Abstract
Objective
Delays in the diagnosis and treatment of pulmonary tuberculosis (PTB) can increase the risk of transmission, thereby posing a significant risk to public health. Early diagnosis is considered to play a crucial role in eliminating TB. Rapid testing, active case finding, and health education are effective strategies for reducing tuberculosis diagnosis delays (TDDs). This study aimed to quantitatively compare the impact of reducing the TDD on incidence rates among student and non-student groups, thus exploring the efficacy of shortening the TDD for ending the TB epidemic and providing a reference for achieving the target incidence rate for ending TB.
Methods
We used unsupervised hierarchical clustering analysis and non-parametric tests to characterize the epidemiological characteristics of TDD. Additionally, a dynamic transmission model was used to quantify the impact of shortening the TDD on the incidence rates of TB among the two groups.
Results
There was an initial increase in the TDD, followed by a decrease. Longer TDDs were observed in the northeastern region of China. Farmers, middle and high school students, middle-aged, elderly individuals and males exhibited relatively longer TDDs. A significant reduction in the incidence rate of PTB was observed when the TDD was decreased by 50 %. However, only reducing the TDD among non-students could achieve the goal of ending TB (i.e., achieving a minimum reduction of 63.00 %).
Conclusions
TDD remains a serious risk to public health, and non-students were shown to experience longer TDD. Shortening the TDD is crucial for reducing the incidence rates of TB, especially among non-students. It is essential to develop a highly sensitive and effective system for eliminating TB among non-students.
Keywords: Diagnostic delays, Pulmonary tuberculosis, Dynamic model, End tuberculosis, Active case finding, Students
1. Background
Tuberculosis (TB) is an infectious disease that is one of the leading causes of death worldwide. Prior to the COVID-19 pandemic, TB had the highest mortality rate of any infectious disease, exceeding that of HIV/AIDS. It was reported that 1.5 million people died from TB in 2020. Although TB is preventable and curable, only 42 % of individuals with TB-related symptoms seek medical assistance promptly [1]. A delay in diagnosis and treatment can lead to a higher risk of transmission and even drug-resistant pulmonary tuberculosis (PTB), which poses an enormous risk to public health [2].
In 2015, the World Health Organization (WHO) proposed the “End TB” strategy, which aims to reduce the incidence of TB by 80 % by 2030 and by 90 % by 2035 (compared to the rates observed in 2015). Although China has made significant progress in preventing and controlling TB, the current situation remains highly concerning. The incidence rate of TB in China only decreased by 11 % in 2020, which falls well below the anticipated target for the year [3]. Achieving the goal of eliminating TB in our country will be highly challenging if methods for TB prevention and control do not progress. Countries are conducting research on various approaches, such as developing novel TB vaccines [4], repurposing drugs [5] and optimizing drug regimens [6]. Proactive screening is regarded as the cornerstone of TB prevention and control strategies [7].
The "End TB" strategy explicitly emphasizes the significance of early diagnosis in TB control [8]. Early detection and prompt treatment of TB can effectively prevent disease progression and reduce transmission. However, a significant number of TB patients either live with and die from undiagnosed disease or experience delays in diagnosis and treatment. It is estimated that approximately 3 million TB cases are undetected each year [9]. Current measures to address delays in TB diagnosis and treatment mainly include active case finding (ACF), employing effective diagnostic methods and strengthening TB awareness campaigns.
ACF aims to promptly identify undiagnosed patients in order to minimize avoidable delays in diagnosis and treatment. Studies have shown that TB screening can reduce symptom duration by 33 % [10]. A cross-sectional study conducted in Yunnan [11] compared the effects of ACF and passive case finding (PCF) on shortening tuberculosis diagnosis delays (TDDs). The results indicated that compared to PCF, ACF reduced patient delays by 29 days but increased health system delays by 21 days. While ACF reduced the time from symptom onset to healthcare seeking, limitations in diagnostic methods and complex diagnostic procedures led to increased health system delays [12]. By decentralizing diagnosis and training county-level healthcare workers to simplify diagnostic confirmation processes, ACF diagnostic delays may be further reduced [11]. Additionally, the use of diagnostic methods with higher sensitivity than conventional approaches greatly enhances the effectiveness of ACF [13]. According to Lee et al.'s review [14], compared to sputum microscopy, the use of Xpert for diagnosis can reduce diagnostic delays by 1.79 days (95 % CI - 0.27–3.85). Drobniewski et al.'s study [15] further demonstrated the feasibility and effectiveness of Xpert as a new diagnostic tool for ACF. Compared to conventional methods such as sputum smear microscopy, sputum culture, and drug susceptibility testing (DST), using Xpert for diagnosis not only saves costs but also reduces QALY loss. Moreover, TB awareness campaigns also serve as crucial strategies for reducing diagnostic delays. A community randomized trial [16] employing sputum smear microscopy yielded a 55–60 % reduction in diagnostic delays through the implementation of monthly outreach clinics and continuous mobilization by community health workers. Integrating ACF based on rapid diagnostic tests and TB awareness campaigns will significantly reduce TDDs.
However, most previous studies have focused on analyzing the duration of diagnostic delays in different populations and the factors influencing these delays, and few studies have analyzed the effect of reducing TDD on the incidence and elimination of TB. This study utilized a model fitted with more than 15 years of epidemiological data to evaluate the feasibility of ending TB by shortening TDD in different populations and regions, thus providing insights for achieving the goal of ending TB as soon as possible.
2. Methods
2.1. Study design
This study includes four main steps. The first step describes the characteristics of the temporal, spatial, and demographic distributions of TDD. The second step establishes the model and determines the model parameters. The third step examines the fit of the model using the collected data. The fourth step simulates the effect of shortening different diagnostic delays on the incidence of TB based on the TDD calculated in the first step among the student and non-student groups. This study collected data from TB cases between 2005 and 2019 across four regions in China: the northeast, southwest, southeast, and central regions. The data are derived from a published paper [17].
2.2. Definitions of TDD and PTB
TDD refers to the time from the onset of symptoms in a patient until the diagnosis of TB is made and includes two time intervals: patient delay and health system delay. Patient delay is the time from the onset of symptoms to the first medical consultation. The health system delay is the time from the first medical consultation to the diagnosis of TB. This study calculated the TDD by subtracting the diagnosis time from the onset date recorded in the infectious disease report [18,19].
We defined PTB as bacteriologically or clinically diagnosed TB of the lung tissue, trachea, bronchi, or pleura. The bacteriological diagnosis is based on the results of sputum smears and sputum cultures. Clinical diagnosis is mainly based on pathogenic (including bacteriological and molecular biological) examination combined with the patient’s epidemiological history, clinical manifestations (cough and sputum for ≥2 weeks or hemoptysis), chest imaging, relevant auxiliary examinations and differential diagnosis [20,21].
2.3. Model establishment
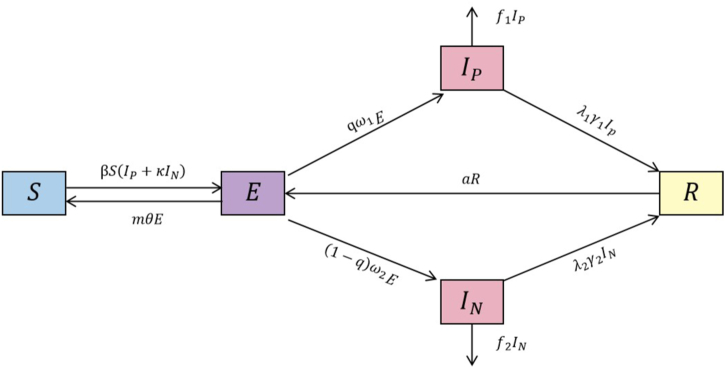
According to the latest criteria issued in China in 2018, TB was classified into four categories: “pathogenicity positive”, “pathogenicity negative”, “rifampicin resistant”, and “no pathogenicity result” [22]. We reclassified the data according to this classification. However, rifampicin resistance and no pathogenetic results accounted for a relatively small proportion (<5 %), so only the “pathogenetic positive” and “pathogenetic negative” classifications were considered in this paper. Based on the underlying SEIR model, the SEINIPR model was constructed by classifying TB cases into pathogenically positive IP and pathogenically negative IN (Fig. 1). The population in the model was classified as susceptible (S), latent (E), TB pathogenetically positive (IP), TB pathogenetically negative (IN) or recovered (R) [17].
Fig. 1.
Schematic diagram of the SEIPINR model.
The model developed in this study is based on the following assumptions.
-
(1)
Population migration and births and deaths are not considered in the model.
-
(2)
Both pathogenetically positive and pathogenetically negative individuals are infectious, but pathogenetically positive individuals are к times more infectious than are pathogenetically negative individuals.
-
(3)
The duration for a latent individual to transition into an active TB patient is represented by the variable 1/ω.The likelihood that a latent person will develop pathologically positive symptoms is denoted as q, while the probability of that person developing pathologically negative symptoms is represented as (1-q).
-
(4)
Latent individuals can clear TB bacteria at an early stage, thereby preventing the development of active TB. The rate at which early TB clearance occurs is denoted as m, and the ratio of early TB clearance is denoted as θ.
-
(5)
A proportion of the recovered population will return to the susceptible population due to reinfection. In this context, α is the proportion of recurrences, and τ is the recurrence rate.
-
(6)
The occurrence of TB-related deaths among TB patients is characterized by a mortality rate of f1 for pathogenetically positive individuals and f2 for pathogenetically negative individuals.
-
(7)
According to previous research, people with non-resistant active TB usually stop being infected after two weeks of this therapy [23]. Therefore, in this study, we set the time to achieve non-infectiousness through treatment to 14 days. The variable 1/γ is defined as the sum of the duration from the initiation of treatment until the TB patient is no longer contagious and the time it takes for TB diagnosis. The probability of successful treatment is denoted by λ.
Differential equations:
The estimation of model parameters was primarily based on the literature review and calculations made using the collected data. The specific details are presented in Table S1.
We conducted segmented simulations based on the respective TDD of the corresponding regions, with the interquartile range (IQR) of each region as the intervention threshold (0–31.5 days). We simulated the variations in incidence rates between 2020 and 2035 across different scenarios for each region and population. Additionally, we compared the predicted incidence rates for 2035 and 2030 with the baseline data from 2015 to assess the feasibility of ending TB.
First, we simulated the number of cases from 2020 to 2035 for each region and population under different scenarios of TDD reduction (0 %, 25 %, 50 %, 75 %, and 100 %). Second, we employed a binary search method to explore the proportion of TDD reduction needed to achieve the target incidence rate for ending TB for each region and population. For instance, among the non-students in the northeast region with a TDD of 31.5 days, we initially considered a 50 % reduction in TDD (X = 0.5). We then observed whether the incidence rates for 2030 and 2035 reached the target. Otherwise, we adjusted the proportion of TDD reduction between 50 % and 100 % (setting X = 0.75). If the target was achieved, we considered a TDD reduction between 0 % and 50 % (setting X = 0.25). We repeated this process until a precise percentage of TDD reduction was identified to achieve the target for ending TB. For example, a TDD reduction of 54 % was needed to achieve the incidence rate target for 2030 in the northeast region, while a reduction of 72 % was required for 2035.
Where γ represents the reciprocal of the duration from infectivity to recovery and the TDD time, X represents the TDD for the corresponding region and population, and p represents the proportion of TDD reduction.
2.4. Statistical analysis
An unsupervised hierarchical clustering analysis was employed to construct a hierarchy of occupational clusters and determining their distances using the Euclidean method. The Mann‒Whitney U test was used to compare two groups, while the Kruskal‒Wallis and Dunn’s tests were employed for comparisons involving two or more groups. Data analyzes were conducted using R (version 4.2.2, R Foundation). Model fitting was performed using the “deSolve” and “bbmle” packages in R version 4.2.2. The degree of model fit was assessed using the R-squared (R2) value and p value. The “ggplot2” package in R version 4.2.2 was utilized to create the graphical plots for visual representation.
3. Results
We collected data from 225,993 patients, including 14,756 students and 211,237 non-students. The male-to-female ratio was approximately 7:3, and the ages ranged from 1 to 105 years. The median TDD among the total population was 21.0 (interquartile range [IQR]:7.0–44.0) days; among students, it was 16.0 (IQR: 6.0–34.4) days, and among non-students, it was 21.0 (IQR: 7.0–45.0) days. Both the student and non-student groups experiences severe TDD, with the non-student group bearing the greatest burden.
3.1. Epidemiological characteristics of TDD
3.1.1. Spatiotemporal characteristics of TDD vary across populations and regions
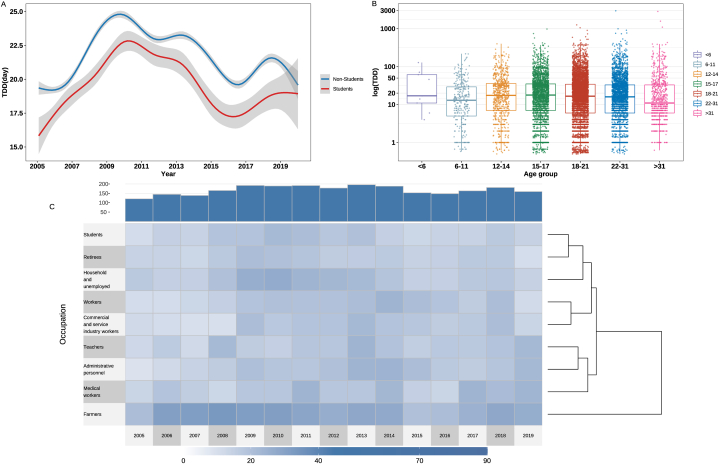
Throughout the study period, the TDD among both the student and non-student groups exhibited an initial increase, followed by a decrease. Additionally, the overall TDD was shorter among students than among non-students during each stage of the study (Fig. 2A).
Fig. 2.
Temporal trends and occupational and age distribution characteristics of TDD. A) The trend of TDD among student and non-student groups. B) Age distribution among student and non-student groups. C) Unsupervised hierarchical cluster analysis of occupation.
The similarity of TDD among the four regions was analyzed by comparing the students with the non-students in the corresponding. In the southwest region, no significant difference was found between the TDD among the students and non-students (p = 0.108); however, significant differences were observed in the other three regions (p < 0.001). As detailed in Table 1.
Table 1.
TDD in four regions.
| Region | Occupation | Median | IQR | p-value |
|---|---|---|---|---|
| Northeast region | Students | 24.6 | (5.4–38.6) | <0.001 |
| Non-students | 31.5 | (9.4–61.4) | ||
| Southwest region | Students | 19.0 | (7.0–43.0) | 0.108 |
| Non-students | 22.0 | (7.0–57.0) | ||
| Southeast region | Students | 12.0 | (6.0–32.0) | <0.001 |
| Non-students | 18.0 | (7.0–47.0) | ||
| Central region | Students | 15.0 | (6.0–31.0) | <0.001 |
| Non-students | 16.0 | (7.0–35.0) |
When analyzing TDD across different regions, we observed a significant difference in TDD (p < 0.001). Multiple comparisons between regions were subsequently made. The results revealed that among the students, there was no significant difference between the southeast region and the central region. Among the non-students, there was a statistically significant difference in the multiple comparisons of all regions (p < 0.05) (Table S3). The northeast region exhibited the longest delay in TB diagnosis, while the central and southeast regions had shorter delays in diagnosing TB. Notably, within the non-student group of the northeast region in 2006, there was a high TDD, with the median TDD for that year being 62.5 (IQR: 31.0–98.0) days (Fig. S1).
3.1.2. Differences in TDD among occupations, age groups, and genders
Overall, the median TDD was 16.0 (IQR: 6.0–34.4) days among students and 21.0 (IQR: 7.0–45.0) days among non-students. There was a significant difference among the distinct occupational groups (p < 0.001) (Table 2). The burden of TDD was more pronounced among the non-students than among the students.
Table 2.
Occupational distribution of TDD.
| Occupations | Median | IQR | p value |
|---|---|---|---|
| Farmers | 29.0 | (8.0–59.6) | <0.001 |
| Household and unemployed | 19.0 | (6.5–41.0) | |
| Workers (including migrant workers) | 16.0 | (6.0–37.0) | |
| Students | 16.0 | (6.0–34.4) | |
| Retirees | 16.0 | (6.0–36.0) | |
| Commercial and service industry workers (including commercial services, food and beverage industry, and public service personnel) | 16.0 | (6.0–36.0) | |
| Administrative personnel | 17.0 | (6.0–36.0) | |
| Teachers | 17.0 | (6.0–39.9) | |
| Medical workers | 18.0 | (7.0–38.0) | |
| Others | 22.6 | (8.0–43.0) | |
| Unknown | 19.0 | (7.0–40.0) |
When performing unsupervised hierarchical cluster analysis of TDD based on different occupations, it was evident that farmers experienced the most prolonged TB diagnostic delays, with a median TDD of 29.0 days (IQR: 8.0–59.6). When divided into two groups, farmers are divided into a separate group. When divided into three groups, farmers constituted a standalone cluster. Teachers, administrative personnel, and medical workers formed another cluster, and students, retirees and other inactive people, homemakers and unemployed people, workers, and the rest were grouped together (Fig. 2C).
There were statistically significant disparities in different age groups among the students (p = 0.002). High school students (aged 15–17) exhibited the most significant diagnostic delay (Fig. 2B), with a median TDD of 17.8 (IQR: 7.0–35.0) days (Table S4). Similarly, among the non-students, the diagnostic delays of middle-aged and older adults (aged 46–50) were more severe, with a median TDD of 23.1 (IQR: 7.0–48.0) days (p < 0.001). In contrast, the diagnostic delays for the young and elderly patients were relatively small, with median diagnostic delays less than 15 days (Table S5).
Moreover, the study revealed statistically significant differences in the gender-specific TDD among both the student (p = 0.005) and non-student groups (p = 0.021) (Table S4 and Table S5). Among the student and non-student groups, the median TDDs for female TB patients were 17.0 days (IQR: 6.0–35.0) and 22.0 days (IQR: 7.0–46.0), respectively. For males, it was 16.0 days (IQR: 6.0–33.6) and 21.0 days (IQR: 7.0–44.4), respectively. Diagnostic delays for female TB patients were greater than those for male patients.
3.2. Model fitting and simulation of interventions
The number of TDDs calculated among students and non-students was entered into the model for simulation (16 and 21 days, respectively). Table S2 displays the goodness of fit of the model, which was satisfactory.
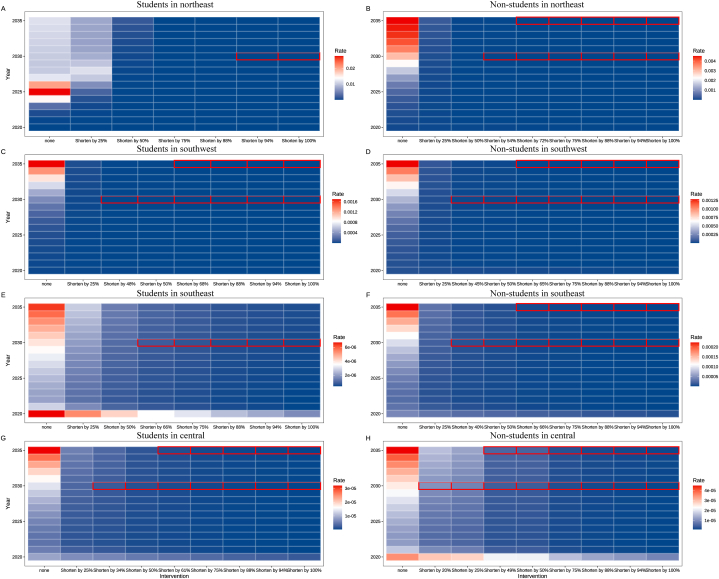
In the first phase of the study, we calculated the TDD for each region. In the second phase, we conducted segmented simulations based on the specific diagnostic delay of each region. The model projects that, except for students in the northeast, by reducing TB diagnosis delays by 50 %, the incidence of TB in 2030 and 2035 will be significantly lower than that in 2015. By 2030, student incidence in the southwest, southeast, and central regions is projected to decrease by 16.92 %, 26.27 %, and 10.92 %, respectively. By 2035, the expected decreases in incidence rates for these three regions will be 26.80 %, 33.14 %, and 14.88 %, respectively. Among the non-students, the projected decreases in incidence rates for the northeast, southwest, southeast, and central regions by 2030 will be 24.26 %, 15.08 %, 13.40 %, and 7.40 %, respectively. By 2035, the projected decreases in incidence rates for these regions will be 37.01 %, 21.72 %, 18.16 %, and 9.42 %, respectively.
Fig. 3A–H illustrates the projected outcomes concerning the attainment of ending TB among the student and non-student groups. Intensifying efforts to mitigate the delay in TDD among non-students can notably augment the prospects of accomplishing TB eradication. Among the students, to surpass an 80 % reduction in the TB incidence rate by 2030 relative to 2015, a minimum reduction of at least 60.05 % in TDD is necessary. Only the southwest and central regions can achieve the goal of ending TB by reducing the delay in TB diagnosis in the region. For these regions, the percentages of TDD reduction needed were 68.00 % and 61.00 %, respectively. Among the non-students, achieving a greater than 80 % reduction in the incidence rate by 2030 compared to 2015 requires at least a 39.75 % reduction in TDD. However, to attain the goal of ending TB, which also requires reducing incidence by 90 % by 2035, a minimum reduction of 63.00 % in TDD is imperative (Table S6).
Fig. 3.
Predicting the likelihood of ending TB by shortening TDD. The heatmap within the outlined boxes represents the incidence rates over various years, ranging from blue (low incidence) to white to red (high incidence). (A) Students in the northeast region. (B) Non-students in the northeast region. (C) Students in the southwest region. (D) Non-students in the southwest region. (E) Students in the southeast region. (F) Non-students in the southeast region. (G) Students in the central region. (H) Non-students in the central region. Red circles represent regions where the student/non-student groups are able to achieve the target for ending TB.
Moreover, our findings revealed a slight increase in TB incidence rates around the year 2030. The projections indicate that by 2035, the incidence rates among student and non-student groups will be higher than those estimated for 2030.
4. Discussion
This study aimed to analyze the differences in TDDs between students and non-students and attempted to simulate and evaluate the effect of reducing TDDs on ending TB.
4.1. TDD cannot be ignored, and the diagnostic delay tends to be more severe among the non-students
According to the analysis of the time trend of TDD, the overall trend of TDD in both the student and non-student groups has shown an upwards trend since 2005, followed by a decreasing trend and an upwards trend. This pattern aligns with the overall change trend in Guangzhou City from 2008 to 2018 [24,25]. The gradual decrease of TDD may be related to the gradual improvement of the reporting system and the improvement of the treatment. However, the upwards trend between 2017 and 2019 suggests that efforts to reduce TDD must continue. As a result, it is critical to continuously strengthen methods such as TB screening and absence registration due to sickness, allowing for early detection of tuberculosis cases. Disease prevention and control agencies should also use the "Infectious Disease Automatic Alert Information System" to monitor the population and identify TB cases in a timely manner. At the same time, key medical institutions should increase detection sensitivity.
In this research, the TDD in the student group was lower than that in the non-student group. This finding is in good agreement with a study conducted in Gambia, which confirmed that the median total delay in treatment varied by occupation: the shortest delay was reported among students (4 (IQR: 3–8) weeks), followed by traders (7 (IQR: 4–11) weeks) and military or police personnel (8 (IQR: 4–10) weeks) [26]. Given that schools routinely screen new students and conduct medical exams, aiding in the early discovery of cases. Furthermore, surveillance systems among students are more reliable than those among non-students [27]. Since 2010, the school TB epidemic surveillance system has undergone continuous improvement, and various TB epidemic contingency plans have been implemented to effectively prevent the transmission and outbreaks of TB within schools. In addition, schools usually carry out health promotion initiatives to enhance their health and disease prevention awareness, which may also help to reduce delays in diagnosis.
4.2. Reducing the diagnostic delay among the non-students holds greater significance in attaining the goal of ending TB but is a more formidable challenge in terms of TB control
Reducing the diagnostic delay of TB by 50 % can notably diminish the incidence rate of TB. Previous experience has shown that key strategies for improving early case detection, mitigating delays include active case finding, the use of new rapid tuberculosis diagnostic tools, and communication campaigns [28]. These measures effectively curb the spread and transmission of the disease within the community. Furthermore, Zhang et al.'s study has demonstrated the effectiveness of ACF among high-risk populations, revealing that it can substantially increase the TB patient detection rate, reaching up to 86 % [29]. In the broader context, enhancing the long-term implementation of these interventions is of great significance in controlling the TB epidemic.
Although reducing diagnostic delay is crucial for preventing TB outbreaks within school settings, targeting non-students holds greater significance in attaining the goal of ending TB entirely. Our study indicates that even without diagnostic delays among student groups, the goal of ending TB remains elusive in many regions. While screening students helps minimize outbreaks within schools, their proportion in the overall population is relatively small, limiting the impact of ending TB across the entire population. This finding aligns with the research conducted by Romain et al. [7]. However, addressing diagnostic delays among the non-student groups presents considerable challenges. Non-students have diverse occupations and complexities, leading to limitations in implementing targeted control measures. Moreover, several vulnerabilities exist within the existing prevention and control system, including inadequate functional division within comprehensive hospitals and insufficient diagnosis and treatment efficiency. Considering the trajectory of China’s TB prevention and control system, we recommend directing attention towards non-students and instituting strategies tailored to the distinct characteristics of various occupational groups. Forming a new mechanism to expedite the amalgamation of medical care and prevention, and establishing comprehensive prevention and treatment strategies will be pivotal [30,31]. Furthermore, developing novel approaches to provide timely prevention, early diagnosis, and rapid treatment is imperative.
4.3. Eliminating TB requires the comprehensive implementation of multiple measures
While shortening the TDD is crucial for achieving the goal of ending TB, our research findings indicate that a reduction of at least 63 % in the TDD is required to achieve the target incidence rate for the year 2035. This poses significant demands on current prevention and control systems. Therefore, in addition to reducing diagnostic delays, comprehensive interventions are needed to address this challenge.
The United Nations High-Level Meeting in 2018 identified latent TB infection(LTBI) screening and preventive therapy as crucial indicators for global TB control [32]. Adequate diagnosis and treatment of LTBI are pivotal elements in the effort to halt TB epidemics [3]. A study conducted by Romain et al. emphasized not only the early identification of active TB patients but also the screening and treatment of individuals with LTBI [7]. The inclusion of LTBI screening in ACF programs is crucial. Failure to incorporate LTBI screening in these programs may impede the accomplishment of the “End TB” strategy by 2035, irrespective of the frequency of interventions considered. Our future research endeavors will delve deeper into screening and treatment strategies for LTBI.
Strengthening the development of TB-free communities is also a useful future direction. In 2022, the Chinese Center for Disease Control and Prevention released a plan for establishing TB-free communities [33]. This initiative involves implementing health education programs, screening, and treating susceptible individuals, administering preventive treatment to those with LTBI, and providing care for TB patients within the community. Currently, this measure has been piloted nationwide, yielding favorable results. However, there is a lack of research on the effectiveness of implementing corresponding strategies in TB-free communities to reduce PTB incidence rates. Assessing the feasibility of TB-free communities in ending PTB could serve as one of our future research directions.
Moreover, thorough treatment of patients is essential to prevent recurrence. Our model projected that even if the TDD decreases, a slight increase in incidence rates would occur approximately 2030. This finding aligns with previous research conducted in Africa [34]. As more individuals recover, the underlying number of relapses increases, with reinfection and reactivation of LTBI contributing to higher incidence rates. According to the 2020 WHO report, the TB relapse rate is 6.8 % [35]. Patients experiencing TB relapse exhibit a higher likelihood of developing drug resistance, presenting a risk factor 4.2 times greater than that observed in newly diagnosed patients. These individuals may become a significant source of persistent TB infection. Hence, it is imperative to implement strategies aimed at preventing relapse, such as continuous TB prophylaxis and improved cure programs [36].
This study focused solely on reducing the TDD on TB transmission capacity at the macro level without delving into specific interventions. Our research is based on data from four regions, which limits its generalizability to other areas. Economic disparities and policy differences across regions may influence the implementation of measures aimed at reducing TDD to achieve the goal of ending TB, thus necessitating contextualization. Our analysis is based on data from the National Notifiable Disease Reporting System from 2005 to 2019. However, there may be underreporting of cases, potentially resulting in an underestimation of TDD. Furthermore, our study does not account for potential changes in TDD during the COVID-19 pandemic. The diagnostic delays for TB during this period may differ from those before the onset of COVID-19 due to the influence of the virus. We plan to further explore the comprehensive intervention effects of various measures, including LTBI screening, preventive therapy, and recurrence prevention, on TB transmission. We will also conduct further research using data collected from field surveys.
5. Conclusion
Although tuberculosis diagnostic delays have decreased over the past few years, they remain very serious among both student and non-student groups, exhibiting regional disparities. Compared to students, non-students have longer TDDs, and it is more difficult to shorten TDDs, but it is of greater value for eliminating TB. Moving forwards, it is essential to establish a highly sensitive and effective system of interventions targeting non-students to achieve the goal of ending TB.
Funding
This study was supported by Self-supporting Program of Guangzhou Laboratory (grant number: No. SRPG22-007). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Ethics statement
This study was approved by the Ethics Committee of the School of Medicine, Xiamen University. Consent requirement, either verbal or written, was waived by the ethics Committee of the School of Medicine on the following grounds: (1) only anonymized records were used without the need for direct involvement nor active participation of patients; (2) neither medical intervention nor biological samples were involved; (3) study procedures and results would not affect clinical management of patients in any form.
Data availability statement
Research-related data are not deposited into publicly available repositories. Data will be made available on request.
CRediT authorship contribution statement
Qiao Liu: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Formal analysis, Data curation, Conceptualization. Qiuping Chen: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Conceptualization. Yichao Guo: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. Shanshan Yu: Writing – review & editing, Validation, Methodology. Jia Rui: Writing – review & editing, Validation, Supervision. Kangguo Li: Writing – review & editing, Validation, Software, Methodology. Huimin Qu: Writing – review & editing, Validation. Laurent Gavotte: Writing – review & editing, Validation, Supervision. Roger Frutos: Writing – review & editing, Validation, Supervision, Methodology. Tianmu Chen: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e35016.
Contributor Information
Roger Frutos, Email: frutossmt@gmail.com.
Tianmu Chen, Email: chentianmu@xmu.edu.cn.
Abbreviations
- TB
Tuberculosis
- ACF
Active case finding
- TDD
tuberculosis diagnostic delays
- PTB
pulmonary tuberculosis
- IQR
interquartile range
- LTBI
latent TB infection
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Y., et al. Factors associated with patient, and diagnostic delays in Chinese TB patients: a systematic review and meta-analysis. BMC Med. 2013;11(1):1–15. doi: 10.1186/1741-7015-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang S., Squire S.B. What lessons can de drawn from tuberculosis (TB) Control in China in the 1990s?: an analysis from a health system perspective. Health Pol. 2005;72(1):93–104. doi: 10.1016/j.healthpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Wei S., et al. Tuberculosis research and innovation: interpretation of the WHO global tuberculosis report 2021. Chinese Journal of Antituberculosis. 2022;44(1):45. [Google Scholar]
- 4.Weerasuriya C., et al. New tuberculosis vaccines: advances in clinical development and modelling. J. Intern. Med. 2020;288(6):661–681. doi: 10.1111/joim.13197. [DOI] [PubMed] [Google Scholar]
- 5.Kim L.-H., et al. Novel antibacterial activity of febuxostat, an FDA-approved antigout drug against Mycobacterium tuberculosis infection. Antimicrob. Agents Chemother. 2022;66(9) doi: 10.1128/aac.00762-22. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee B.-Y., et al. Drug regimens identified and optimized by output-driven platform markedly reduce tuberculosis treatment time. Nat. Commun. 2017;8(1) doi: 10.1038/ncomms14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragonnet R., et al. Estimating the long-term effects of mass screening for latent and active tuberculosis in the Marshall Islands. Int. J. Epidemiol. 2022;51(5):1433–1445. doi: 10.1093/ije/dyac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The end TB strategy. https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19 (accessed 10 April 2024)].
- 9.Arja A., et al. Patient delay and associated factors among tuberculosis patients in Gamo zone public health facilities, Southern Ethiopia: an institution-based cross-sectional study. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0255327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verver S., Bwire R., Borgdorff M W. Screening for pulmonary tuberculosis among immigrants: estimated effect on severity of disease and duration of infectiousness. Int. J. Tubercul. Lung Dis. 2001;5(5):419–425. [PubMed] [Google Scholar]
- 11.Chen J.-O., et al. Role of community-based active case finding in screening tuberculosis in Yunnan province of China. Infectious Diseases of Poverty. 2019;8:1–12. doi: 10.1186/s40249-019-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikell A., et al. Diagnostic pathways and delay among tuberculosis patients in Stockholm, Sweden: a retrospective observational study. BMC Publ. Health. 2019;19:1–10. doi: 10.1186/s12889-019-6462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kranzer K., et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review [State of the art series. Case finding/screening. Number 2 in the series] Int. J. Tubercul. Lung Dis. 2013;17(4):432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.H., et al. Impact of molecular diagnostic tests on diagnostic and treatment delays in tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 2022;22(1):940. doi: 10.1186/s12879-022-07855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drobniewski F., et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol. Assess. 2015;19(34):1–viii. doi: 10.3310/hta19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shargie E.B., Mørkve O., Lindtjørn B. Tuberculosis case-finding through a village outreach programme in a rural setting in southern Ethiopia: community randomized trial. Bull. World Health Organ. 2006;84(2):112–119. doi: 10.2471/blt.05.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q., et al. Transmissibility of tuberculosis among students and non-students: an occupational-specific mathematical modelling. Infectious Diseases of Poverty. 2022;11(6):5–26. doi: 10.1186/s40249-022-01046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Organization W.H. 2011. Early Detection of Tuberculosis: an Overview of Approaches, Guidelines and Tools. [Google Scholar]
- 19.Li J., et al. The strategic framework of tuberculosis control and prevention in the elderly: a scoping review towards End TB targets. Infectious diseases of poverty. 2017;6(3):16–27. doi: 10.1186/s40249-017-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khadka P., et al. Diagnosis of tuberculosis from smear-negative presumptive TB cases using Xpert MTB/Rif assay: a cross-sectional study from Nepal. BMC Infect. Dis. 2019;19:1–7. doi: 10.1186/s12879-019-4728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuberculosis diagnosis (replaces WS 288 2008. http://www.nhc.gov.cn/wjw/s9491/201712/a452586fd21d4018b0ebc00b89c06254.shtml
- 22.China's Technical Specifications for Tuberculosis Prevention and Control (2020 Edition). http://www.zgflzz.cn/CN/news/news329.shtml (accessed 30 November 2023)].
- 23.Ahmed N., Hasnain S.E. Molecular epidemiology of tuberculosis in India: moving forward with a systems biology approach. Tuberculosis. 2011;91(5):407–413. doi: 10.1016/j.tube.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Daiquan C., et al. Patient delay and related factors among tuberculosis patient in Fujian Province, 2010—2019. Chinese Journal of Antituberculosis. 2023;45(1):96. [Google Scholar]
- 25.Jianxiong L., et al. Influencing factors of pulmonary tuberculosis diagnosis delay in Guangzhou, 2008—2018. Chinese Journal of Antituberculosis. 2021;43(1):80. [Google Scholar]
- 26.Lienhardt C., et al. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of the Gambia. Int. J. Tubercul. Lung Dis. 2001;5(3):233–239. [PubMed] [Google Scholar]
- 27.Regarding the issuance of the school tuberculosis prevention and control code. http://www.moe.gov.cn/srcsite/A17/moe_943/s3285/201707/t20170727_310182.html 2017 Edition)
- 28.GBD 2021 Tuberculosis Collaborators. Global, regional, National age-specific progress towards the 2020 milestones of the WHO End TB Strategy: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024;24(7):698–725. doi: 10.1016/S1473-3099(24)00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., et al. Prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population based cross-sectional study. Infectious Diseases of Poverty. 2019;8(1):26–35. doi: 10.1186/s40249-019-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du X., et al. Health facilities and treatment service models of the national tuberculosis program—China, 2010− 2020. China CDC Weekly. 2021;3(13):274. doi: 10.46234/ccdcw2021.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., et al. Patient pathway analysis of tuberculosis diagnostic delay: a multicentre retrospective cohort study in China. Clin. Microbiol. Infection. 2021;27(7):1000–1006. doi: 10.1016/j.cmi.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Global Tuberculosis Report. 2018. https://www.who.int/publications/i/item/9789241565646 [Google Scholar]
- 33.Organization W.H. World Health Organization; 2013. Systematic Screening for Active Tuberculosis: Principles and Recommendations. [PubMed] [Google Scholar]
- 34.Houben R.M., et al. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc. Natl. Acad. Sci. USA. 2014;111(14):5325–5330. doi: 10.1073/pnas.1317660111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Global Tuberculosis Report. 2020. https://www.who.int/publications/i/item/9789240013131 [Google Scholar]
- 36.Lenaerts A.J., Chapman P.L., Orme I.M. Statistical limitations to the Cornell model of latent tuberculosis infection for the study of relapse rates. Tuberculosis. 2004;84(6):361–364. doi: 10.1016/j.tube.2004.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research-related data are not deposited into publicly available repositories. Data will be made available on request.