b | 1.43 ± 0.08c | 2.17 ± 0.41c |
| Tyramine | 14.8 ± 0.87d | 37.3 ± 1.04b | 27.9 ± 1.37c | 33.2 ± 2.16bc | 49.1 ± 1.37a |
| Spermidine | 6.20 ± 0.14a | 5.31 ± 0.22b | 2.06 ± 0.08c | 5.13 ± 0.10b | 5.37 ± 0.23b |
| Spermine | 114 ± 2.14b | 116 ± 3.83b | 134 ± 3.74a | 139 ± 3.56a | 138 ± 4.34a |
| Total | 340 ± 9.57a | 175 ± 5.85d | 239 ± 7.98b | 190 ± 6.74cd | 207 ± 7.16c |
a-d Means within the same row with different lowercase letters differ significantly (P < 0.05);
Control: noninoculation; LP: Lactiplantibacillus plantarum S8; LP + SC: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + SC + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8 + Pichia kudriavzevii M6.
Histamine is formed by enzymatic decarboxylation of histidine, which is the most toxic of the eight BAs, and excessive intake can cause toxic reactions (e.g., headaches and diarrhea, (Sun et al., 2016)). The histamine content of the control treatment (188.51 mg/kg) was much higher than the recommended maximum daily intake (50 mg) proposed by the European food safety authority (FDA, 2011). Inoculation of these starter cultures could reduce the content of histamine (99.70%, 66.41%, 99.20%, and 98.56% for the LP, LP + SC, LP + PK, and LP + PK + SC treatments, respectively), thereby promoting the safety of dry sausages. Sun et al. (2016) found that L. plantarum can compete with other bacteria with amino acid decarboxylase in dry sausages and reduce the accumulation of histamine. Thus, the results further confirmed that inoculation with starter cultures can reduce histamine content of fermented meat products.
Tyramine, formed by tyrosine decarboxylation, is a common BA in fermented foods (Chen et al., 2023). The tyramine contents of the inoculated treatments (27.91–49.07 mg/kg) were significantly higher than that of the control treatment (14.77 mg/kg, p < 0.05), probably because inoculation of L. plantarum S8 contributed to tyramine production. The stimulation effects are probably a result of the synergistic effect of LAB and foodborne pathogens (Ye et al., 2021). Similar results were observed in the tyrosine decarboxylase broth test, in which the inoculation of Leuconostoc mesenteroides subsp, Lactococcus lactis subsp. Lactis in the broth increased the generation of tyramine by S. aureus and L. monocytogenes (Özogul & Hamed, 2018). The stimulation effect is probably a result of the synergistic effect of LAB and foodborne pathogens. According to the European Food Safety Authority (EFSA), healthy individuals should not consume >600 mg of tyramine daily (Emer, Marques, Colla, & Reinehr, 2021). Therefore, the tyramine contents in all dry sausages were considered safe.
Spermidine and spermidine are naturally occurring BAs that are found in fresh pork (Kalač, 2006). The spermidine contents in the inoculated treatment (2.06–5.37 mg/kg) were significantly lower than that in the control treatment, which was consistent with the results of Rubén, Paulo, Rubén, and José (2016). The spermine contents in the coinoculated treatments (133.73–138.72 mg/kg) were higher than that in the single inoculated treatment (115.85 mg/kg) and the control treatment (114.23 mg/kg), which may be due to the interaction between these strains.
3.4. Correlation between free amino acids and biogenic amines
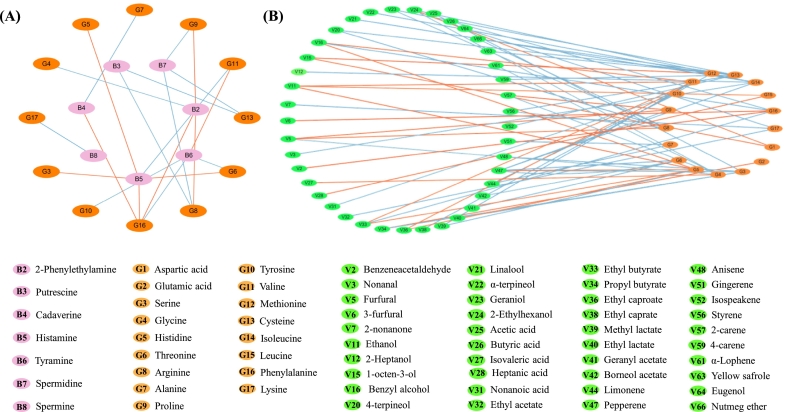
To elucidate the relationship between BAs and their precursor FAAs, a visual correlation network was established (Fig. 2A). The strongest positive correlations (0.925) were found between phenylalanine (G16) and phenylethylamine (B2), and histidine (G5) and histamine (B5), which were similar to the findings of Dabadé et al. (2021). Histamine is a common BA in fermented foods and can easily cause histamine poisoning (Sun et al., 2016). Therefore, more attention should be paid to histidine-rich foods. A significant negative correlation (0.977) was found between lysine (G17) and spermine (B8). The negative correlations were found between arginine (G8) and putrescine (B3), arginine (G8) and spermidine (B7), and tyrosine (G10) and tyramine (B6). Dai et al. (2021) reported the same correlations between FAAs and BAs in bighead carp. However, lysine, as the precursor of cadaverine, did not correlate with cadaverine. Although the formation of BAs is extremely complex, significant correlations can be seen between the major BAs and their precursor FAAs from dry sausages in this study. Further studies should be undertaken to validate these relationships and clarify their contribution to BA formation.
Fig. 2.
Correlations between free amino acids and biogenic amines (A) and volatile compounds (B) in dry sausages. The highly correlated part (|ρ| ≥ 0.8) with p value <0.05 is visualized. The pink, orange, and green nodes represent the biogenic amines, free amino acids, and volatile compounds in dry sausages, respectively. Blue lines represent the negative correlations and red lines represent the positive correlations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Volatile compound analysis
As listed in Table 3, 66 volatile compounds were detected in all dry sausages, which were further classified into 7 categories. These volatiles include 6 aldehydes, 4 ketones, 14 alcohols, 7 acids, 11 esters, 20 terpenes, and 4 others, which were mainly derived from protein degradation, lipid oxidation, carbohydrate metabolism, wine, and spices (Wen et al., 2023). The highest total aldehyde content (103.31 μg/kg) was observed in the control treatment, followed by the LP, LP + SC, LP + PK, and LP + SC + PK treatments (85.37, 95.50, 56.10, and 72.43 μg/kg, respectively; p < 0.05). Compared with the other treatments, the LP + SC + PK treatment had higher total contents of ketones, alcohols, and acids (p < 0.05). The highest total ester content was observed in the LP + PK treatment (996.70 μg/kg), followed by the control, LP, LP + SC, and LP + SC + PK treatments (542.45, 829.45, 861.93, and 848.68 μg/kg, respectively, p < 0.05). Compared with the other treatments, the LP treatment had a higher total content of terpenes (p < 0.05). Notably, the inoculation with different starter cultures can change the contents of aldehydes, ketones, alcohols, acids, esters, and terpenes in dry sausages, resulting in different flavor profiles. The changes of each volatile compound contents are specifically described and discussed as follows.
Table 3.
Volatile compound contents (μg/kg) of the dry sausages coinoculated with different autochthonous starter cultures after a nine-day ripening.
| Number | Volatile compound | CAS number | Control | LP | LP + SC | LP + PK | LP + SC + PK |
|---|---|---|---|---|---|---|---|
| Aldehyde | |||||||
| V1 | Hexanal | 66–25-1 | 57.18 ± 0.72b | 60.23 ± 0.45b | 64.04 ± 2.23a | 38.97 ± 0.42c | 40.23 ± 0.89c |
| V2 | Benzene acetaldehyde | 122–78-1 | 4.94 ± 0.55 | n.d. | n.d. | n.d. | n.d. |
| V3 | Nonanal | 124–19-6 | 19.54 ± 0.37b | 25.14 ± 0.27a | 21.68 ± 0.99ab | 17.13 ± 0.14b | 26.22 ± 0.63a |
| V4 | Trans-2-octenal | 2548-87-0 | n.d. | n.d. | 8.23 ± 0.39a | n.d. | 5.98 ± 0.32b |
| V5 | Furfural | 98–01-1 | n.d. | n.d. | 1.55 ± 0.94 | n.d. | n.d. |
| V6 | 3-Furfural | 498–60-2 | 16.61 ± 0.71a | n.d. | n.d. | n.d. | n.d. |
| Total | 103.31 ± 7.13a | 85.37 ± 6.80c | 95.50 ± 7.87b | 56.10 ± 5.81e | 72.43 ± 7.35d | ||
| Ketone | |||||||
| V7 | 2-Nonanone | 821–55-6 | n.d. | n.d. | 5.08 ± 0.14b | 5.73 ± 0.13ab | 6.10 ± 0.20a |
| V8 | 3-Hydroxy-2-butanone | 513–86-0 | 30.82 ± 0.92b | 45.88 ± 0.99a | 33.64 ± 0.74b | n.d. | 47.17 ± 0.38a |
| V9 | 2,5-Octanedione | 3214-41-3 | n.d. | 1.97 ± 0.78 | n.d. | n.d. | n.d. |
| V10 | Fenchone | 4695-62-9 | 95.92 ± 5.76a | 96.10 ± 6.43a | 86.60 ± 8.65b | 75.68 ± 6.05c | 93.76 ± 9.75a |
| Total | 135.94 ± 12.86c | 154.26 ± 14.61b | 133.06 ± 12.01c | 85.62 ± 5.96d | 162.79 ± 22.28a | ||
| Alcohol | |||||||
| V11 | Ethanol | 64–17-5 | 245.58 ± 18.66e | 355.36 ± 13.95c | 337.20 ± 21.36d | 428.67 ± 11.23a | 362.29 ± 6.71b |
| V12 | 2-Heptanol | 543–49-7 | n.d. | 3.26 ± 0.25a | n.d. | n.d. | 3.05 ± 0.59a |
| V13 | n-Pentanol | 71–41-0 | n.d. | n.d. | 4.09 ± 0.39a | n.d. | 4.42 ± 0.19a |
| V14 | Isopentenol | 15,575–04-9 | n.d. | 2.87 ± 0.54 | n.d. | n.d. | n.d. |
| V15 | 1-Octen-3-ol | 3391-86-4 | 10.61 ± 0.41ab | 9.31 ± 0.19b | 9.17 ± 1.71b | 11.38 ± 0.54a | 10.23 ± 0.46ab |
| V16 | Benzyl alcohol | 3391-86-4 | 1.64 ± 0.05d | 3.32 ± 0.06c | 5.50 ± 0.26b | 9.07 ± 0.53a | 3.06 ± 0.09c |
| V17 | Phenylethanol | 1321-27-3 | 8.11 ± 0.52b | 16.92 ± 0.75a | 9.20 ± 1.24b | 5.83 ± 0.34c | 8.94 ± 1.23b |
| V18 | Dodecanol | 27,342–88-7 | 10.09 ± 1.06b | n.d. | n.d. | n.d. | 13.13 ± 0.35a |
| V19 | Lauryl alcohol | 112–53-8 | 5.99 ± 0.42b | 15.30 ± 1.04a | n.d. | n.d. | n.d. |
| V20 | Terpine-4-ol | 10,482–56-1 | 63.26 ± 2.65a | 61.34 ± 1.03a | 42.71 ± 2.89c | 46.54 ± 1.83b | 62.34 ± 2.88a |
| V21 | Linalool | 78–70-6 | 50.66 ± 1.05b | 58.95 ± 0.54a | 46.47 ± 0.75c | 44.86 ± 1.13c | 61.87 ± 0.76a |
| V22 | α-Terpineol | 20,126–76- | 28.58 ± 1.42c | 31.51 ± 0.12b | 23.26 ± 0.57e | 25.80 ± 0.86d | 38.66 ± 1.98a |
| V23 | Geraniol | 106–24-1 | 7.01 ± 0.42a | 4.03 ± 0.24b | n.d. | n.d. | 5.28 ± 0.31b |
| V24 | 2-Ethylhexanol | 104–76-7 | n.d. | 5.31 ± 0.20b | 8.58 ± 1.09a | n.d. | n.d. |
| Total | 421.65 ± 13.98e | 586.46 ± 18.98b | 475.05 ± 15.74d | 567.63 ± 11.39c | 603.46 ± 22.73a | ||
| Acid | |||||||
| V25 | Acetic acid | 64–19-7 | 37.17 ± 0.94d | 113.36 ± 0.68a | 68.51 ± 0.99c | 75.60 ± 0.68b | 74.00 ± 0.40b |
| V26 | Butyric acid | 107–92-6 | 12.72 ± 0.93d | 28.75 ± 1.10a | 23.08 ± 1.38b | 19.66 ± 1.27c | 25.07 ± 0.93ab |
| V27 | Isovaleric acid | 503–74-2 | n.d. | n.d. | n.d. | 5.39 ± 0.82a | 6.00 ± 0.68a |
| V28 | Heptanic acid | 111–14-8 | n.d. | n.d. | n.d. | 4.40 ± 0.48 | n.d. |
| V29 | Octanoic acid | 124–07-2 | 6.59 ± 0.63c | 9.36 ± 1.11b | 5.58 ± 0.45d | 7.40 ± 0.60c | 21.64 ± 1.37a |
| V30 | Decanoic acid | 334–48-5 | 5.38 ± 0.94b | n.d. | 4.09 ± 0.08c | 4.55 ± 0.25c | 6.11 ± 0.36a |
| V31 | Nonanoic acid | 112–05-0 | n.d. | n.d. | n.d. | n.d. | 22.31 ± 1.26 |
| Total | 69.40 ± 10.66e | 160.89 ± 13.32b | 113.59 ± 17.44d | 122.52 ± 7.81c | 176.26 ± 29.88a | ||
| Esters | |||||||
| V32 | Ethyl acetate | 141–78-6 | 81.51 ± 4.45e | 151.82 ± 6.04d | 267.38 ± 7.75b | 320.71 ± 13.61a | 223.27 ± 9.17c |
| V33 | Ethyl butyrate | 105–54-4 | 36.87 ± 1.40c | 63.51 ± 0.40b | 64.68 ± 1.23b | 73.56 ± 1.48a | 61.38 ± 0.82b |
| V34 | Propyl butyrate | 105–66-8 | n.d. | 8.90 ± 0.23a | n.d. | 8.51 ± 0.82a | n.d. |
| V35 | Ethyl valerate | 123–66-0 | n.d. | 5.08 ± 0.26 | n.d. | n.d. | n.d. |
| V36 | Ethyl caproate | 539–82-2 | 210.65 ± 8.01e | 303.21 ± 11.03d | 333.04 ± 6.72b | 384.52 ± 9.20a | 313.52 ± 4.93c |
| V37 | Ethyl octanoate | 106–32-1 | 19.06 ± 1.81b | 23.16 ± 0.36a | 23.52 ± 1.28a | 20.36 ± 0.68b | 16.07 ± 0.89c |
| V38 | Ethyl caprate | 110–38-3 | 7.82 ± 1.29c | 15.13 ± 0.25a | 13.96 ± 0.67b | 8.11 ± 0.74c | 7.09 ± 0.95d |
| V39 | Methyl lactate | 547–64-8 | 3.10 ± 0.28bc | 4.36 ± 0.25b | 6.54 ± 0.25a | n.d. | 2.80 ± 0.37c |
| V40 | Ethyl lactate | 97–64-3 | 16.02 ± 2.35e | 84.60 ± 4.33a | 35.73 ± 4.77c | 45.73 ± 3.77b | 25.60 ± 1.06d |
| V41 | Geranyl acetate | 105–87-3 | 11.62 ± 1.82b | 22.21 ± 1.57a | 13.52 ± 1.42b | 16.66 ± 1.34ab | 21.70 ± 2.36a |
| V42 | Borneol acetate | 76–49-3 | 107.49 ± 16.88b | 166.45 ± 6.13ab | 123.45 ± 4.63bc | 141.35 ± 5.74b | 196.67 ± 7.01a |
| Total | 542.45 ± 18.32d | 829.45 ± 24.84c | 861.93 ± 27.89b | 996.70 ± 22.25a | 848.68 ± 27.64bc | ||
| Terpene | |||||||
| V43 | Laurene | 123–35-3 | 56.26 ± 6.45d | 68.34 ± 5.67b | 62.06 ± 6.53c | 43.78 ± 3.49e | 74.90 ± 6.88a |
| V44 | Limonene | 5989-27-5 | 760.54 ± 36.46d | 1022.41 ± 20.6b | 974.22 ± 35.75c | 788.17 ± 29.53d | 1178.71 ± 28.85a |
| V45 | γ-Terpinene | 99–85-4 | 89.98 ± 14.02b | 132.41 ± 8.78a | 77.16 ± 13.38bc | 55.16 ± 9.55d | 70.02 ± 12.12c |
| V46 | P-Isopropyl toluene | 99–87-6 | 37.60 ± 4.96bc | 39.81 ± 2.45b | 39.19 ± 4.02b | 44.01 ± 4.52a | 33.33 ± 3.42c |
| V47 | Piperenone | 57,625–31-7 | n.d. | 9.15 ± 1.18a | n.d. | 8.38 ± 1.66b | 9.13 ± 1.80a |
| V48 | Anisene | 4180-23-8 | 100.61 ± 11.49e | 135.25 ± 7.27a | 106.52 ± 9.56d | 117.64 ± 10.56c | 120.76 ± 10.84b |
| V49 | (Z)-β-Ocimene | 13,877–91-31 | n.d. | 1.81 ± 0.25a | n.d. | n.d. | 1.56 ± 0.33a |
| V50 | β-Caryophyllene | 87–44-5 | 148.71 ± 18.91b | 155.04 ± 12.18a | 115.68 ± 14.71e | 133.18 ± 16.93d | 137.78 ± 17.52c |
| V51 | Gingerene | 495–60-3 | n.d. | 9.04 ± 1.14a | 9.52 ± 1.20a | n.d. | 9.36 ± 1.18a |
| V52 | Isospeakene | 95,910–36-4 | 24.89 ± 3.88a | 23.5 ± 2.31a | 19.55 ± 3.05b | 23.73 ± 3.70a | 23.21 ± 3.62a |
| V53 | α-Curcumene | 4176-17-4 | 20.21 ± 1.98a | 12.99 ± 1.27c | 15.23 ± 1.49bc | 18.03 ± 1.77b | 20.04 ± 1.96a |
| V54 | α-Pinene | 80–56-8 | 2.89 ± 0.25c | 5.76 ± 0.49a | 3.96 ± 0.34b | 4.17 ± 0.35b | n.d. |
| V55 | β-Pinene | 2437-95-8 | 3.58 ± 0.42b | 5.01 ± 0.58a | n.d. | n.d. | n.d. |
| V56 | Styrene | 69,011–19-4 | 7.66 ± 0.63a | 5.52 ± 0.21b | n.d. | 4.26 ± 0.28b | 4.46 ± 0.29b |
| V57 | 2-Carene | 4497-92-1 | 13.89 ± 1.78b | 7.96 ± 1.02d | 34.71 ± 4.44a | 7.38 ± 0.95d | 10.20 ± 1.31c |
| V58 | 3-Carene | 13,466–78-9 | 4.37 ± 0.15a | 2.22 ± 0.08c | n.d. | 3.35 ± 0.11b | 4.27 ± 0.14a |
| V59 | 4-Carene | 29,050–33-7 | 24.85 ± 5.34a | 21.84 ± 3.49b | 13.55 ± 2.17d | 19.64 ± 3.14c | 25.63 ± 4.09a |
| V60 | α-Cubebene | 17,699–14-8 | 6.10 ± 1.06a | 3.79 ± 0.66c | 4.24 ± 0.74ab | 4.46 ± 0.77ab | 5.47 ± 0.95a |
| V61 | α-Humulene | 6753-98-6 | n.d. | 11.71 ± 1.30a | 19.51 ± 2.35b | n.d. | n.d. |
| V62 | Sabinene | 3387-41-5 | n.d. | 8.66 ± 1.47 | n.d. | n.d. | n.d. |
| Total | 1405.12 ± 113.08c | 1800.10 ± 44.88a | 1529.31 ± 44.69b | 1298.43 ± 28.98d | 1786.77 ± 57.33a | ||
| Others | |||||||
| V63 | Safrole | 120–58-1 | 16.03 ± 0.94a | 10.20 ± 0.36b | n.d. | n.d. | 9.79 ± 7.70b |
| V64 | Eugenol | 97–53-0 | n.d. | 1.40 ± 0.08b | 2.72 ± 0.16a | 2.60 ± 0.15a | 2.29 ± 0.14ab |
| V65 | n-Heptadecane | 629–78-7 | 28.10 ± 3.57b | 49.52 ± 2.30a | 30.63 ± 3.89b | 22.79 ± 2.89c | 28.09 ± 3.57b |
| V66 | Nutmeg ether | 607–91-0 | 17.53 ± 1.75a | 13.03 ± 1.66b | 9.05 ± 1.15c | 12.30 ± 1.56d | 16.06 ± 2.04a |
| Total | 66.38 ± 6.67b | 76.12 ± 2.78a | 43.64 ± 1.76d | 40.99 ± 4.67d | 60.03 ± 5.80c | ||
n.d.: not detected.
a-e Means within the same row with different lowercase letters differ significantly (P < 0.05).
Control: noninoculation; LP: Lactiplantibacillus plantarum S8; LP + SC: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + SC + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8 + Pichia kudriavzevii M6.
Six aldehydes were detected in dry sausages, which could give fat flavor to fermented meat products and reflect the degree of lipid oxidation (Hu, Tian, et al., 2022). Nonanal imparts carnation, citrus, and laurel odors (Chen et al., 2017), whereas high amounts of hexanal generally result in a rancid flavor (Gómez & Lorenzo, 2013). The highest nonanal content was observed in the LP + SC + PK treatment (26.22 μg/kg), followed by the LP, LP + SC, control, and LP + PK treatments (25.14, 21.68, 19.54, and 17.13 μg/kg, respectively; p < 0.05). The hexanal content in the LP + PK and LP + SC + PK treatments (38.97 and 40.23 μg/kg) were significantly lower than that in the other treatments (57.18, 60.23, and 64.04 μg/kg for the control, LP, and LP + SC treatments, respectively, p < 0.05), likely because yeast inoculation inhibited the degree of lipid oxidation and reduced the hexanal contents, which was similar to the results of Flores, Durá, Marco, and Toldrá (2004). Therefore, inoculation of suitable mixed starter cultures can promote the hydrolysis of proteins to produce FAAs and inhibit the excessive oxidation of lipids, thereby improving the flavor formation of dry sausages.
A total of four ketones were detected in dry sausages. The higher contents of 3-hydroxy-2-butanone were found in the LP and LP + SC + PK treatments (p < 0.05). Several studies have demonstrated that 3-hydroxy-2-butanone has the odor of dairy products and overripe fruits, which is related to citric acid, lactose metabolism, and amino acid decomposition (Hu, Wang, et al., 2022). The chemical oxidation of 2,3-butanediol can also produce 3-hydroxy-2-butanone, and its relative low odor threshold is very important for contribution to dry sausage flavor (Sidira, Kandylis, Kanellaki, & Kourkoutas, 2015). 2-Nonanone is mainly derived from the β-oxidation of unsaturated fatty acids by microorganisms (Li et al., 2022). In the control and LP treatments, 2-nonanone was not detected. Further, the contents of fenchone were high in all dry sausages, mainly from spices.
During sausage fermentation, alcohols are mainly derived from the metabolism of carbohydrates, amino acids, and lipids during fermentation and the addition of wine during sausage preparation (Sun, Zhao, Zhao, Zhao, & Yang, 2010). Among the 13 alcohols detected, ethanol had the highest contents (p < 0.05). Ethanol is mainly derived from the addition of Daqu wine to the raw material and the metabolites of the added starter culture on carbohydrates, which are also involved in the formation of esters as substrates (Xu et al., 2018). Ethanol content in the inoculated treatments (337.20–428.67 μg/kg) was significantly higher than that in the control treatment (245.58 μg/kg; p < 0.05), which may be ascribed to the carbohydrate fermentation and lipid oxidation that occurred with microbial participation (Sun et al., 2010). Benzyl alcohol is one of the most important aromatic alcohols, which is often present in the form of esters in dry sausages. The benzyl alcohol content in the inoculated treatments (3.06–9.07 μg/kg) was significantly higher than that in the control treatment (1.64 μg/kg; p < 0.05). This may be due to the synergistic effect of LAB and yeast, which promoted the conversion of some amino acids in dry sausages (Hu, Tian, et al., 2022).
Seven acids were detected in dry sausages. Acids have a higher threshold and a relatively small contribution to flavor; however, they can participate in forming esters as important precursors and affect the fermented flavor (Hu et al., 2021). The total acid content in the inoculated treatments (113.59–176.26 μg/kg) was significantly higher than that in the control treatment (69.40 μg/kg; p < 0.05), especially in the LP + SC + PK treatment. Acetic acid and butyric acid are also derived from the metabolism of carbohydrates and microbial oxidation of lipids (Li, Cao, Yu, Zhu, & Zhao, 2023) and have the highest contents among these acids (p < 0.05). Heptanoic acid was detected in the LP + PK treatment, whereas nonanoic acid was detected in the LP + SC + PK treatment. Heptanoic acid, octanoic acid, and nonanoic acid, which are mainly derived from lipid degradation, may act as precursors of compounds that could indirectly influence the flavor development of dry sausages because of their high odor threshold values (Domínguez, G'omez, Fonseca, & Lorenzo, 2014).
Esters are mainly derived from the esterification of alcohols and acids, most of which have a unique aroma and can give dry sausages unique fruit and wine aromas (Marusic, Vidacek, Janci, Petrak, & Medic, 2014). A total of 11 esters were detected in dry sausages, and the total content of esters in the LP + PK treatment (996.70 μg/kg) was significantly higher than that in the other treatments (542.45–861.93 μg/kg; p < 0.05). Related studies have shown that yeasts have esterase activity and promote the formation of esters (Jiang et al., 2021). The ethyl caprate content was higher in the LP and LP + SC treatments than in the other treatments; The higher contents of ethyl butyrate and ethyl caproate in the LP + PK treatment may be due to its formation from the esterase activity of yeast (Chen & Liu, 2016).
In addition, 20 terpenes and other compounds were detected in dry sausages, most of which primarily originate from the added spices during sausage preparation (Wen et al., 2023). These compounds were concentrated during fermentation due to the loss of water, ultimately shaping flavor profile of dry sausages. The LP treatment had the highest terpene content (1800.10 μg/kg; p < 0.05). Hu, Tian, et al. (2022) similarly found that the content of terpene in dry sausage inoculated with LAB was higher than that in the noninoculated dry sausage. On the one hand, due to the lowest moisture content in the LP treatment, the terpene was more concentrated. On the other hand, LAB inhibited the activity of lipoxygenase, which inhibits the decomposition of terpene substances by lipoxygenase (Wang & Hammond, 2010).
3.6. Correlation between free amino acids and volatile compounds
FAAs are crucial in the flavor development of dry sausage. LAB possess the ability to enzymatically convert valine and leucine into their respective alpha-keto acids through aminotransferase activity (Olesen, Meyer, & Stahnke, 2004). These alpha-keto acids subsequently contribute to the generation of aroma compounds, thereby imparting the characteristic flavor profile observed in fermented sausages. To further determine the contribution of FAAs to the formation of volatile compounds, the correlation between them was evaluated by Pearson correlation analysis. Seventeen FAAs were significantly correlated with 41 of 66 volatile compounds (Fig. 2B). Among them, the key flavor substance phenylacetaldehyde (V2) was significantly positively correlated with phenylalanine (G16), primarily due to the catalysis of phenylalanine into styrene and subsequent formation of phenacetaldehyde by dehydrogenase (Xie et al., 2024); 1-octen-3-ol (V15) was positively correlated with aspartic acid (G1), primarily due to the conversion of aspartic acid into alcohols catalyzed by aminotransferase and dehydrogenase (Xie et al., 2024); Ehanol (V11) was significantly positively correlated with histidine (G5). Ethyl butyrate (V33) was positively correlated with valine (G11). Other volatile compounds such as 2-ethylhexanol (V24), propyl butyrate (V34), ethyl lactate (V40), isovaleric acid (V27), ethyl caproate (V36), furfural (V5), and benzyl alcohol (V16) were positively correlated with histidine (G5), glutamic acid (G2), glycine (G4), arginine (G8), and phenylalanine (G16). The above results indicate that FAAs are ideal precursors for flavor formation in dry sausages. FAAs can produce volatile flavor compounds through the amino acid metabolism pathway with the assistance of transaminase, dehydrogenase, and oxidase. Additionally, the flavor characteristics of dry sausage could be improved by inoculation of starter cultures.
3.7. Sensory evaluation
Sensory characteristics are crucial in determining the acceptability of a final product. The sensory characteristics of dry sausages are susceptible to changes in pH value, moisture content, and protein hydrolysis during fermentation (Seleshe & Kang, 2021). The color, hardness, taste, and odor attributes of the dry sausages inoculated with different starter cultures were evaluated by a sensory panel (Fig. 3). The highest color score was observed in the LP + SC treatment, followed by the LP + SC + PK, LP, LP + PK, and control treatments. This may be due to the fact that reductase produced by Staphylococcus promotes the formation of nitrosomyoglobin (Hu et al., 2019). Concerning hardness, the LP treatment had the highest hardness score, possibly due to it having the lowest moisture content. The score of salty taste was higher in the inoculated treatments, especially, the LP + PK treatment, than in the control treatment. However, higher scores of sour tastes were observed in the inoculated treatments than the control treatment (p < 0.05), which indicated that L. plantarum S8 plays a specific role in the acidification of sausages. With regard to the odor, higher scores of fermented odor were observed in the inoculated treatments, which may be due to their higher alcohol and ester contents. The scores of fatty odor of the inoculated treatments were significantly lower than those of the control treatment (p < 0.05). Notably, the LP + SC + PK treatment exhibited the highest fermented odor and lowest fatty odor among the the treatments. As a result, the inoculation of autochthonous starter cultures changed the flavor profiles of dry sausages. The dry sausages coinoculated with L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 have more positive taste and odor profiles and exceptional sensory quality.
Fig. 3.
Sensory evaluation of the dry sausages coinoculated with different autochthonous starter cultures after a nine-day ripening. Different lowercase letters (a–c) among the different treatments at the same time indicate significant differences (p < 0.05). Control: noninoculation; LP: Lactiplantibacillus plantarum S8; LP + SC: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8; LP + SC + PK: Lactiplantibacillus plantarum S8 + Staphylococcus carnosus L8 + Pichia kudriavzevii M6.
4. Conclusions
Coinoculation with autochthonous starter cultures had a significant effect on improving the formation of FAAs and flavor, and inhibition of BA accumulation. Notably, the coinoculation of L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 promoted the formation of FAAs and volatile compounds, improved the comprehensive sensory characteristics, and inhibited BA accumulation. In addition, a Pearson correlation analysis between FAAs and volatile compounds, and FAAs and BAs, confirmed that FAAs are crucial in the formation of flavor compounds and BAs. Overall, L. plantarum S8, S. carnosus L8, and P. kudriavzevii M6 are promising compound starter cultures for inhibiting BA accumulation and improving the flavor profiles and sensory characteristics of dry sausage. This study provided valuable information for improving the flavor profile and safety of Harbin dry sausages.
Ethical statement
No coercion to participate, full disclosure of study requirements and risks, written or verbal consent of participants, no release of participant data without their knowledge, ability to withdraw from the study at any time.
CRediT authorship contribution statement
Yumeng Sui: Writing – original draft, Methodology, Investigation, Data curation. Jiaqi Liu: Methodology, Investigation. Jiasheng Lu: Software. Yuan Gao: Methodology. Iftikhar Hussain Badar: Investigation. Xiang-ao Li: Data curation. Qian Chen: Project administration, Funding acquisition. Baohua Kong: Supervision. Ligang Qin: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (32172232 and U22A20547).
Data availability
Data will be made available on request.
References
- AOAC . Association of Official Analytical Chemists; Arlington, VA: 1995. AOAC, association of official methods of analysis methods 925.04. [Google Scholar]
- Cavalheiro C.P., Ruiz-Capillas C., Herrero A.M., Pintado T. Dry-fermented sausages inoculated with Enterococcus faecium CECT 410 as free cells or in alginate beads. LWT - Food Science and Technology. 2020;139 doi: 10.1016/j.lwt.2020.110561. [DOI] [Google Scholar]
- Chen D., Liu S. Impact of simultaneous and sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on non-volatiles and volatiles of lychee wines. LWT - Food Science and Technology. 2016;65:53–61. [Google Scholar]
- Chen Q., Kong B.H., Han Q., Xia X.F., Xu L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT - Food Science and Technology. 2017;77:389–396. doi: 10.1016/j.lwt.2016.11.075. [DOI] [Google Scholar]
- Chen Q., Liu Q., Sun Q.X., Kong B.H., Xiong Y.L. Flavour formation from hydrolysis of pork sarcoplasmic protein extract by a unique lab culture isolated from Harbin dry sausage. Meat Science. 2015;100:10–117. doi: 10.1016/j.meatsci.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Chen Z.G., Chen H., Du H., Chen C., Lu K.X., Xue Q.L., Hu Y.J. Dynamic changes in physicochemical property, biogenic amines content and microbial diversity during the fermentation of sanchuan ham. Food Science and Human Wellness. 2023;13(1):506–516. doi: 10.26599/FSHW.2022.9250044. [DOI] [Google Scholar]
- China National Standard . 2016. Determination of biogenic amines in food. GB 5009.208. [Google Scholar]
- Dabadé S.D., Jacxsens L., Miclotte L., Abatih E., Devlieghere F., Meulenaer B.D. Survey of multiple biogenic amines and correlation to microbiological quality and free amino acids in foods. Food Control. 2021;120:107497. doi: 10.1016/j.foodcont.2020.107497. [DOI] [Google Scholar]
- Dai W.L., Gu S.Q., Xu M., Wang W.J., Yao H.Z., Zhou X.X., Ding Y.T. The effect of tea polyphenols on biogenic amines and free amino acids in bighead carp (Aristichthys nobilis) fillets during frozen storage. LWT - Food Science and Technology. 2021;150 doi: 10.1016/J.LWT.2021.111933. [DOI] [Google Scholar]
- Domínguez R., G’omez M., Fonseca S., Lorenzo J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. Lebensmittel-Wissenschaft und -Technologie- Food Science and Technology. 2014;58(2):439–445. doi: 10.1016/j.lwt.2014.04.006. [DOI] [Google Scholar]
- Dong C.H., Shi S., Pan N., Du X., Li H.J., Xia X.X. Inhibitory mechanism of tyramine-degrading strains on reducing tyramine accumulation in Harbin dry sausage during fermentation. Food Control. 2022;137 [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ) Scientific opinion on risk-based control of biogenic amine formation in fermented foods. EFSA Journal. 2011;9(10):2393. doi: 10.2903/j.efsa.2011.2393. [DOI] [Google Scholar]
- Emer C.D., Marques S., Colla L.M., Reinehr C.O. Biogenic amines and the importance of starter cultures for malolactic fermentation. Australian Journal of Grape and Wine Research. 2021;27(1):26–33. doi: 10.1111/ajgw.12462. [DOI] [Google Scholar]
- Essid I., Hassouna M. Effect of inoculation of selected Staphylococcus xylosus and Lactobacillus plantarum strains on biochemical, microbiological and textural characteristics of a tunisian dry fermented sausage. Food Control. 2013;32(2):707–714. doi: 10.1016/j.foodcont.2013.02.003. [DOI] [Google Scholar]
- FDA Guidance for industry on fish and fishery products hazards and controls, fourth edition; availability. US Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition. 2011;76(082) [Google Scholar]
- Flores M., Durá M.A., Marco A., Toldrá F. Effect of debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Science. 2004;68(3):439–446. doi: 10.1016/j.meatsci.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Giello M., Storia A.L., Filippis F.D., Ercolini D., Villani F. Impact of Lactobacillus curvatus 54M16 on microbiota composition and growth of listeria monocytogenes in fermented sausages. Food Microbiology. 2018;72(JUN.):1. doi: 10.1016/j.fm.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Gómez M., Lorenzo J.M. Effect of fat level on physicochemical, volatile compounds and sensory characteristics of dry-ripened “chorizo” from celta pig breed. Meat Science. 2013;95(3):658–666. doi: 10.1016/j.meatsci.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Hu Y.Y., Chen Q., Wen R.X., Wang Y., Qin L.G., Kong B.H. Quality characteristics and flavor profile of Harbin dry sausages inoculated with lactic acid bacteria and Staphylococcus xylosus. LWT - Food Science and Technology. 2019;114 doi: 10.1016/j.lwt.2019.108392. [DOI] [Google Scholar]
- Hu Y.Y., Li Y.J., Li X.A., Zhang H.W., Chen Q., Kong B.H. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT - Food Science and Technology. 2021;154 doi: 10.1016/J.LWT.2021.112723. [DOI] [Google Scholar]
- Hu Y.Y., Tian Y., Zhu J.M., Wen R.X., Chen Q., Kong B.H. Technological characterization and flavor-producing potential of lactic acid bacteria isolated from traditional dry fermented sausages in northeast China. Food Microbiology. 2022;106 doi: 10.1016/J.FM.2022.104059. [DOI] [PubMed] [Google Scholar]
- Hu Y.Y., Wang J.W., Liu Q., Wang Y., Ren J., Chen Q., Kong B.H. Unraveling the difference in flavor characteristics of dry sausages inoculated with different autochthonous lactic acid bacteria. Food Bioscience. 2022;47 doi: 10.1016/J.FBIO.2022.101778. [DOI] [Google Scholar]
- Hua Q., Sun Y.Y., Xu Y.S., Gao P., Xia W.S. Contribution of mixed commercial starter cultures to the quality improvement of fish-chili paste, a Chinese traditional fermented condiment. Food Bioscience. 2022;46 doi: 10.1016/j.fbio.2022.101559. [DOI] [Google Scholar]
- ISO . International Organization for Standardization; Geneva, Switzerland: 2012. Sensory analysis—general guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. Volume ISO 8586. [Google Scholar]
- Jiang X.W., Peng D., Zhang W., Duan M.Y., Ruan Z.Q., Huang S.E.…Fang Q.J. Effect of aroma-producing yeasts in high-salt liquid-state fermentation soy sauce and the biosynthesis pathways of the dominant esters. Food Chemistry. 2021;344(1) doi: 10.1016/j.foodchem.2020.128681. [DOI] [PubMed] [Google Scholar]
- Kalač P. Biologically active polyamines in beef, pork and meat products: A review. Meat Science. 2006;73(1):1–11. doi: 10.1016/j.meatsci.2005.11. [DOI] [PubMed] [Google Scholar]
- Li X.A., Kong B.H., Wen R.X., Wang H.P., Li M.T., Chen Q. Flavour compensation role of yeast strains in reduced-salt dry sausages: Taste and odour profiles. Foods. 2022;11:650. doi: 10.3390/foods11050650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.A., Sui Y.M., Lu J.S., Ren J., Kong B.H., Li Y.J.…Yang W.W. Compensative role of autochthonous lactic acid bacteria in physical properties and taste profiles of dry sausage with partial substitution of NaCl by KCl. LWT - Food Science and Technology. 2024;199 doi: 10.1016/j.lwt.2024.116115. [DOI] [Google Scholar]
- Li Y.X., Cao Z.X., Yu Z.H., Zhu Y., Zhao K.L. Effect of mixed cultures starter of Lactobacillus and Staphylococcus on bacterial communities and volatile flavor in fermented sausages. Food Science and Human Health. 2023;12(1):12. doi: 10.1016/j.fshw.2022.07.010. [DOI] [Google Scholar]
- Lorenzo J.M., Cachaldora A., Fonseca S., G’omez M., Franco I., Carballo J. Production of biogenic amines “in vitro” in relation to the growth phase by Enterobacteriaceae species isolated from traditional sausages. Meat Science. 2010;86(3):684–691. doi: 10.1016/j.meatsci.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Marusic N., Vidacek S., Janci T., Petrak T., Medic H. Determination of volatile compounds and quality parameters of traditional dry-cured ham. Meat Science. 2014;96(4):1409–1416. doi: 10.1016/j.meatsci.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Olesen P.T., Meyer A.S., Stahnke L.H. Generation of flavour compounds in fermented sausages―the influence of curing ingredients, Staphylococcus starter culture and ripening time. Meat Science. 2004;3:675–687. doi: 10.1016/s0309-1740(03)00189-x. [DOI] [PubMed] [Google Scholar]
- Özogul F., Hamed I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Critical Reviews in Food Science and Nutrition. 2018;10:1660–1670. doi: 10.1080/10408398.2016.1277972. [DOI] [PubMed] [Google Scholar]
- Ren H.Y., Deng Y.P., Wang X.H. Effect of a compound starter cultures on bacterial profile and biogenic amine accumulation in Chinese Sichuan sausages. Food Science and Human Health. 2022;11(2):8. doi: 10.1016/j.fshw. [DOI] [Google Scholar]
- Rubén D., Paulo E.M., Rubén A., José M.L. Effect of commercial starter cultures on free amino acid, biogenic amine and free fatty acid contents in dry-cured foal sausage. LWT - Food Science and Technology. 2016;71:47–53. doi: 10.1016/j.lwt.2016.03.016. [DOI] [Google Scholar]
- Seleshe S., Kang S.N. Effect of different pediococcus pentosaceus and Lactobacillus plantarum strains on quality characteristics of dry fermented sausage after completion of ripening period. Food Science of Animal Resources. 2021;41(4):636–649. doi: 10.5851/KOSFA.2021.E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidira M., Kandylis P., Kanellaki M., Kourkoutas Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chemistry. 2015;178:201–207. doi: 10.1016/j.foodchem. [DOI] [PubMed] [Google Scholar]
- Sun Q.X., Chen Q., Li F.F., Zheng D.M., Kong B.H. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Control. 2016;68:358–366. doi: 10.1016/j.foodcont.2016.04.021. [DOI] [Google Scholar]
- Sun W.Z., Zhao Q.Z., Zhao H.F., Zhao M., Yang B. Volatile compounds of cantonese sausage released at different stages of processing and storage. Food Chemistry. 2010;121(2):319–325. doi: 10.1016/j.foodchem.2009.12.031. [DOI] [Google Scholar]
- Sun Y.Y., Hua Q., Tian X.Y., Xu Y.S., Gao P., Xia W.S. Effect of starter cultures and spices on physicochemical properties and microbial communities of fermented fish (suanyu) after fermentation and storage. Food Research International, (Ottawa, Ont.) 2022;159 doi: 10.1016/j.foodres.2022.111631. [DOI] [PubMed] [Google Scholar]
- Wang D.B., Zhao L.H., Su R.N., Jin Y. Effects of different starter culture combinations onmicrobial counts and physico-chemical properties in dry fermented mutton sausages. Food Science & Nutrition. 2019;7(6):1957–1968. doi: 10.1002/fsn3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.P., Zhang H.W., Liu S.T., Qin L.G., Chen Q., Kong B.H. Analysis of biogenic amine in dry sausages collected from northeast China: From the perspective of free amino acid profile and bacterial community composition. Food Research International. 2022;162 doi: 10.1016/J.FOODRES.2022.112084. [DOI] [PubMed] [Google Scholar]
- Wang T., Hammond E.G. Lipoxygenase and lipid oxidation in foods. Oxidation in Foods and Beverages and Antioxidant Applications. 2010;105-121 doi: 10.1533/9780857090447.1.105. [DOI] [Google Scholar]
- Wen R.X., Dong Z.M., Lv Y.C., Liu H.T., Bayana B., Kong B.H., Chen Q. Comparative evaluation of the flavour-promoting role of autochthonous yeast strains on dry sausages. LWT - Food Science and Technology. 2023;184(15) doi: 10.1016/J.LWT.2023.115032. [DOI] [Google Scholar]
- Xiao Y.Q., Liu Y.N., Chen C.G., Xie T.T., Li P.J. Effect of Lactobacillus plantarum and Staphylococcus xylosus on flavour development and bacterial communities in Chinese dry fermented sausages. Food Research International. 2020;135(1) doi: 10.1016/j.foodres.2020.109247. [DOI] [PubMed] [Google Scholar]
- Xie C.Z., Qin L.K., Liu N., Wen A.Y., Zeng H.Y., Miao S. Flavor formation by amino acid catabolism in low-salt sufu paste, a Chinese fermented soybean food. Food Bioscience. 2024;59 doi: 10.1016/j.fbio.2024.104228. [DOI] [Google Scholar]
- Xu Y.S., Li L., Regenstein J.M., Gao P., Zang J.H., Xia W.S., Jiang Q.X. The contribution of autochthonous microflora on free fatty acids release and flavor development in low-salt fermented fish. Food Chemistry. 2018;256:259–267. doi: 10.1016/j.foodchem.2018.02.142. [DOI] [PubMed] [Google Scholar]
- Ye H.Q., Lang X.S., Ji Y.Y., Li S.N., Xin N.C., Meng X.R.…Zhao C.H. The interaction between Lactobacillus plantarum sc-5 and its biogenic amine formation with different salt concentrations in chinese dongbei suancai. Food Research International. 2021;150 doi: 10.1016/j.foodres.2021.110813. [DOI] [PubMed] [Google Scholar]
- Zang J.H., Xu Y.S., Xia W.S, Regenstein, J.M., Yu D.W., Yang F., Jiang Q.X. Correlations between microbiota succession and flavor formation during fermentation of chinese low-salt fermented common carp (Cyprinus carpio L.) inoculated with mixed starter cultures. Food Microbiology. 2020;90 doi: 10.1016/j.fm.2020.103487. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Qin Y.X., Wang Y., Li P.L. Lactobacillus plantarum LPL-1, a bacteriocin producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT - Food Science and Technology. 2020;128 doi: 10.1016/j.lwt.2020.109385. [DOI] [Google Scholar]
- Zhao D.D., Hu J., Chen W.X. Analysis of the relationship between microorganisms and flavour development in dry-cured grass carp by high throughput sequencing, volatile flavour analysis and metabolomics. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.