Abstract
Serious infection is common in patients with multiple myeloma due to immune deficiency from the underlying disease and/or its treatment. Immunoglobulin replacement is one approach to reduce infection risk in these patients. However, few real‐world data exist on its use in patients with myeloma. We investigated immunoglobulin use in Australia, New Zealand and Asia‐Pacific using registry data and explored its association with survival outcomes. A total of 2374 patients with a median follow‐up time of 29.5 months (interquartile range 13.3–54.3 months) were included in the analysis – 1673 from Australia, 313 Korea, 281 New Zealand and 107 Singapore. Overall, 7.1% of participants received immunoglobulin replacement within 24 months of diagnosis. Patients who received immunoglobulin replacement were likely to be younger, had lower baseline IgG levels (excluding paraprotein), were more likely to have baseline hypogammaglobulinaemia, baseline severe hypogammaglobulinaemia and abnormal baseline fluorescent in‐situ hybridisation status, receive first‐line myeloma treatment with immunomodulatory drugs or anti‐CD38 therapy and undergo upfront autologous stem cell transplant. In our patient cohort, the use of immunoglobulin was not associated with overall survival benefit at the time of last follow‐up (adjusted hazard ratio 0.72, 95% CI 0.46–1.14, p = 0.16). Understanding treatment approaches in clinical practice can help support future planning and provision of immunoglobulin resources.
Keywords: immunoglobulin, infection, multiple myeloma
1. INTRODUCTION
Serious infection is common in patients with multiple myeloma (MM) due to a multifactorial immune deficiency attributable to both the underlying disease and/or its treatment. Targeted therapies that deplete plasma cells, including monoclonal antibodies, chimeric antigen receptor T‐cells and proteasome inhibitors (PIs) have improved disease‐specific survival, but these therapies also contribute to secondary hypogammaglobulinaemia [1].
Immunoglobulin replacement is used to reduce the frequency and/or severity of infections in these patients. The most recent Cochrane systematic review evaluating the use of immunoglobulin prophylaxis in haematological malignancies was conducted in 2008 and demonstrated a significant reduction in infections but no difference in all‐cause mortality in patients with haematological malignancies [2]. Only one of the included trials evaluated patients with MM, which recruited patients with stable disease and was conducted before widespread use of novel therapies [3]. A 2022 systematic review identified only one new single‐centre trial evaluating subcutaneous immunoglobulin in 46 MM patients, which showed reduction in severe infections in patients receiving Ig [4]. Two other recent retrospective studies in MM patients undergoing autologous stem cell transplant (ASCT) did not show a reduction in infections with immunoglobulin prophylaxis in the peri‐transplant or post‐transplantation setting [5, 6].
Given the lack of high‐quality randomised controlled trial data, there are differing guidelines internationally about immunoglobulin replacement. In Australia, immunoglobulin replacement therapy is government‐funded if there is significant hypogammaglobulinaemia (serum IgG < 4 g/L excluding paraprotein) or the presence of hypogammaglobulinaemia (serum IgG < lower limit of reference range) with at least one life‐threatening infection in the previous 12 months, or at least two serious infections in the last 6 months requiring more than standard courses of antibiotics [7]. In the United Kingdom, patients may be commenced on immunoglobulin if there is hypogammaglobulinaemia (serum IgG < 5 g/L excluding paraprotein), recurrent or severe bacterial infection despite continuous oral antibiotic therapy for 3 months, and documented failure of serum antibody response to pneumococcal vaccine [8]. In New Zealand, there are no set guidelines for immunoglobulin use but Australian and/or UK guidelines are generally followed. Similarly, in Singapore and Korea, there are no set national guidelines, but patients generally require a history of infection and/or transplantation prior to immunoglobulin administration.
As patients live longer and access to targeted anti‐myeloma therapies expands, the demand and costs for immunoglobulin, which are already considerable, are likely to rise. Indeed, global demand is increasing, with an estimated growth of 6%–8% per year, and higher rise in emerging markets because of lower starting consumption levels [1, 9]. Given the increasing demand for immunoglobulin products and challenges for blood services to meet this demand, urgent attention is required to understand current practice with regard to immunoglobulin use.
This study aims to describe the use of immunoglobulin replacement in the ‘real‐world MM’ setting using established clinical registries in Australia, New Zealand and the Asia‐Pacific. Specifically, we aimed to describe Ig use in patients with MM, identify potential variation in immunoglobulin usage between states in Australia and between countries, predictors for immunoglobulin use, and describe potential associations between immunoglobulin use and survival outcomes.
2. MATERIALS AND METHODS
We performed a retrospective review of patients registered on the Australian and New Zealand Myeloma and Related Diseases Registry (ANZ MRDR) and the Asia‐Pacific MRDR (APAC MRDR). These are prospective clinical quality registries of patients with MM, monoclonal gammopathy of undetermined significance (MGUS), smouldering myeloma, plasma cell leukaemia and solitary plasmacytoma. The ANZ MRDR was established in 2012 and has enrolled over 7000 patients at over 50 sites in Australia and New Zealand (ANZ) (see Supporting Information). The APAC MRDR was established in 2018 and has enrolled over 1700 patients from countries, including Korea, Singapore, Malaysia, China and Taiwan. Both these registries are managed by Monash University's School of Public Health and Preventive Medicine. The design, development and implementation of the ANZ MRDR have been previously described [10, 11].
We included patients with a diagnosis of MM or plasma cell leukaemia from ANZ and APAC MRDR sites with complete immunoglobulin administration data using a data cut‐off date of 30 July 2021. MM and plasma cell leukaemia were defined according to International Myeloma Working Group diagnostic criteria [12]. We excluded patients with diagnoses of MGUS, smouldering myeloma and solitary plasmacytoma, and sites without verified immunoglobulin administration data.
2.1. Data collection
Data were sourced from the MRDR. The MRDR collects patient demographics, laboratory and radiology investigations, disease staging and comorbidities at diagnosis, as well as detailed data on initial treatment, ASCT, maintenance therapy and subsequent lines of therapy. Patient outcomes (including response status, survival status and date and cause of death) are reviewed and recorded every 4 months.
To confirm the accuracy of immunoglobulin administration, we confirmed that data were recently updated from either medical or laboratory records, national Australian immunoglobulin online authorisation portal (Bloodstar) or from the New Zealand Blood Service online prescribing portal.
The ANZ MRDR conducts annual linkage with the Australian National Death Index and the New Zealand Death Registry to ensure the accuracy of mortality data. Date and cause of death (including primary and secondary causes of death) are provided for participants in the registry, including those lost to follow‐up at the time of analysis. Linkage with national death registries is not yet available for Korea, Singapore, Malaysia and Taiwan.
2.2. Statistical analysis
Descriptive statistics are presented as median (interquartile range, IQR) for continuous variables and frequency (percentage) for categorical variables. Baseline patient and disease characteristics and upfront therapy were presented for all patients, and by immunoglobulin usage within 24 months of MM diagnosis, using chi‐square tests for categorical variables and rank‐sum tests for continuous variables, with a p‐value <0.05 considered statistically significant. When reporting characteristics of the group of patients who did not receive immunoglobulin therapy within 24 months of diagnosis, we excluded patients who had died to eliminate survival bias. However, all patients were included in subsequent analyses.
Baseline patient characteristics, including age, sex, European Cooperative Oncology Group status, comorbidities and disease characteristics, including baseline myeloma disease stage (international staging system [ISS] and revised‐ISS), immunoglobulin levels and fluorescent in‐situ hybridisation (FISH) status (defined as any abnormality detected on FISH) were presented. Upfront therapy received including the use of immunomodulatory drugs (IMiDs) (mainly thalidomide‐based), PIs (mainly bortezomib‐based), anti‐CD38 therapy (mainly daratumumab‐based) and upfront ASCT were presented. The choice of upfront therapy in these patients was based on access to anti‐myeloma treatment at the time of MM diagnosis in each country.
Immunoglobulin commencement was assessed using incidence rates, defined as events per person‐year. Incidence rates were compared between countries, and within one country (between states/territories within Australia), which had the highest number of included sites.
To evaluate the potential association between the impact of immunoglobulin replacement and overall survival, we used the Cox proportional hazards model with immunoglobulin therapy included as a time‐varying covariate adjusting for age and ISS for all patients. To assess the robustness of the estimated hazard ratio (HR), we performed sensitivity analysis by censoring survival at specific timepoints (at 2, 3, 4 and 5 years). We also repeated the analysis for infection‐related survival in which deaths caused by other reasons were treated as a competing risk.
We assessed the association between baseline hypogammaglobulinaemia (defined as IgG < 7 g/L excluding paraprotein) and overall survival, and between baseline severe hypogammaglobulinaemia (defined as IgG < 4 g/L excluding paraprotein) and overall survival. To evaluate potential association between immunoglobulin use and overall survival in these patients specifically, we repeated the time‐dependent analysis in these patients with immunoglobulin use as a time‐varying covariate adjusting for age and ISS. We also assessed the potential association between the use of immunoglobulin and progression‐free survival (PFS, defined as time from commencement of therapy to disease progression or death) using a time‐varying Cox analysis.
All statistical analysis was completed on STATAv16.1, College Station, TX.
2.3. Ethics
The study has approval from the Human Research Ethics Committee (HREC) at Monash University, Alfred Hospital and HRECs or independent Institutional Review Boards at all participating ANZ and APAC sites.
3. RESULTS
3.1. Participant baseline characteristics
A total of 2374 patients with a median follow‐up time of 29.5 months (IQR 13.3–54.3 months) were included in this analysis – 1673 from Australia (12 sites), 313 Korea (3 sites), 281 New Zealand (1 site) and 107 Singapore (3 sites).
Table 1 presents the baseline patient and disease characteristics, and upfront anti‐myeloma therapy received by immunoglobulin usage. Patients who received immunoglobulin replacement within 24 months of MM diagnosis were more likely to be younger (median age 62.62 vs. 65.28 years, p = 0.01), had lower baseline immunoglobulin (20.35 g/L vs. 23.00 g/L, p = 0.006) and IgG levels (excluding paraprotein) (5.20 g/L vs. 6.00 g/L, p = 0.002), were more likely to have baseline hypogammaglobulinaemia (72.1% vs. 56.0%, p < 0.001), baseline severe hypogammaglobulinaemia (35.0% vs. 24.7%, p = 0.013), abnormal baseline FISH status (79.3% vs. 65.4%, p = 0.003), have received first‐line MM treatment with IMiDs (44.7% vs. 19.3%, p < 0.001) or first‐line treatment with anti‐CD38 therapy (3.9% vs. 1.7%, p = 0.041) and undergone upfront ASCT (68.1% vs. 53.3%, p < 0.001). Presence of comorbidities, including respiratory or cardiac disease and diabetes mellitus, did not appear to influence the likelihood of receiving immunoglobulin replacement within 24 months post‐MM‐diagnosis. Upfront use of PIs also did not appear to influence the likelihood of receiving immunoglobulin replacement within 24 months post‐MM‐diagnosis.
TABLE 1.
Patient demographics, disease characteristics and upfront treatment, by immunoglobulin use at 24 months.
| Characteristics: median (IQR) or percentage (%) | No Immunoglobulin within 24 months of MM diagnosis (1031 patients)* | Immunoglobulin within 24 months of MM diagnosis (209 patients) | All patients (2374 patients) | p‐Value |
|---|---|---|---|---|
| Age at diagnosis (years) | 65.28 (58.00, 72.60) | 62.62 (55.59, 69.50) | 66.43 (58.41, 73.50) | 0.01 |
| Female gender | 411/1031 (39.9%) | 86/209 (41.1%) | 968/2373 (40.8%) | 0.73 |
| ECOG 2–4 | 102/674 (15.1%) | 20/159 (12.6%) | 301/1638 (18.4%) | 0.41 |
| Abnormal FISH | 399/610 (65.4%) | 96/121 (79.3%) | 1015/1422 (71.4%) | 0.003 |
| ISS‐3 | 230/802 (28.7%) | 45/172 (26.2%) | 581/1857 (31.3%) | 0.51 |
| Serum Immunoglobulin (excluding paraprotein) (g/L) | 23.00 (19.00, 40.00) | 20.35 (16.60, 24.55) | 23.00 (18.20, 32.00) | 0.006 |
| Serum IgA levels (excluding paraprotein) (g/L) | 0.50 (0.28, 1.10) | 0.41 (0.21, 1.10) | 0.50 (0.27, 1.20) | 0.42 |
| Serum IgM levels (excluding paraprotein) (g/L) | 0.20 (0.18, 0.40) | 0.20 (0.13, 0.36) | 0.20 (0.15, 0.40) | 0.11 |
| Serum IgG levels (excluding paraprotein) (g/L) | 6.00 (4.00, 10.30) | 5.20 (3.25, 7.15) | 6.10 (3.90, 10.10) | 0.002 |
| Baseline hypogammaglobulinaemia (Serum IgG < 7 g/L excluding paraprotein) | 345/616 (56.0%) | 101/140 (72.1%) | 842/1485 (56.7%) | <0.001 |
| Baseline severe hypogammaglobulinaemia (Serum IgG < 4 g/L excluding paraprotein) | 152/616 (24.7%) | 49/140 (35.0%) | 374/1485 (25.2%) | 0.013 |
| Comorbidity – Cardiac disease | 97/924 (10.5%) | 15/186 (8.1%) | 248/2121 (11.7%) | 0.31 |
| Comorbidity – Pulmonary disease | 44/924 (4.8%) | 7/186 (3.8%) | 104/2121 (4.9%) | 0.55 |
| Comorbidity – Diabetes | 122/924 (13.2%) | 19/186 (10.2%) | 300/2121 (14.1%) | 0.26 |
| Comorbidity – Liver disease | 11/924 (1.2%) | 2/186 (1.1%) | 35/2121 (1.7%) | 0.89 |
| First‐line ASCT | 550/1031 (53.3%) | 124/182 (68.1%) | 983/1998 (49.2%) | <0.001 |
| First‐line PI‐based therapy (mainly bortezomib) | 883/1016 (86.9%) | 187/206 (90.8%) | 1983/2309 (85.9%) | 0.13 |
| First‐line IMiD‐based therapy (mainly thalidomide) | 196/1016 (19.3%) | 92/206 (44.7%) | 772/2309 (31.3%) | <0.001 |
| First‐line Anti‐CD38 therapy (mainly daratumumab) | 17/1016 (1.7%) | 8/206 (3.9%) | 79/2309 (3.4%) | 0.041 |
| First‐line treatment with regimen containing dexamethasone | 952/1016 (93.7%) | 197/206 (95.6%) | 2150/2309 (93.1%) | 0.29 |
Note: Patients who survived and completed 24 months duration of follow‐up are included in the groups comparing immunoglobulin versus no immunoglobulin administration within 24 months of MM diagnosis. There were 301 patients excluded from the no immunoglobulin group (as they had died and not yet started immunoglobulin before death) to eliminate survival bias. All patients included in subsequent analyses and are reported in column “All patients.”
Abbreviations: ASCT, autologous stem cell transplant; ECOG, European Cooperative Oncology Group; FISH, fluorescent in‐situ hybridisation; IMiDs, immunomodulatory drugs; IQR, interquartile range; ISS, international staging system; MM, multiple myeloma.
3.2. Commencement of immunoglobulin
The administration of immunoglobulin varied significantly by country, varying between 5 and 132 events per 1000 person‐years (Table 2). Administration was also found to vary widely within Australia, which had the highest number of sites, with the commencement rate of immunoglobulin varied between 25 and 103 events per 1000 person‐years between states/territories (Table 3).
TABLE 2.
Inter‐country variation in immunoglobulin use.
| Country | Immunoglobulin commencement rate, incidence rate (events per 1000 person‐years) (95% CI) |
|---|---|
| Australia | 61 (53–69) |
| Korea | 132 (99–176) |
| Singapore | 17 (5–53) |
| New Zealand | 5 (2–14) |
TABLE 3.
Intra‐country variation in immunoglobulin use within Australia.
| State/territory | Immunoglobulin commencement rate, incidence rate (events per 1000 person‐years) (95% CI) |
|---|---|
| A | 103 (84–127) |
| B* | 80 (58–111) |
| C* | 65 (31–137) |
| D | 45 (37–56) |
| E* | 25 (12–49) |
| * 3 or fewer sites | |
Figure 1 shows the estimated time from diagnosis to first use of immunoglobulin by country. The probabilities of receiving immunoglobulin at 12 months and at 24 months post‐diagnosis were 6.9% and 7.1%, respectively. Figure 2 shows the time spent on and off immunoglobulin in participants who received immunoglobulin.
FIGURE 1.
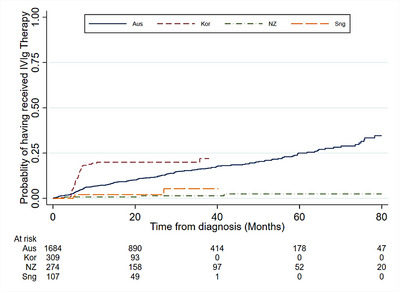
Time from diagnosis of multiple myeloma (MM)/ plasma cell leukaemia (PCL) to first use of immunoglobulin, by country.
FIGURE 2.
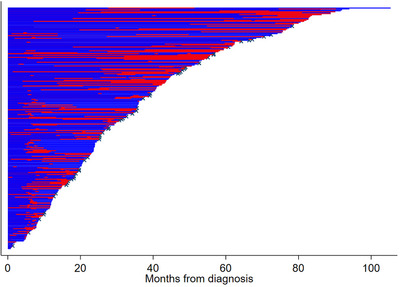
Time spent on immunoglobulin amongst patients who received immunoglobulin. Blue = time not on immunoglobulin, Red = time on immunoglobulin, x = censored due to death.
The majority of Ig‐users (69%) were estimated to receive over 24‐month duration of Ig administration.
3.3. Association between hypogammaglobulinaemia, immunoglobulin use and survival outcomes
The presence of baseline hypogammaglobulinaemia (HR = 1.02, 95% CI 0.83–1.25) and baseline severe hypogammaglobulinaemia (HR = 1.03, 95% CI 0.82–1.30) were not found to be associated with overall survival.
In all patients, the use of immunoglobulin was not associated with overall survival benefit, with HR for the time‐varying use of immunoglobulin of 0.73 (95% CI 0.46–1.16, p = 0.18). When adjusted for age and ISS, findings were similar with no association found between immunoglobulin use and overall survival benefit during follow‐up (adjusted HR [aHR] = 0.72, 95% CI 0.46–1.14, p = 0.16). Sensitivity analysis done by censoring survival at specific timepoints (at 2, 3, 4 and 5 years) did not alter the significance of the HR, and there was not much change in the HR over time. We further explored if there was a difference in survival if immunoglobulin was administered to patients with baseline hypogammaglobulinaemia (IgG < 7 g/L) or baseline severe hypogammaglobulinaemia (IgG < 4 g/L). There was no overall survival benefit in the use of immunoglobulin in patients with baseline hypogammaglobulinaemia (aHR = 0.80, 95% CI 0.39–1.63, p = 0.54) and in patients with baseline severe hypogammaglobulinaemia (aHR = 0.91, 95% CI 0.40–2.10, p = 0.83) when adjusted for age and ISS.
Use of immunoglobulin was not associated with PFS, with HR for the time‐varying use of immunoglobulin of 0.85 (95% CI 0.57–1.28, p = 0.44).
At the time of last follow‐up, there were 623 deaths (26.2%) overall. Data on cause of death was available for Australian patients (318 patients). Of these, 110 (34.6%) had infection‐related death, with infection listed as either a primary or secondary cause of death. When using a time‐dependent Cox regression, immunoglobulin use was not associated with the risk of infection‐related death in Australian patients (HR = 1.30, 95% CI 0.50–3.30).
4. DISCUSSION
In this real‐world prospective cohort of myeloma patients across four countries in the Asia‐Pacific, the use of immunoglobulin varied between countries and between states in Australia. This may be in part due to differences in access to immunoglobulin by region but may also reflect differences in the approach to replacement therapy in clinical practice.
Patients with lower baseline IgG (excluding paraprotein) and baseline serum immunoglobulin levels were more likely to receive immunoglobulin replacement. Immunoglobulin therapy was also associated with first‐line MM therapy with anti‐CD38 monoclonal antibodies. Uses of these monoclonal antibodies have been reported to be associated with neutropenia, lymphopenia and hypogammaglobulinaemia [13, 14]. As access to targeted therapies, including anti‐CD38 directed therapy, expands, this carries significant implications for future expected demand and costs of immunoglobulin therapy.
There was a high burden of deaths associated with infection as either primary or contributing cause of death in patients in ANZ. Our findings are similar to other population‐based MM studies, including an analysis of early mortality using data from the ANZ MRDR [15]. In a Swedish population‐based study of over 9000 patients, infection was the underlying cause in 22% of deaths at 1‐year follow‐up [16]. The Danish MM registry reported that for non‐transplant eligible patients, infection was the cause of death in approximately half of all early mortality cases [17], and in a multicentre Korean study, infection accounted for 36% of deaths at 12 months [18]. In contrast, a US registry study found that only 13% of deaths were due to infection [19]. In our study, we did not find that immunoglobulin replacement was associated with a reduced risk of infection‐related death or all‐cause survival; however, the confidence intervals were wide and did not exclude a clinically important benefit.
In addition, baseline hypogammaglobulinaemia and severe hypogammaglobulinaemia were not found to be associated with overall survival in our patient cohort. This finding adds to the reports from other population‐based and retrospective studies. Our study findings showed similar findings to the Danish MM registry where baseline hypogammaglobulinaemia was not independently associated with overall survival in over 2000 patients with newly diagnosed myeloma [20]. In a single‐centre Turkish study of newly diagnosed MM patients, there was no significant association of immunoparesis with overall survival in patients treated with novel regimens [21]. In contrast, a multi‐centre Greek study found that patients without hypogammaglobulinaemia had significantly improved survival when adjusting for other prognostic factors, but the majority of patients in this study did not receive upfront treatment with novel agents [22].
To our knowledge, no other studies have described rates of immunoglobulin use in real‐world patients with MM and its impact on survival. In countries with comprehensive registries, such as Denmark and Sweden, there are published studies regarding predictors of infection and hypogammaglobulinaemia in patients with newly diagnosed MM. However, descriptions regarding the rates of immunoglobulin replacement therapy in these patients have not been reported, noting that immunoglobulin replacement in Denmark is not routine regardless of immunoparesis status [20], and in Sweden, patients generally require three or more severe infections per season before receiving immunoglobulin [16]. Other observational studies have described association between immunoglobulin replacement and infection risk [23, 24, 25] but rates of immunoglobulin replacement are not known.
We found substantial variation between countries, and within Australia, on the use of immunoglobulin replacement. This variation in clinical practice may reflect local access to immunoglobulin products. However, although Australia has a national blood supply with similar access to immunoglobulin products, there was still significant variation between states/territories. This may be partly attributable to limited evidence for immunoglobulin and the consequent uncertainty in guidelines. Previous clinician practice surveys have reported differences in approaches to commencing, dosing, monitoring and ceasing immunoglobulin, as well as the use of alternative agents, such as antibiotics [26, 27].
4.1. Limitations and strengths
Our study had several limitations: First, limited follow‐up duration in our patient cohort and the lack of routine capture of infection episodes or hospitalisations on our registry meant we were unable to explore association between baseline hypogammaglobulinaemia or immunoglobulin replacement with infection episodes and hospitalisations. Indication for immunoglobulin replacement and route of immunoglobulin received by patient were not captured on the registry. In addition, uses of other types of infection prophylaxis, such as antibiotic prophylaxis and immunisations, were not routinely captured on our registry. Although we did not find an association between immunoglobulin replacement and survival, our confidence intervals were wide and do not exclude a clinically important effect on either benefit or harm.
Additionally, as is common to many registry‐based studies, there was incomplete data for some data fields. To help overcome this, accuracy was enhanced by crosschecking immunoglobulin data with other sources, including medical or laboratory records and national online immunoglobulin tools where available, and only sites with verifiable immunoglobulin data were included in the analysis. There were also limitations in the coding of deaths, which was available in the ANZ database only. Although data from the ANZ national death registries provides reliable cause‐of‐death data, there is often a lag‐time of up to 18 months in the availability of cause‐of‐death data, and so this was only available for a proportion of deaths. As this is a registry‐based observational study, immunoglobulin replacement was determined by local access criteria and funding, which may limit the generalisability to other countries with different access to immunoglobulin. Finally, the observational study design limits the ability to determine causal effects.
Our study has a number of strengths, including its prospective design, a large number of enrolled patients and inclusion of 19 sites across four countries, which contribute to the generalisability of our findings. We had comprehensive data on immunoglobulin administration. Data linkage with national death registries and corroboration of primary cause of death data with a review of the medical record also ensure the robustness of our survival data.
5. CONCLUSION
To our knowledge, this is the first description of real‐world immunoglobulin replacement practice in MM – in particular, reporting the proportions, types and outcomes of patients who receive immunoglobulin. This analysis incorporates current data from a large region within Australia, New Zealand and Asia‐Pacific, utilising data infrastructure from multiple registries.
Establishing clinical practice patterns and outcome data using established clinical registries provides valuable insights into treatment approaches used in everyday clinical practice and can help support the future planning and provision of immunoglobulin resources. Given the high burden of infection‐related mortality in MM patients and the rising costs and demand for immunoglobulin, there is a pressing need for well‐designed contemporary studies to inform patient selection for immunoglobulin replacement therapy and evaluation of alternate infection prevention strategies.
AUTHOR CONTRIBUTIONS
Erica M Wood, Zoe K McQuilten, Cameron Wellard and Khai Li Chai conceived the research idea. Cameron Wellard, Khai Li Chai, LTP Thao and Zoe K. McQuilten developed the statistical analysis plan. Cameron Wellard performed the data analysis. Khai Li Chai drafted the first version of the manuscript. Cameron Wellard, Erica M Wood, LTP Thao and Zoe K. McQuilten edited the first version of the manuscript. All authors reviewed, edited and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
This research project did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors. However, the ANZ MRDR has received funding from Abbvie, Amgen, Antengene, Bristol‐Myers Squibb, Celgene, Gilead, GSK, Janssen, Novartis, Sanofi and Takeda. The APAC MRDR has received funding from Janssen Asia‐Pacific. Monash University has received funding from CSL Behring for other projects.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
PATIENT CONSENT STATEMENT
The authors have confirmed patient consent statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Supporting information
ACKNOWLEDGEMENTS
We thank the patients, hospitals, clinicians and research staff at participating institutions for supporting this study. Members of the Australian and New Zealand and Asia‐Pacific Myeloma and Related Diseases Registries are listed in the Supporting Information. We thank Dr Ai Leen Ang, Health Sciences Authority and Singapore General Hospital, Singapore, for helpful discussion. KLC is supported by a PhD scholarship from the Leukaemia Foundation, the Haematology Society of Australia and New Zealand (HSANZ) and Monash University. ZKM and EMW are supported by NHMRC Investigator Grants (#1194811 and #1177784). This research was supported by the NHMRC‐funded Blood Synergy program (#1189490).
Chai KL, Wellard C, Thao LTP, Aoki N, Moore EM, Augustson BM, et al. Variation in immunoglobulin use and impact on survival in myeloma. eJHaem. 2024;5:690–697. 10.1002/jha2.938
DATA AVAILABILITY STATEMENT
The data from the Myeloma and Related Diseases Registries that support the findings of this study are available on request and following approval from the corresponding author and the registries. The data are not publicly available due to privacy or ethical restrictions. The Data Request Form is in the Data Access Policy available at www.mrdr.net.au and apacmrdr.org.
REFERENCES
- 1. Wyndham A, Vogan A, Newton S, Schubert C. Immunoglobulin for acquired hypogammaglobulinaemia secondary to haematological malignancies, or post‐haemopoietic stem cell transplantation. MSAC Assessment Report 2019. Commonwealth of Australia, Canberra: ACT; 2019. [Google Scholar]
- 2. Raanani P, Gafter‐Gvili A, Paul M, Ben‐Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta‐analysis. Leuk Lymphoma. 2009;50(5):764–772. [DOI] [PubMed] [Google Scholar]
- 3. Chapel HM, Lee M. The use of intravenous immune globulin in multiple myeloma. Clin Exp Immunol. 1994;97(Suppl 1):21–24. [PMC free article] [PubMed] [Google Scholar]
- 4. Chai KL, Wong JWK, Weinkove R, Keegan A, Crispin PJ, Stanworth SJ, et al. Interventions to reduce infections in patients with hematological malignancies: a systematic review and meta‐analysis. Blood Adv. 2022;7:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blombery P, Prince HM, Worth LJ, Main J, Yang M, Wood EM, et al. Prophylactic intravenous immunoglobulin during autologous haemopoietic stem cell transplantation for multiple myeloma is not associated with reduced infectious complications. Ann Hematol. 2011;90(10):1167–1172. [DOI] [PubMed] [Google Scholar]
- 6. Park S, Jung CW, Jang JH, Kim SJ, Kim WS, Kim K. Incidence of infection according to intravenous immunoglobulin use in autologous hematopoietic stem cell transplant recipients with multiple myeloma. Transpl Infect Dis. 2015;17(5):679–687. [DOI] [PubMed] [Google Scholar]
- 7. NBA . Criteria for the clinical use of immunoglobulin in Australia. Canberra: NBA; 2020. [Google Scholar]
- 8. NHS England . Commissioning criteria for the use of therapeutic immunoglobulin (Ig) in immunology, haematology, neurology and infectious diseases in England, January 2019. Leeds: NHS England. Available from: http://igd.mdsas.com/wp‐content/uploads/Ig‐PWG‐Guidance‐for‐the‐use‐of‐Ig‐V1.3‐12022019.pdf [Google Scholar]
- 9. Prevot J, Jolles S. Global immunoglobulin supply: steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergin K, Moore E, McQuilten Z, Wood E, Augustson B, Blacklock H, et al. Design and development of the Australian and New Zealand (ANZ) myeloma and related diseases registry. BMC Med Res Methodol. 2016;16(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergin K, Wellard C, Moore E, McQuilten Z, Blacklock H, Harrison SJ, et al. The myeloma landscape in Australia and New Zealand: the first 8 years of the Myeloma And Related Diseases Registry (MRDR). Clin Lymphoma Myeloma Leuk. 2021;21(6):e510–e520. [DOI] [PubMed] [Google Scholar]
- 12. International Myeloma Working G . Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 13. Lancman G, Sastow D, Aslanova M, Moshier E, Cho HJ, Jagannath S, et al. Effect of intravenous immunoglobulin on infections in multiple myeloma (MM) patients receiving daratumumab. Blood. 2020;136:6–7.32614958 [Google Scholar]
- 14. Palumbo A, Chanan‐Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. [DOI] [PubMed] [Google Scholar]
- 15. McQuilten Z, Wellard C, Moore E, Augustson B, Bergin K, Blacklock H, et al. Predictors of early mortality in multiple myeloma: Results from the Australian and New Zealand Myeloma and Related Diseases Registry (MRDR). Br J Haematol. 2022;198(5):830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cecilie B, Erik H, Ulf‐Henrik M, Ola L, Magnus B, Malin H, et al. Multiple myeloma and infections: a population‐based study on 9253 multiple myeloma patients. Haematologica. 2015;100(1):107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holmström MO, Gimsing P, Abildgaard N, Andersen NF, Helleberg C, Clausen NA, et al. Causes of early death in multiple myeloma patients who are ineligible for high‐dose therapy with hematopoietic stem cell support: a study based on the nationwide Danish Myeloma Database. Am J Hematol. 2015;90(4):E73–E74. [DOI] [PubMed] [Google Scholar]
- 18. Jung SH, Cho MS, Kim HK, Kim SJ, Kim K, Cheong JW, et al. Risk factors associated with early mortality in patients with multiple myeloma who were treated upfront with a novel agents containing regimen. BMC Cancer. 2016;16:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Terebelo H, Srinivasan S, Narang M, Abonour R, Gasparetto C, Toomey K, et al. Recognition of early mortality in multiple myeloma by a prediction matrix. Am J Hematol. 2017;92(9):915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sørrig R, Klausen TW, Salomo M, Vangsted AJ, Frølund UC, Andersen KT, et al. Immunoparesis in newly diagnosed multiple myeloma patients: effects on overall survival and progression free survival in the Danish population. PLoS ONE. 2017;12(12):e0188988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sarı M, Sarı S, Nalçacı M. The effect of suppressed levels of uninvolved immunoglobulins on the prognosis of symptomatic multiple myeloma. Turk J Haematol. 2017;34(2):131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kastritis E, Zagouri F, Symeonidis A, Roussou M, Sioni A, Pouli A, et al. Preserved levels of uninvolved immunoglobulins are independently associated with favorable outcome in patients with symptomatic multiple myeloma. Leukemia. 2014;28(10):2075–2079. [DOI] [PubMed] [Google Scholar]
- 23. Legendre P, Chahwan D, Marjanovic Z, Vignon M, Hermine O, Lortholary O, et al. Utilization of intravenous or subcutaneous immunoglobulins in secondary immune deficiency (ULTIMATE): a retrospective multicenter study. Clin Immunol. 2020;215:108419. [DOI] [PubMed] [Google Scholar]
- 24. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reiser M, Borte M, Huscher D, Baumann U, Pittrow D, Sommer C, et al. Management of patients with malignancies and secondary immunodeficiencies treated with immunoglobulins in clinical practice: long‐term data of the SIGNS study. Eur J Haematol. 2017;99(2):169–177. [DOI] [PubMed] [Google Scholar]
- 26. Wong J, Wood EM, Crispin P, Weinkove R, McQuilten ZK, obotA Leukaemia, et al. Managing hypogammaglobulinaemia secondary to haematological malignancies in Australia and New Zealand: a clinician survey. Int Med J. 2019;49(3):358–363. [DOI] [PubMed] [Google Scholar]
- 27. Na IK, Buckland M, Agostini C, Edgar JDM, Friman V, Michallet M, et al. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur J Haematol. 2019;102(6):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from the Myeloma and Related Diseases Registries that support the findings of this study are available on request and following approval from the corresponding author and the registries. The data are not publicly available due to privacy or ethical restrictions. The Data Request Form is in the Data Access Policy available at www.mrdr.net.au and apacmrdr.org.


