Abstract
Immune thrombocytopenic purpura (ITP) is an immune disorder characterized by thrombocytopenia. Fostamatinib is an orally administered spleen tyrosine kinase inhibitor intended to treat refractory ITP. To evaluate the efficacy and safety of fostamatinib as a subsequent‐line therapy for ITP in adults. We searched four electronic databases for primary studies of any design. Primary efficacy outcomes included proportions of patients achieving overall (≥30 × 109 cells/L), partial (≥50 × 109 cells/L), and stable (as defined in original studies) platelet response. Safety outcomes included rescue medication use and other adverse events. We used narrative synthesis and Mantel–Haenszel random effect meta‐analysis to summarize results. Our systematic review included 11 studies for analyses (n = 722). Weighted mean proportions of patients achieving overall, partial, and stable responses with fostamatinib treatment were 0.70 [0.62, 0.76], 0.48 [0.36, 0.61], and 0.28 [0.16, 0.44], respectively. Fostamatinib was favored over placebo for partial (relative risk [RR] = 3.04, 95% confidence interval [CI] [1.53, 6.06]) and stable (RR = 6.43, 95% CI [1.58, 26.23]) responses. Patients on fostamatinib required less rescue medication and were more likely to experience hypertension. Fostamatinib is a viable subsequent‐line therapy option for refractory ITP. Given the heterogeneous data and large number of small studies, these results should be interpreted cautiously.
Keywords: fostamatinib, immune thrombocytopenic purpura, ITP, platelet disorders, systematic review
1. INTRODUCTION
Immune thrombocytopenic purpura (ITP) is an acquired immune disorder characterized by decreased platelet counts and increased bleeding risk [1]. Treatment of ITP aims to increase and maintain the platelet count to stop or prevent bleeding. First‐line treatments include corticosteroids, intravenous immunoglobulin, and anti‐D immunoglobulin [1]. These interventions provide rapid benefits that are often transient. Second‐line treatments include splenectomy, thrombopoietin receptor agonists (TPO‐RAs), and rituximab [1].
Splenectomy is known for its potential to induce long‐term remission and has a high initial response rate. However, splenectomy introduces risks of surgical complications and infection and requires lifetime postsplenectomy care [2, 3]. TPO‐RAs may improve bone marrow platelet production, and rituximab targets B lymphocytes to reduce ITP‐associated autoantibody production [4, 5]. Both are widely used treatments, are expensive, and rarely induce long‐term, off‐therapy improvements in the platelet count [6].
Fostamatinib is an orally administered spleen tyrosine kinase (SYK) inhibitor that received U.S. Food and Drug Administration approval in 2018 for treatment in adult patients with refractory ITP [7]. The target of fostamatinib, SYK, is an essential protein involved in the phagocytosis of platelets that antibodies have opsonized [8]. This therapy has demonstrated potential for the treatment of patients with refractory ITP without increasing the risks of bleeding [9]. This paper aims to synthesize the evidence for fostamatinib as a subsequent‐line treatment for ITP with regard to increased platelet count and adverse events (AEs).
1.1. Objectives
To systematically review the safety and efficacy of fostamatinib as a subsequent‐line treatment for ITP in adults.
2. METHODS
This review was prospectively registered on PROSPERO (CRD42023425690) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (checklist in Supporting Information 1) [10].
2.1. Eligibility criteria
We included all primary studies (trials, observational cohorts, and case series) of adult patients (≥18 years of age) with ITP who were treated with fostamatinib. We did not exclude based on language or abstract‐only publications. Studies were excluded if patients were on concomitant medication that altered platelet function or coagulation.
2.2. Information sources and search strategy
Electronic database searches from inception to December 12, 2023, were conducted in MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL). Key terms included “fostamatinib” and “ITP.” Full search strategies are available in Supporting Information 2. The references of relevant review articles captured in the literature search were manually reviewed to identify any articles not identified in the original search strategy.
2.3. Study selection and data extraction
Study records were imported into Covidence (Veritas Health Information). Screening for eligible records and data extraction (study characteristics and outcome data) were conducted in duplicate by pairs of independent reviewers (Roger Kou, Lucy Zhao, and Daniel Tham). Disagreements were resolved by consensus.
2.4. Outcomes
Primary efficacy outcomes included the proportion of patients who achieved an overall platelet response (≥30 × 109 cells/L), partial response (≥50 × 109 cells/L), or a complete platelet response (as defined by the original study) while receiving fostamatinib. Secondary efficacy outcomes included time to and duration of response. Safety outcomes included rescue medication use, venous thromboembolism (VTE), major, minor, and clinically relevant nonmajor bleeding (CRNMB) events as defined by the International Society on Thrombosis and Haemostasis (ISTH) criteria [11, 12], and other AEs (hypertension, diarrhea, nausea, neutropenia, dizziness, fatigue, abdominal pain, transaminitis, and infection).
2.5. Risk of bias assessment
The risk of bias assessment was conducted using the RoB2 tool for randomized controlled trials (RCTs) and the ROBINS‐I tool for observational studies [13, 14]. We evaluated the methodological quality of the case series using guidance developed by Murad et al. [15]. Abstracts were excluded from the risk of bias assessments.
2.6. Statistical analysis
We reported the primary and secondary outcomes by narrative synthesis and pooled proportion estimates (with associated 95% confidence interval [CI]). Case series and abstract‐only publications were excluded from all primary meta‐analyses. Secondary analyses included these articles and results were compared qualitatively to primary analysis results. Logit transformation was applied to proportions. The weighted mean proportions of patients with partial, overall, and stable responses to fostamatinib treatment were pooled separately using random effects models. We conducted additional post hoc comparisons of fostamatinib to placebo using Mantel–Haenszel random effects models to calculate risk ratios for efficacy and safety outcomes when possible. The restricted maximum likelihood method was used to estimate the between‐study variance, τ 2. Heterogeneity among studies was tested with the Cochran Q statistic, qualified by visual inspection of the forest plots, and quantified by indicator I 2. An I 2 <30% was considered nonsignificant heterogeneity, I 2 of 30%–70% as moderate heterogeneity, and I 2 >70% as considerable heterogeneity. All p values <0.05 were considered statistically significant. All meta‐analyses were performed using R software (version 4.1.2).
3. RESULTS
3.1. Study selection
We identified 2118 articles through our literature search, of which 91 were examined for full‐text review and 11 were included for analysis (Figure 1). These included two RCT reports (representing three separate trials), five observational cohorts (three abstracts and one follow‐up to the RCTs), and four case series (one of which was an abstract), representing a total of 722 patients. Among the RCTs, one study included results from two separate trials (FIT‐1 and FIT‐2) [16]. For meta‐analyses, data from FIT‐1 and FIT‐2 were treated as distinct studies when it was possible to extract individual study data (e.g., for partial, stable, and rescue medication use). The FIT‐3 study, which included follow‐up data of patients from these two trials, was only used in analyses when data from the original trials was unavailable (pooled proportion of overall response). Secondary analyses that include data from abstracts and case series are reported in Table S1.
FIGURE 1.
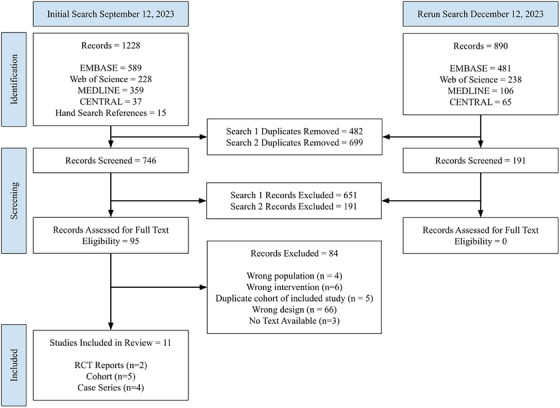
Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) flowchart. RCT, randomized controlled trial.
3.2. Risk of bias
All RCTs were judged to be low risk for bias for all outcomes. All nonrandomized studies were judged to be at high risk for bias primarily due to a lack of confounder adjustment. Figure 2 illustrates the traffic‐light plots for full‐text RCTs and observational studies [17]. All case series were determined to have low methodological quality. Publication bias was suspected, given the high number of small studies.
FIGURE 2.
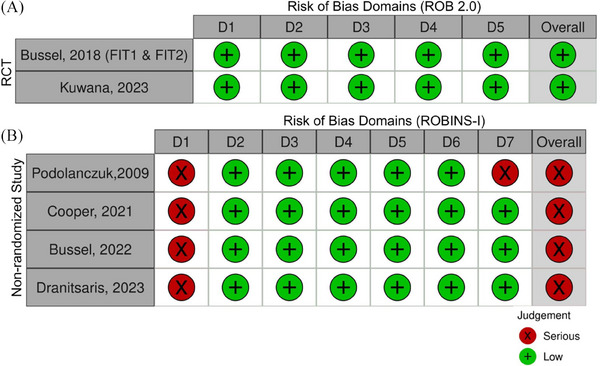
Traffic‐light plots for (A) randomized and (B) nonrandomized studies, not including case series or abstracts. RCT, randomized controlled trial.
3.3. Description of study participants
Patient ages ranged from 19 to 100 years, with a duration of ITP between <1 and 53 years. Most studies included patients who had undergone a median of 3 prior therapies for ITP. These prior therapies were categorized accordingly: corticosteroids, IVIG/IV Anti D, TPO agents, immunosuppressants, splenectomy, rituximab, danazol, chemotherapy, and others. All studies with available data reported median baseline platelets at therapy initiation of <40 × 109 cells/L. Patients in all RCTs were permitted to take one concomitant ITP medication throughout the study [16, 18]. Table 1 summarizes the characteristics of the studies included in this review.
TABLE 1.
Characteristics of included studies. a
| Study, year | Design | Funding | Patients on fostamatinib | Country | Age, years b | Male, n (%) | Duration of ITP, years b | Prior treatments b , c | Splenectomy (%) | Baseline platelets, ×109 cells/L b |
|---|---|---|---|---|---|---|---|---|---|---|
| Kuwana, 2023 [18] | Phase III RCT | Industry | 22 | Japan | 61 (25–81) | 4 (18) | 12 (1–41) | 2 (1–7) d | 5 (23) | 19 (3–28) |
| Bussel, 2018 [16] | Two Phase III RCTs | Industry | 101 | International | 54 (20–88) | 40 (40) | 8.7 (0.3–53) | 3.0 (1–13) | 34 (34) | 16.1 (1–51) |
| FIT‐1 | 51 | North America, Europe, Australia | 57 (20–88) | 21 (41) | 7.5 (0.6–53) | 3 (1–9) | 20 (39) | 16.2 (1–51) | ||
| FIT‐2 | 50 | Europe | 50 (21–82) | 19 (38) | 8.8 (0.3–50.2) | 3 (1–13) | 14 (28) | 15.9 (1–33) | ||
| Dranitsaris, 2023 [30] | Retrospective cohort | Industry | 51 | United States | 59 (21–88) | 16 (31) | 4.5 (1–21) | 3 (2–6) | 20 (39) | 21 (4–46) |
| Bussel, 2022 [23, 36] | Retrospective cohort abstract | Industry | 318 | NR | 67 (19–100) | NR | NR | NR | NR | 32 (NR) |
| Moezi, 2022 [37] | Retrospective cohort abstract | Industry | 46 | United States | 58 (NR) | 16 (35) | NR | 2 (NR) | 16 (35) | 19 (3–70) |
| Cooper, 2021 e [20] | Prospective cohort (FIT‐3) | Industry | 146 | NR | 53 (20–88) | 58 (40) | 8.4 (<1–53) | 3 (1–13) | 51 (35) | 16 (NR) |
| Podolanczuk, 2009 [19] | Prospective cohort | Industry | 16 | United States | 66 (31–81) | 6 (38) | 9 (1–≥29) | 5 (2–≥3) | 11 (69) | 16 (2–28) |
| Grantab, 2022 [38] | Case series abstract | NR | 5 | NR | 77 (56–94) | 3 (60) | 5 (0.6–18) | 2 (2–5) | 0 (0) | 14 (<10–26) |
| Liu, 2022 [21] | Case series | None | 7 | United Kingdom | 80 (63–94) | 3 (43) | 6 (0.5–30) | 2 (1–6) | 1 (14) | 25 (10–193) |
| Mehta, 2022 [22] | Case series | Industry | 5 | NR | 79 (60–90) | 4 (80) | 6 (0.2–8.3) | 2 (1–6) | NR | 39 (17–59) |
| Hughes, 2021 [39] | Case series | NR | 5 | NR | 69 (22–75) | 3 (60) | 6 (1–8) | 3 (3–6) | 1 (20) | 35 (4–264) |
Abbreviations: ITP, immune thrombocytopenic purpura; RCT, randomized controlled trial.
NR, not reported.
Unless otherwise noted, values are the median (range [i.e., minimum, maximum]).
Prior unique treatments counted as falling within the following categories: corticosteroid, IVIG/IV Anti D, thrombopoietin (TPO) agents, immunosuppressants, splenectomy, rituximab, danazol, chemotherapy, and other (i.e., dapsone).
Kuwana et al. report splenectomy separately and is not included in this count.
Cooper et al. (FIT‐3) is a prospective cohort that includes follow‐up of participants from the FIT‐1 and FIT‐2 randomized trials.
3.4. Description of fostamatinib dosing and schedule
All studies, except for the open‐label pilot [19], initiated fostamatinib at 100 mg BID, with dose modifications up to 150 mg BID permitted. Dose modification protocols were clearly described in all trials [16, 18] and the two prospective studies (i.e., escalation permitted after 4 weeks, depending on platelet count) [19, 20]. The reported duration of treatment varied between studies; all trials used fostamatinib for a minimum of 24 weeks.
3.5. Efficacy outcomes
3.5.1. Platelet count response on fostamatinib
The proportion of patients achieving an overall platelet count response (≥30 × 109 cells/L) was 0.70 (95% CI: 0.62, 0.76; I 2 = 0%; N = 162). The pooled proportion of partial responders (≥50 × 109 cells/L) was 0.48 (95% CI: 0.36, 0.61; I 2 = 89%; N = 139). The pooled proportion of stable responders was 0.28 (95% CI: 0.16, 0.44, I 2 = 95%; N = 139). Table 2 summarizes the pooled proportions for platelet response outcomes. Definition of stable responses was similar across the three RCTs and more varied across the observational studies; definitions are available in Table S2. Table S3 summarizes platelet response data and includes forest plots to illustrate these results (Figures S1–S3).
TABLE 2.
Summary of pooled proportions for efficacy and safety outcomes on fostamatinib. a
| Outcome | Contributing studies | Number of patients | Pooled proportion | 95% CI | I 2 c |
|---|---|---|---|---|---|
| Overall response | 2 | 162 | 0.70 | 0.62, 0.76 | 0% |
| Partial response | 4 | 139 | 0.48 | 0.36, 0.61 | 53% |
| Stable response | 4 | 139 | 0.28 | 0.16, 0.44 | 67% |
| Any adverse event b | 3 | 174 | 0.86 | 0.80, 0.91 | 0% |
| Severe adverse event b | 2 | 123 | 0.15 | 0.10, 0.22 | 0% |
| Adverse event causing treatment withdrawal b | 3 | 174 | 0.10 | 0.06, 0.15 | 0% |
| Any bleeding b | 2 | 123 | 0.08 | 0.03, 0.20 | 52% |
| Rescue therapy b | 4 | 190 | 0.30 | 0.24, 0.37 | 30% |
| Hypertension b | 4 | 190 | 0.24 | 0.13; 0.40 | 67% |
| Diarrhea b | 4 | 190 | 0.30 | 0.21, 0.40 | 43% |
| Nausea b | 4 | 190 | 0.16 | 0.10; 0.24 | 37% |
| Neutropenia b | 3 | 174 | 0.07 | 0.02; 0.21 | 64% |
| Dizziness b | 2 | 117 | 0.11 | 0.07; 0.18 | 0% |
| Fatigue b | 3 | 168 | 0.15 | 0.04; 0.44 | 87% |
| Abdominal pain | 3 | 89 | 0.09 | 0.04; 0.22 | 38% |
| Transaminitis b | 3 | 139 | 0.15 | 0.07; 0.29 | 61% |
| Infection b | 2 | 123 | 0.17 | 0.11; 0.24 | 0% |
Abbreviation: CI, confidence interval.
No data from case series or abstracts were included in this primary analysis.
Data from FIT‐1 and FIT‐2 were treated as one study in meta‐analysis.
The I 2 statistic may be biased when the number of contributing studies is small, and the 95% CI should be included in the interpretation of a point estimate.
3.5.2. Time to response and response duration
Reporting of the time to response was sparse and could not be meta‐analyzed. The FIT‐1 and FIT‐2 trials reported that the median platelet counts of both overall and stable responders reached 50 × 109 cells/L by the second week of treatment [16]. These results were similar to those in the two case series where data regarding time to response was available; the seven patients in Liu and Hsia achieved an overall response by a median of 19 (range: 0–181) days, and the five patients in Mehta et al. all achieved partial responses within 1 month [21, 22]. Few studies formally reported on the duration of response; the duration of treatment was more often quantified, ranging from <1 month to 61.7 months. Of note, however, the 2019 results from the FIT‐3 open‐label study reported that among the 64 overall/stable responders (44% of the total cohort), response was maintained for a median duration ≥28 months while on fostamatinib [23]. Table S4 summarizes the time to response and duration outcomes across all included studies.
3.5.3. Relative efficacy compared to placebo
The FIT‐1, FIT‐2, and trials by Kuwana et al. represented 123 patients on fostamatinib and 61 on placebo [16, 18]. We could only compare fostamatinib and placebo for two efficacy outcomes: partial platelet response by 12 weeks of treatment and stable response by 24 weeks. Fostamatinib was favored in both comparisons, with a relative risk (RR) of 3.04 (95% CI: 1.53, 6.06, I 2 = 0%, p < 0.01) for partial response (Figure 3) and a RR of 6.43 (95% CI: 1.58, 25.23, I 2 = 0%, p < 0.01) for stable response (Figure 4).
FIGURE 3.
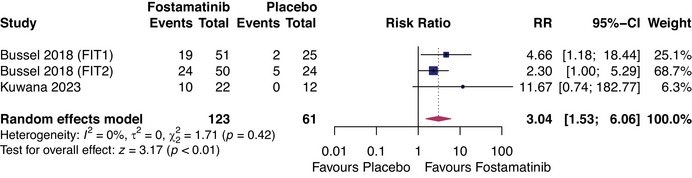
Forest plot of comparison between fostamatinib and placebo for outcome: partial platelet response (≥50 × 109 cells/L) within 12 weeks of treatment. RR, relative risk.
FIGURE 4.
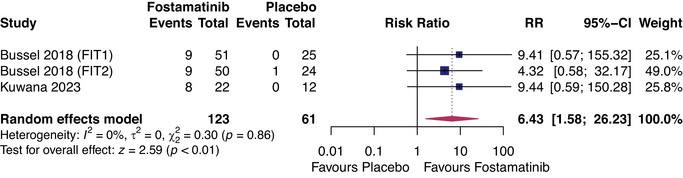
Forest plot of comparison between fostamatinib and placebo for outcome: stable platelet response within 24 weeks of treatment. RR, relative risk.
3.6. Safety outcomes
3.6.1. Mortality, bleeding, and VTE on fostamatinib
All‐cause mortality and VTE were both infrequent events that were not be meta‐analyzed. Across 10 studies, 8/404 (2%) deaths occurred among patients on fostamatinib and 1/61 (1%) among placebo. Across seven studies, 4/207 (2%) VTE events were reported for fostamatinib and 0/61 (0%) for placebo. Bleeding events were reported across eight studies, where no studies explicitly stated the use of ISTH criteria for bleeding events. Compared to placebo, patients on fostamatinib were less likely to experience any bleeding events (RR = 0.41, 95% CI: 0.17, 0.99) during 24 weeks of treatment (Table 3). Pooled proportion of bleeding events from the RCTs was 0.08 (95% CI: 0.03, 0.20), and the long‐term follow‐up recorded 67/146 (46%) minor events over 229.4 patient years.
TABLE 3.
Summary of adverse event comparisons between fostamatinib and placebo (24 weeks).
| Outcome a | Number of studies | Fostamatinib (event/total) | Placebo (event/total) | Relative risk | 95% CI | I 2 c | p |
|---|---|---|---|---|---|---|---|
| Any AE b | 2 | 105/123 | 44/61 | 1.18 | 0.99, 1.40 | 0% | 0.46 |
| Any severe AE b | 2 | 18/123 | 8/61 | 1.11 | 0.51, 2.40 | 0% | 0.80 |
| AE causing withdrawal from treatment | 3 | 13/123 | 4/61 | 1.37 | 0.47, 4.03 | 0% | 0.57 |
| Rescue medication b | 2 | 31/123 | 26/61 | 0.59 | 0.39, 0.90 | 0% | 0.01 |
| Any bleeding b | 2 | 8/123 | 10/61 | 0.41 | 0.17, 0.99 | 0% | 0.05 |
| Hypertension b | 2 | 37/123 | 7/61 | 2.57 | 1.21, 5.43 | 0% | 0.01 |
| Nausea b | 2 | 20/123 | 4/61 | 2.23 | 0.84, 5.90 | 0% | 0.11 |
| Diarrhea b | 2 | 41/123 | 7/61 | 2.69 | 0.98, 7.32 | 13% | 0.05 |
| Neutropenia b | 2 | 10/123 | 0/61 | 5.63 | 0.75, 42.14 | 0% | 0.09 |
Abbreviation: CI, confidence interval.
AE, adverse event.
Data from FIT‐1 and FIT‐2 were treated as one study in meta‐analysis.
The I 2 statistic may be biased when the number of contributing studies is small, and the 95% CI should be included in the interpretation of a point estimate.
3.6.2. Rescue therapy and other AEs on fostamatinib
The most common AEs for patients on fostamatinib included the need for rescue medication (proportion = 0.30, 95% CI: 0.24, 0.37), diarrhea (proportion = 0.30, 95% CI: 0.21, 0.40), and hypertension (proportion = 0.24, 95% CI: 0.13, 0.40). Pooled proportions for all AEs are summarized in Table 2. Compared to placebo, patients on fostamatinib were less likely to require rescue medication (RR = 0.59, 95% CI: 0.39, 0.90, p = 0.01) and more likely to experience hypertension (RR = 2.57, 95% CI: 1.21, 5.43, p = 0.01) during 24 weeks of treatment. Table 3 and Figures S14–S22 summarize the comparisons between fostamatinib and placebo for other AEs during the 24 weeks of treatment. Table S5 summarizes the safety data across all included studies.
4. DISCUSSION
This systematic review summarizes the published data regarding the efficacy and safety of fostamatinib for treating adults with chronic or refractory ITP. Compared to placebo, patients on fostamatinib were 3.04 (95% CI: 1.53, 6.06) times more likely to achieve a partial response (≥50 × 109 cells/L at any point) by 12 weeks of treatment and 6.43 (95% CI: 1.58, 25.23) times more likely to achieve a stable response (≥50 × 109 cells/L at minimum of 66% of follow‐up visits) by 24 weeks. Mortality, bleeding, and VTE events were rare. The most common AEs included rescue therapy given to 30% (95% CI: 24%, 37%) of patients, diarrhea in 30% (95% CI: 21%, 40%), and hypertension in 24% (95% CI: 13%, 40%). Fostamatinib was associated with a 0.59 (95% CI: 0.39, 0.90) times lower risk of requiring rescue medication and a 2.57 (95% CI: 1.21, 5.43) times higher risk of hypertension in 24 weeks. The evidence is limited by heterogeneous outcomes reported across multiple small studies with no direct comparisons to other second‐ or third‐line therapies. The current data suggest that fostamatinib is a viable option for the treatment of ITP that has failed to respond to other treatments.
A recent systematic review provided an overview of clinical trials investigating tyrosine kinase inhibitors, with FIT1 and FIT2 trials as the only data for fostamatinib [24]. Our review is the first to focus on data for fostamatinib and estimate the proportion of patients who achieve clinically relevant platelet thresholds while on treatment. Our results for efficacy are similar to those from the FIT‐1 and FIT‐2 trials alone, with the observation that the proportion of partial and stable responders was higher among nonrandomized studies compared to the RCTs. This may be due to different participant characteristics; most RCT participants had already undergone multiple lines of therapy and may be affected by a disease that is inherently more refractory to treatment compared to the general population of ITP patients. The safety data of nonrandomized studies tended to concord with those of the RCTs and indicate a relatively high incidence of AEs while on fostamatinib (i.e., requiring rescue medication, hypertension, diarrhea, infection, nausea, fatigue, transaminitis, and dizziness). While these side effects may be successfully managed, they remain bothersome and may inform the decision to choose fostamatinib as a therapy.
Two network meta‐analyses have compared fostamatinib data from FIT1 and FIT2 to TPO‐RAs and ranked fostamatinib as the least efficacious but with a relatively high safety profile [25, 26]. This was theorized to be due to a combination of the fostamatinib trials’ patient composition and the single mechanism of ITP that fostamatinib targets compared to the TPO‐RAs [25, 26]. In the analyses of a network meta‐analysis (abstract only) that compared data from the FIT‐1 and FIT‐2 trials to four rituximab studies, the investigators identified fostamatinib as being associated with improved overall platelet response relative to rituximab [27]. Using data primarily from the FIT‐1 and FIT‐2 trials, major guideline groups have either positioned fostamatinib as a subsequent‐line therapy [28], or made formal recommendations for when fostamatinib may be used as a third‐line agent for refractory ITP patients nonresponsive to TPO‐RAs [29], or as a second‐line agent for some chronic ITP patients [30].
The cost of therapies for chronic ITP remains an important factor in determining the clinical treatment plan for patients. From our literature search, only one study evaluated the cost‐effectiveness of fostamatinib [30]. This economic analysis considered the costs of AEs in addition to the base cost of therapy and determined the total mean cost per patient on fostamatinib to be similar to eltrombopag and less costly compared to avatrombopag or romiplostim [30]. While underpowered for noninferiority, the results suggest that there may be economic rationale for including fostamatinib as an alternative to TPOs. However, the higher efficacy of TPO‐RAs suggests that fostamatinib will remain as a therapy subsequent to TPO‐RAs for most patients.
To support the development of guidelines, further investigation of fostamatinib with direct comparisons to other third‐ or second‐line therapies (i.e., rituximab and mycophenolate) with standardized reporting of outcomes that include duration of response will be worthwhile. The reporting of data stratified by important subgroups (e.g., duration of ITP and splenectomy status) may also clarify whether there are greater benefits to initiating fostamatinib sooner rather than later. There also remains a need for evidence‐based guidance for practitioners regarding the optimal strategies to transition patients between ITP therapies and whether there is benefit to the combination of therapies that utilize different underlying mechanisms (e.g., fostamatinib and TPO‐RAs). Ongoing studies registered to clinicaltrials.gov include NCT05502783 and NCT05509582 for fostamatinib in post‐transplant cytopenia [31, 32], NCT06071520 [33], and NCT05613296 [34].
4.1. Limitations
All studies except the RCTs were determined to have a serious risk of bias. We attempted to reduce the impact of bias by excluding case series and abstracts from primary statistical analyses, as case studies tended to report extreme proportions for efficacy and were subject to selection biases, while abstracts were not peer‐reviewed. The risk for confounding within our estimates remains high; we could not make statistical adjustments due to limited sample size and lack of stratified data within individual reports. The entire body of evidence is also at risk of publication bias due to the predominance of small studies. Although industry sources funded the majority of the included studies, a bibliographic analysis of trials in VTE prevention suggested that there may be no difference in the reporting of favorable outcomes between commercially and noncommercially funded studies [35]. Our meta‐analyses also demonstrated moderate to significant heterogeneity for nearly all pooled outcomes, as indicated by the I 2 value and visual inspection of the forest plots with results stratified by study design. Even with an I 2 value of 0% for our comparisons between fostamatinib and placebo, we cannot exclude heterogeneity due to the small number of studies and wide CIs. Due to the limited sample size, we did not conduct metaregression to explore causes of heterogeneity.
5. CONCLUSION
This review found that fostamatinib offers an efficacy and safety profile that makes it a viable option for providers and adult patients to consider in treating ITP refractory to previous treatments. Given the heterogeneous data and many small contributing studies, these results should be interpreted cautiously. Further prospective studies comparing fostamatinib to other third‐ and second‐line treatments are needed to clarify how fostamatinib may be used to optimize the management of ITP in this patient population.
AUTHOR CONTRIBUTIONS
Roger Kou, Lucy Zhao, and Daniel Tham conducted the research. Roger Kou, Giovanna Schünemann, and Mark Crowther designed the study. Roger Kou conducted the analysis and drafted the paper. Rachael Principato, Aqib Mannan, and Giovanna Schünemann piloted the screening and extraction phases. Roger Kou, Lucy Zhao, Daniel Tham, and Mark Crowther contributed to the editing and review of the paper.
CONFLICTS OF INTEREST STATEMENT
Roger Kou, Lucy Zhao, Daniel Tham, Rachael Principato, Aqib Mannan, and Giovanna Schünemann have no conflicts of interest to disclose. This work received no funding. In the last 36 months, Dr. Crowther has received personal funding, including but not limited to preparation of educational material, participation in Advisory Boards, or providing expert testimony for Bayer, Astra Zeneca, Pfizer, Hemostasis Reference Laboratories, Syneos Health, and Eversana. He has participated in various medicolegal activities relating to thrombosis, anticoagulant drugs, or other aspects of internal medicine and hematological practice. He has also worked with multiple organizations for‐profit and not‐for‐profit entities such as Up To Date and medical communication companies. He holds the Leo Pharma Chair in Thromboembolism, endowed at McMaster University.
FUNDING INFORMATION
This study received no funding.
ETHICS STATEMENT
The authors have confirmed ethical approval statement is not needed for this submission.
PATIENT CONSENT STATEMENT
The authors have confirmed patient consent statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
Supporting information
Supporting Information
Kou R, Zhao L, Tham D, Principato R, Schünemann G, Mannan A, et al. Fostamatinib for immune thrombocytopenic purpura in adult patients: A systematic review and meta‐analysis. eJHaem. 2024;5:651–660. 10.1002/jha2.939
DATA AVAILABILITY STATEMENT
Available upon request.
REFERENCES
- 1. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grace RF, Neunert C. Second‐line therapies in immune thrombocytopenia. Hematol Am Soc Hematol Educ Program. 2016;2016(1):698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuker A. Transitioning patients with immune thrombocytopenia to second‐line therapy: challenges and best practices. Am J Hematol. 2018;93(6):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnold DM, Dentali F, Crowther MA, Meyer RM, Cook RJ, Sigouin C, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;146(1):25. [DOI] [PubMed] [Google Scholar]
- 5. Pulanić D, Bátorová A, Bodó I, Červinek L, Ionita I, Lissitchkov T, et al. Use of thrombopoietin receptor agonists in adults with immune thrombocytopenia: a systematic review and Central European expert consensus. Ann Hematol. 2023;102(4):715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second‐line treatment. Blood. 2012;120(5):960–969. [DOI] [PubMed] [Google Scholar]
- 7. Center for Drug Evaluation . FDA approves fostamatinib tablets for ITP. FDA [Internet]. 2019. [cited 2024 Feb 4]. Available from: https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐approves‐fostamatinib‐tablets‐itp
- 8. Perrella G, Montague SJ, Brown HC, Garcia Quintanilla L, Slater A, Stegner D, et al. Role of tyrosine kinase Syk in thrombus stabilisation at high shear. Int J Mol Sci. 2022;23(1):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cooper N, Altomare I, Thomas MR, Nicolson PLR, Watson SP, Markovtsov V, et al. Assessment of thrombotic risk during long‐term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12:20406207211010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692–694. [DOI] [PubMed] [Google Scholar]
- 12. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. [DOI] [PubMed] [Google Scholar]
- 13. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bussel J, Arnold DM, Grossbard E, Mayer J, Treliński J, Homenda W, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: results of two phase 3, randomized, placebo‐controlled trials. Am J Hematol. 2018;93(7):921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGuinness L. robvis: an R package and web application for visualising risk‐of‐bias assessments [Internet]. 2019. [cited 2023 Dec 31]. Available from: https://github.com/mcguinlu/robvis
- 18. Kuwana M, Ito T, Kowata S, Hatta Y, Fujimaki K, Naito K, et al. Fostamatinib for the treatment of Japanese patients with primary immune thrombocytopenia: a phase 3, placebo‐controlled, double‐blind, parallel‐group study. Br J Haematol. 2023;200(6):802–811. [DOI] [PubMed] [Google Scholar]
- 19. Podolanczuk A, Lazarus AH, Crow AR, Grossbard E, Bussel JB. Of mice and men: an open‐label pilot study for treatment of immune thrombocytopenic purpura by an inhibitor of Syk. Blood. 2009;113(14):3154–3160. [DOI] [PubMed] [Google Scholar]
- 20. Cooper N, Altomare I, Thomas MR, Nicolson PLR, Watson SP, Markovtsov V, et al. Assessment of thrombotic risk during long‐term treatment of immune thrombocytopenia with fostamatinib. Ther Adv Hematol. 2021;12:20406207211010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Hsia CC. The efficacy and safety of fostamatinib in elderly patients with immune thrombocytopenia: a single‐center, real‐world case series. Adv Hematol. 2022;2022:e8119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta AR, Kefela A, Toste C, Sweet D. Real‐world use of fostamatinib in patients with immune thrombocytopenia and thrombotic risk. Acta Haematol. 2022;145(2):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bussel JB, Arnold DM, Boxer MA, Cooper N, Mayer J, Zayed H, et al. Long‐term fostamatinib treatment of adults with immune thrombocytopenia during the phase 3 clinical trial program. Am J Hematol. 2019;94(5):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali MA, Anwar MY, Aiman W, Dhanesar G, Omar Z, Hamza M, et al. Safety and efficacy of tyrosine kinase inhibitors in immune thrombocytopenic purpura: a systematic review of clinical trials. J Xenobiot. 2023;13(1):29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wojciechowski P, Wilson K, Nazir J, Pustułka I, Tytuła A, Smela B, et al. Efficacy and safety of avatrombopag in patients with chronic immune thrombocytopenia: a systematic literature review and network meta‐analysis. Adv Ther. 2021;38(6):3113–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang R, Lin L, Yao H, Ji O, Shen Q. Therapeutic options for adult patients with previously treated immune thrombocytopenia – a systematic review and network meta‐analysis. Hematology. 2019;24(1):290–299. [DOI] [PubMed] [Google Scholar]
- 27. Laws A, Gomez‐Ulloa D, Calvo E, Stacey D, Hamilton J, McDonald V. Comparative effectiveness of fostamatinib vs. rituximab in refractory chronic immune thrombocytopenia: a network meta‐analysis. Blood. 2022;140(Suppl 1):2687–2688. [Google Scholar]
- 28. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Institute for Health and Care Excellence . Fostamatinib for treating refractory chronic immune thrombocytopenia (TA835). [cited 2022 Oct 19]. Available from: https://www.nice.org.uk/guidance/ta835
- 30. Matzdorff A, Alesci SR, Gebhart J, Holzhauer S, Hütter‐Krönke ML, Kühne T, et al. Expert report on immune thrombocytopenia: current diagnostics and treatment – recommendations from an expert group from Austria, Germany, and Switzerland. Oncol Res Treat. 2023;46(Suppl 2):5–44. [DOI] [PubMed] [Google Scholar]
- 31. Dranitsaris G, Peevyhouse A, Wood T, Kreychman Y, Neuhalfen H, Moezi M. Fostamatinib or thrombopoietin for the treatment of chronic immune thrombocytopenia in adult patients: a real‐world assessment of safety, effectiveness and cost. Acta Haematol. 2023;1–11 [DOI] [PubMed] [Google Scholar]
- 32. National Heart, Lung, and Blood Institute (NHLBI) . A phase II study using fostamatinib to treat post‐hematopoietic stem cell transplant immune‐mediated cytopenias [Internet]. clinicaltrials.gov; 2024. [cited 2023 Dec 31]. Report No.: NCT05502783. Available from: https://clinicaltrials.gov/study/NCT05502783
- 33. National Heart, Lung, and Blood Institute (NHLBI) . Extension study (extended access) of Syk‐inhibition using fostamatinib to treat post‐transplant immune‐mediated cytopenias [Internet]. clinicaltrials.gov; 2024. [cited 2023 Dec 31]. Report No.: NCT05509582. Available from: https://clinicaltrials.gov/study/NCT05509582
- 34. Fundación Pública Andaluza para la gestión de la Investigación en Sevilla . Andalusian experience in the use of fostamatinib in patients with ITP. FOSTASUR Study [Internet]. clinicaltrials.gov; 2023. [cited 2023 Dec 31]. Report No.: NCT06071520. Available from: https://clinicaltrials.gov/study/NCT06071520
- 35. Gruppo Italiano Malattie EMatologiche dell'Adulto . Real world evaluation among Italian centers of the activity and safety of fostamatinib in consecutive adult patients with immune thrombocytopenia (ITP) [Internet]. clinicaltrials.gov; 2023. [cited 2023 Dec 31]. Report No.: NCT05613296. Available from: https://clinicaltrials.gov/study/NCT05613296
- 36. Zhao L, Kherani J, Li PY, Zhang K, Horta A, Lin C, et al. Primary prevention of venous thromboembolism for cancer patients in randomized controlled trials: a bibliographical analysis of funding and trial characteristics. Res Pract Thrombos Haemostas. 2024;8(1):102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bussel JB, Hales J, Buxman L, Todd LK. Real‐world experience with fostamatinib for treatment of immune thrombocytopenia (ITP): patient‐reported outcomes. Blood. 2022;140(Suppl 1):13143–13144. [Google Scholar]
- 38. Moezi MM, Peevyhouse A, Wood T, Kreychman Y, Dranitsaris G. The safety and efficacy of fostamatinib in patients with chronic immune thrombocytopenic purpura treated in a real‐world community hematology setting. Blood. 2022;140(Suppl 1):5135–5136. [Google Scholar]
- 39. Grantab R, Markovtsov V, Hsia C. Fostamatinib for the treatment of immune thrombocytopenia in a real‐world setting: a case series of five patients. In: ISTH congress abstracts [Internet]. 2022. [cited 2023 Dec 17]. Available from: https://abstracts.isth.org/abstract/fostamatinib‐for‐the‐treatment‐of‐immune‐thrombocytopenia‐in‐a‐real‐world‐setting‐a‐case‐series‐of‐five‐patients/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Available upon request.


