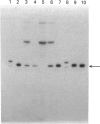
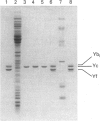
Abstract
Resistance to the carcinogenic effects of aflatoxin B1 (AFB1) in the mouse is due to the constitutive expression of an Alpha-class glutathione S-transferase (GST), YcYc, with high detoxification activity towards AFB1-8,9-epoxide. A cDNA clone (pmusGST Yc) for a murine GST Yc polypeptide has been isolated. Sequencing has shown the cDNA insert of pmusGST Yc to be 922 bp in length, with an open reading frame of 663 bp that encodes a polypeptide of M(r) 25358. The primary structure of the murine GST Yc subunit predicted by pmusGST Yc is in complete agreement with the partial amino acid sequence of the aflatoxin-metabolizing mouse liver GST described previously [McLellan, Kerr, Cronshaw & Hayes (1991) Biochem. J. 276, 461-469]. A plasmid, termed pKK-musGST Yc, which permits the expression of the murine Yc subunit in Escherichia coli, has been constructed. The murine GST expressed in E. coli was purified and found to be catalytically active towards several GST substrates, including AFB1-8,9-epoxide. This enzyme was also found to possess electrophoretic and immunochemical properties closely similar to those of the GST Yc subunit from mouse liver. However, the GST synthesized in E. coli and the constitutive mouse liver Alpha-class GST exhibited small differences in their chromatographic behaviour during reverse-phase h.p.l.c. Automated Edman degradation revealed alanine to be the N-terminal amino acid in the GST Yc subunit expressed in E. coli, whereas the enzyme in mouse liver possesses a blocked N-terminus. Although sequencing showed that the purified Yc subunit from E. coli lacked the initiator methionine, the amino acid sequence obtained over the first eleven N-terminal residues agreed with that predicted from the cDNA clone, pmusGST Yc. Comparison of the deduced amino acid sequence of the mouse Yc polypeptide with the primary structures of the rat Alpha-class GST enzymes revealed that it is more closely related to the ethoxyquin-induced rat liver Yc2 subunit than to the constitutively expressed rat liver Yc1 subunit. The significance of the fact that both mouse Yc and rat Yc2 exhibit high catalytic activity towards AFB1-8,9-epoxide, whereas rat Yc1 possesses little activity towards this compound, is discussed in terms of structure/function.
Full text
PDF




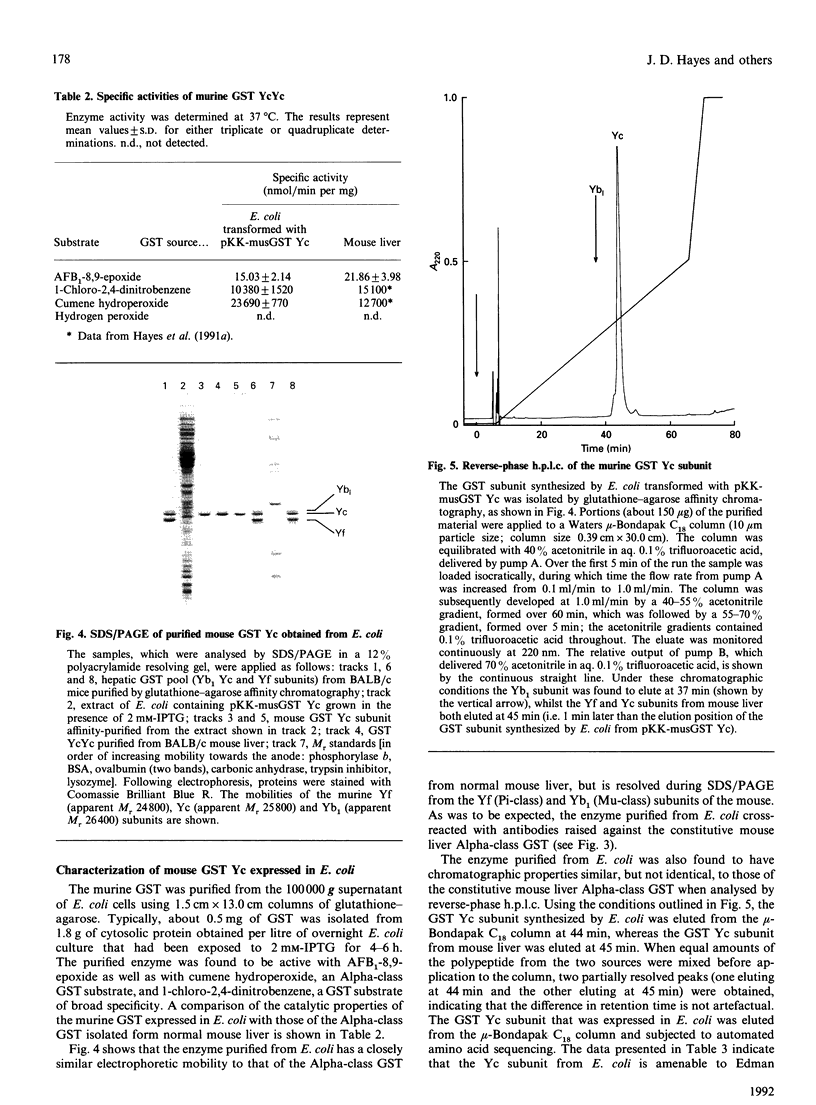
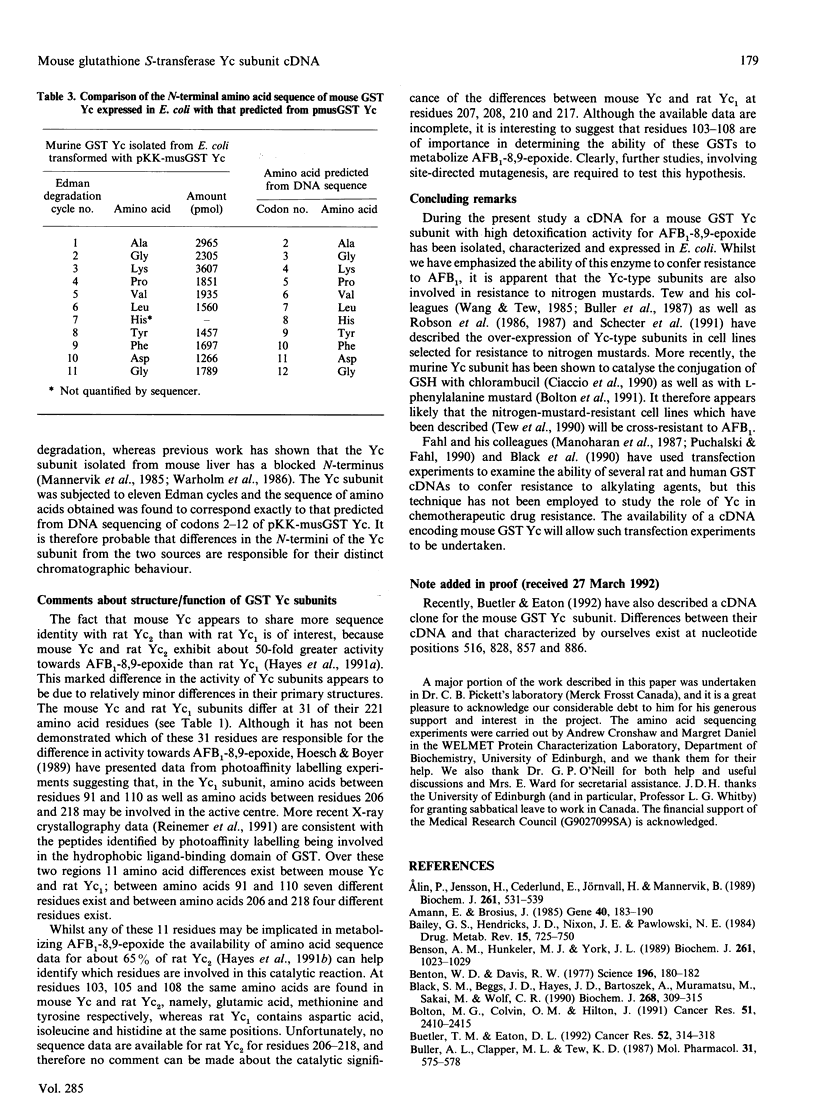

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Jensson H., Cederlund E., Jörnvall H., Mannervik B. Cytosolic glutathione transferases from rat liver. Primary structure of class alpha glutathione transferase 8-8 and characterization of low-abundance class Mu glutathione transferases. Biochem J. 1989 Jul 15;261(2):531–539. doi: 10.1042/bj2610531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Hendricks J. D., Nixon J. E., Pawlowski N. E. The sensitivity of rainbow trout and other fish to carcinogens. Drug Metab Rev. 1984;15(4):725–750. doi: 10.3109/03602538409041078. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., York J. L. Mouse hepatic glutathione transferase isoenzymes and their differential induction by anticarcinogens. Specificities of butylated hydroxyanisole and bisethylxanthogen as inducers of glutathione transferases in male and female CD-1 mice. Biochem J. 1989 Aug 1;261(3):1023–1029. doi: 10.1042/bj2611023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Black S. M., Beggs J. D., Hayes J. D., Bartoszek A., Muramatsu M., Sakai M., Wolf C. R. Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem J. 1990 Jun 1;268(2):309–315. doi: 10.1042/bj2680309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton M. G., Colvin O. M., Hilton J. Specificity of isozymes of murine hepatic glutathione S-transferase for the conjugation of glutathione with L-phenylalanine mustard. Cancer Res. 1991 May 1;51(9):2410–2415. [PubMed] [Google Scholar]
- Buetler T. M., Eaton D. L. Complementary DNA cloning, messenger RNA expression, and induction of alpha-class glutathione S-transferases in mouse tissues. Cancer Res. 1992 Jan 15;52(2):314–318. [PubMed] [Google Scholar]
- Buller A. L., Clapper M. L., Tew K. D. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- Campbell T. C., Chen J. S., Liu C. B., Li J. Y., Parpia B. Nonassociation of aflatoxin with primary liver cancer in a cross-sectional ecological survey in the People's Republic of China. Cancer Res. 1990 Nov 1;50(21):6882–6893. [PubMed] [Google Scholar]
- Ciaccio P. J., Tew K. D., LaCreta F. P. The spontaneous and glutathione S-transferase-mediated reaction of chlorambucil with glutathione. Cancer Commun. 1990;2(8):279–285. doi: 10.3727/095535490820874263. [DOI] [PubMed] [Google Scholar]
- Daniel V., Sharon R., Tichauer Y., Sarid S. Mouse glutathione S-transferase Ya subunit: gene structure and sequence. DNA. 1987 Aug;6(4):317–324. doi: 10.1089/dna.1987.6.317. [DOI] [PubMed] [Google Scholar]
- Degen G. H., Neumann H. G. Differences in aflatoxin B1-susceptibility of rat and mouse are correlated with the capability in vitro to inactivate aflatoxin B1-epoxide. Carcinogenesis. 1981;2(4):299–306. doi: 10.1093/carcin/2.4.299. [DOI] [PubMed] [Google Scholar]
- Garger S. J., Griffith O. M., Grill L. K. Rapid purification of plasmid DNA by a single centrifugation in a two-step cesium chloride-ethidium bromide gradient. Biochem Biophys Res Commun. 1983 Dec 28;117(3):835–842. doi: 10.1016/0006-291x(83)91672-8. [DOI] [PubMed] [Google Scholar]
- Hatayama I., Satoh K., Sato K. A cDNA sequence coding a class pi glutathione S-transferase of mouse. Nucleic Acids Res. 1990 Aug 11;18(15):4606–4606. doi: 10.1093/nar/18.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Coulthwaite R. E., Stockman P. K., Hussey A. J., Mantle T. J., Wolf C. R. Glutathione S-transferase subunits in the mouse and their catalytic activities towards reactive electrophiles. Arch Toxicol Suppl. 1987;10:136–146. doi: 10.1007/978-3-642-71617-1_11. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Kerr L. A., Peacock S. D., Neal G. E. Ethoxyquin-induced resistance to aflatoxin B1 in the rat is associated with the expression of a novel alpha-class glutathione S-transferase subunit, Yc2, which possesses high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1991 Oct 15;279(Pt 2):385–398. doi: 10.1042/bj2790385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Judah D. J., McLellan L. I., Neal G. E. Contribution of the glutathione S-transferases to the mechanisms of resistance to aflatoxin B1. Pharmacol Ther. 1991;50(3):443–472. doi: 10.1016/0163-7258(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Cronshaw A. D. Evidence that glutathione S-transferases B1B1 and B2B2 are the products of separate genes and that their expression in human liver is subject to inter-individual variation. Molecular relationships between the B1 and B2 subunits and other Alpha class glutathione S-transferases. Biochem J. 1989 Dec 1;264(2):437–445. doi: 10.1042/bj2640437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Kerr L. A., Peacock S. D., Cronshaw A. D., McLellan L. I. Hepatic glutathione S-transferases in mice fed on a diet containing the anticarcinogenic antioxidant butylated hydroxyanisole. Isolation of mouse glutathione S-transferase heterodimers by gradient elution of the glutathione-Sepharose affinity matrix. Biochem J. 1991 Jul 15;277(Pt 2):501–512. doi: 10.1042/bj2770501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Anomalous electrophoretic behaviour of the glutathione S-transferase Ya and Yk subunits isolated from man and rodents. A potential pitfall for nomenclature. Biochem J. 1986 Aug 1;237(3):731–740. doi: 10.1042/bj2370731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Wolf C. R. Molecular mechanisms of drug resistance. Biochem J. 1990 Dec 1;272(2):281–295. doi: 10.1042/bj2720281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesch R. M., Boyer T. D. Localization of a portion of the active site of two rat liver glutathione S-transferases using a photoaffinity label. J Biol Chem. 1989 Oct 25;264(30):17712–17717. [PubMed] [Google Scholar]
- Krishnamachari K. A., Bhat R. V., Nagarajan V., Tilak T. B. Hepatitis due to aflatoxicosis. An outbreak in Western India. Lancet. 1975 May 10;1(7915):1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoharan T. H., Puchalski R. B., Burgess J. A., Pickett C. B., Fahl W. E. Promoter-glutathione S-transferase Ya cDNA hybrid genes. Expression and conferred resistance to an alkylating molecule in mammalian cells. J Biol Chem. 1987 Mar 15;262(8):3739–3745. [PubMed] [Google Scholar]
- McLellan L. I., Hayes J. D. Differential induction of class alpha glutathione S-transferases in mouse liver by the anticarcinogenic antioxidant butylated hydroxyanisole. Purification and characterization of glutathione S-transferase Ya1Ya1. Biochem J. 1989 Oct 15;263(2):393–402. doi: 10.1042/bj2630393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L. I., Hayes J. D. Sex-specific constitutive expression of the pre-neoplastic marker glutathione S-transferase, YfYf, in mouse liver. Biochem J. 1987 Jul 15;245(2):399–406. doi: 10.1042/bj2450399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L. I., Kerr L. A., Cronshaw A. D., Hayes J. D. Regulation of mouse glutathione S-transferases by chemoprotectors. Molecular evidence for the existence of three distinct alpha-class glutathione S-transferase subunits, Ya1, Ya2, and Ya3, in mouse liver. Biochem J. 1991 Jun 1;276(Pt 2):461–469. doi: 10.1042/bj2760461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe D. H., Eaton D. L. Comparative effects of butylated hydroxyanisole on hepatic in vivo DNA binding and in vitro biotransformation of aflatoxin B1 in the rat and mouse. Toxicol Appl Pharmacol. 1987 Sep 30;90(3):401–409. doi: 10.1016/0041-008x(87)90132-3. [DOI] [PubMed] [Google Scholar]
- Monroe D. H., Eaton D. L. Effects of modulation of hepatic glutathione on biotransformation and covalent binding of aflatoxin B1 to DNA in the mouse. Toxicol Appl Pharmacol. 1988 Jun 15;94(1):118–127. doi: 10.1016/0041-008x(88)90342-0. [DOI] [PubMed] [Google Scholar]
- Moss E. J., Judah D. J., Przybylski M., Neal G. E. Some mass-spectral and n.m.r. analytical studies of a glutathione conjugate of aflatoxin B1. Biochem J. 1983 Jan 15;210(1):227–233. doi: 10.1042/bj2100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal G. E., Nielsch U., Judah D. J., Hulbert P. B. Conjugation of model substrates or microsomally-activated aflatoxin B1 with reduced glutathione, catalysed by cytosolic glutathione-S-transferases in livers of rats, mice and guinea pigs. Biochem Pharmacol. 1987 Dec 15;36(24):4269–4276. doi: 10.1016/0006-2952(87)90669-1. [DOI] [PubMed] [Google Scholar]
- Newberne P. M., Butler W. H. Acute and chronic effects of aflatoxin on the liver of domestic and laboratory animals: a review. Cancer Res. 1969 Jan;29(1):236–250. [PubMed] [Google Scholar]
- O'Brien K., Moss E., Judah D., Neal G. Metabolic basis of the species difference to aflatoxin B1 induced hepatotoxicity. Biochem Biophys Res Commun. 1983 Jul 29;114(2):813–821. doi: 10.1016/0006-291x(83)90854-9. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Reinhart J., Sisk S. C., Anderson K. S., Adler P. N. Tissue-specific induction of murine glutathione transferase mRNAs by butylated hydroxyanisole. J Biol Chem. 1988 Sep 15;263(26):13324–13332. [PubMed] [Google Scholar]
- Pearson W. R., Windle J. J., Morrow J. F., Benson A. M., Talalay P. Increased synthesis of glutathione S-transferases in response to anticarcinogenic antioxidants. Cloning and measurement of messenger RNA. J Biol Chem. 1983 Feb 10;258(3):2052–2062. [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Puchalski R. B., Fahl W. E. Expression of recombinant glutathione S-transferase pi, Ya, or Yb1 confers resistance to alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2443–2447. doi: 10.1073/pnas.87.7.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn B. A., Crane T. L., Kocal T. E., Best S. J., Cameron R. G., Rushmore T. H., Farber E., Hayes M. A. Protective activity of different hepatic cytosolic glutathione S-transferases against DNA-binding metabolites of aflatoxin B1. Toxicol Appl Pharmacol. 1990 Sep 15;105(3):351–363. doi: 10.1016/0041-008x(90)90139-l. [DOI] [PubMed] [Google Scholar]
- Ramsdell H. S., Eaton D. L. Mouse liver glutathione S-transferase isoenzyme activity toward aflatoxin B1-8,9-epoxide and benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide. Toxicol Appl Pharmacol. 1990 Sep 1;105(2):216–225. doi: 10.1016/0041-008x(90)90183-u. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson C. N., Alexander J., Harris A. L., Hickson I. D. Isolation and characterization of a Chinese hamster ovary cell line resistant to bifunctional nitrogen mustards. Cancer Res. 1986 Dec;46(12 Pt 1):6290–6294. [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter R. L., Woo A., Duong M., Batist G. In vivo and in vitro mechanisms of drug resistance in a rat mammary carcinoma model. Cancer Res. 1991 Mar 1;51(5):1434–1442. [PubMed] [Google Scholar]
- Simons P. C., Vander Jagt D. L. Purification of glutathione S-transferases from human liver by glutathione-affinity chromatography. Anal Biochem. 1977 Oct;82(2):334–341. doi: 10.1016/0003-2697(77)90169-5. [DOI] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Townsend A. J., Goldsmith M. E., Pickett C. B., Cowan K. H. Isolation, characterization, and expression in Escherichia coli of two murine Mu class glutathione S-transferase cDNAs homologous to the rat subunits 3 (Yb1) and 4 (Yb2). J Biol Chem. 1989 Dec 25;264(36):21582–21590. [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- Warholm M., Jensson H., Tahir M. K., Mannervik B. Purification and characterization of three distinct glutathione transferases from mouse liver. Biochemistry. 1986 Jul 15;25(14):4119–4125. doi: 10.1021/bi00362a020. [DOI] [PubMed] [Google Scholar]