Abstract
Background
We aimed to evaluate the cardiac adverse events (AEs) in hospitalized patients with coronavirus disease 2019 (COVID-19) who received remdesivir plus standard of care (SoC) compared with SoC alone (control), as an association was noted in some cohort studies and disproportionality analyses of safety databases.
Methods
This post hoc safety analysis is based on data from the multicenter, randomized, open-label, controlled DisCoVeRy trial in hospitalized patients with COVID-19. Any first AE that occurred between randomization and day 29 in the modified intention-to-treat (mITT) population randomized to either remdesivir or control group was considered. Analysis was performed using Kaplan-Meier survival curves, and Kaplan-Meier estimates were calculated for event rates.
Results
Cardiac AEs were reported in 46 (11.2%) of 410 and 48 (11.3%) of 423 patients in the mITT population (n = 833) enrolled in the remdesivir and control groups, respectively. The difference between both groups was not significant (hazard ratio [HR], 1.0; 95% confidence interval [CI], .7–1.5; P = .98), even when serious and nonserious cardiac AEs were evaluated separately. The majority of reports in both groups were of arrhythmic nature (remdesivir, 84.8%; control, 83.3%) and were associated with a favorable outcome. There was no significant difference between the two groups in the occurrence of cardiac AE subclasses, including arrhythmic events (HR, 1.1; 95% CI, .7–1.7; P = .68).
Conclusions
Remdesivir treatment was not associated with an increased risk of cardiac AEs compared with control in patients hospitalized with moderate or severe COVID-19. These results are consistent with other randomized, controlled trials and meta-analyses.
Clinical Trials Registration. NCT 04315948; EudraCT 2020-000936-23.
Keywords: COVID-19, remdesivir, cardiac adverse events, randomized controlled trials, antiviral therapy
The safety analysis from the randomized DisCoVeRy trial designed for hospitalized patients with moderate to severe coronavirus disease 2019 showed no significant association between remdesivir treatment compared with control in the occurrence of cardiac adverse events, including arrhythmias.
(See the Editorial Commentary by Gottlieb and Kalil on pages 392–4.)
Remdesivir, now used in more than 50 countries as the standard of care (SoC) for the treatment of coronavirus disease 2019 (COVID-19), is an RNA-dependent RNA polymerase (RdRp) inhibitor originally developed against several RNA viruses, including Ebola and hepatitis C virus [1]. Although none of the completed COVID-19 randomized, controlled trials (RCTs) have demonstrated cardiac events as a safety concern associated with remdesivir [2–5], some case reports [6–8], cohort studies [9–11], and disproportionality analyses from safety databases [12–14] suggest that arrhythmias may be associated with remdesivir administration, mostly mild to moderate and reversible upon discontinuation. Based on this, the Pharmacovigilance Risk Assessment Committee of the European Medicines Agency initiated a new safety signal procedure for sinus bradycardia, concluding in June 2021 that a causal relationship is at least a reasonable possibility [15].
The focus on arrhythmias and bradycardia, in particular, was motivated by the adenosine analog properties of remdesivir. Meanwhile, the potential role of remdesivir in the occurrence of cardiac events and the mechanism of sinus bradycardia induction are not yet clear, notably due to the complex confounding factors. It is well established that COVID-19 disease itself can induce cardiac manifestations as common complications in hospitalized patients with COVID-19 [16].
In addition to efficacy [17, 18], understanding the potential associated risks and safety profile of remdesivir is essential in the context of its widespread use.
Our primary aim in this work was to compare the occurrence of cardiac adverse events (CAEs) in the DisCoVeRy RCT between remdesivir and control groups from randomization to day (D) 29 in patients hospitalized with moderate or severe COVID-19.
METHODS
DisCoVeRy is a phase 3, randomized, controlled, open-label study sponsored by Inserm and initiated at the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in March 2020 in 48 sites in Europe. The study was primarily designed to evaluate the efficacy and safety of 4 repurposed drugs, including remdesivir, in combination with SoC (treatment group) vs SoC alone (control group) in hospitalized patients (aged ≥18 years) with laboratory-confirmed SARS-CoV-2 infection. Data collection included medical history, baseline cardiac disorders, concomitant treatments, and electrocardiogram (EKG) findings at screening with calculation of corrected QT. A total of 1308 patients were enrolled in the 5 groups, first randomly assigned in a 1:1:1:1:1 ratio, then 1:1 once the other 3 treatment groups were stopped for futility to control or remdesivir group, which were enrolled during the whole study. Randomization was stratified on the center and the severity of the disease at inclusion (moderate or severe). Patients assigned to the remdesivir group received a loading dose of 200 mg intravenously on D1, followed by a maintenance dose of 100 mg once daily for up to 9 days. Patients in the control group received the applicable local SoC for COVID-19 management.
Safety data were collected until D29 after enrollment, and all AEs were graded using the Division of Acquired Immunodeficiency Syndrome table [19] to ensure consistency across study sites. The study was approved by all relevant ethics committees and regulatory authorities and registered under EudraCT.
Patient Population
This post hoc safety analysis was done on the modified intention-to-treat (mITT) population from the remdesivir and control groups that included participants who received at least 1 dose of the treatment allocated by randomization.
Data Collection and Management
To evaluate the remdesivir-associated cardiotoxicity risk, the following data were collected for each patient from the mITT population: individual factors (age, sex, body mass index), comorbidities, documented cardiac history (cardiac diseases including chronic, eg, myocardial infarction, acute coronary syndrome, atrial fibrillation, arrhythmias, stent or pacemaker holder; peripheral vascular diseases, eg, peripheral obliterative arteriopathy, aortic aneurysm; and cerebrovascular diseases), cardiac disorders at baseline (rhythm, conduction, EKG), other risk factors for cardiovascular diseases, relevant concomitant treatments including those from the SoC therapies, and COVID-19 severity (severe or moderate, ventilation status at inclusion).
All AEs and CAEs, both serious and nonserious, that occurred between randomization and D29 were taken into account. If a participant experienced multiple AEs or CAEs, only the first occurrence during the period of analysis was considered.
For descriptive purposes, we collected additional variables for each CAE, such as the time to onset, resolution date, and outcome, including fatal events and those that resulted from remdesivir discontinuation due to the CAE (ie, dechallenge).
Serious and nonserious CAEs included the Preferred Terms encoded in the Medical Dictionary for Regulatory Activities (MedDRA), “Cardiac Disorders” and “Investigations” System Organ Classes,” and those extracted from broad cardiac Standardized MedDRA Queries (validated, standard sets of MedDRA terms used as a tool to support signal detection and safety monitoring). All CAEs not considered to be of cardiac origin were excluded from the analysis after review (ie, dyspnea due to desaturation associated with COVID-19 pulmonary progression, cardiac arrest immediately following a context of respiratory failure such as post-extubation and acute respiratory distress syndrome [ARDS], blood creatine phosphokinase increase due to mild rhabdomyolysis associated with COVID-19 with normal EKG and troponin, hyponatremia due to history of kidney disease and orthostatic hypotension considered as a vascular disorder).
Endpoints
The primary safety endpoint was the CAEs (serious or nonserious, possibly related to study drug or not, and regardless of severity) that occurred between randomization and D29 in the mITT population.
Secondary safety endpoints included subclasses of CAEs and overall serious AE (SAE) occurrences. The number of participants who experienced at least 1 SAE in the mITT population is slightly higher than in the DisCoVeRy final results following secondary safety data reconciliation.
To complete the analysis, some baseline parameters that could influence the occurrence of CAEs were presented. For descriptive purposes, times to onset, outcomes including deaths, CAEs that led to remdesivir discontinuation, and the impact of concomitant treatments identified as cardiotoxic that were used prior to the occurrence of a CAE were examined.
Statistical Analyses
Descriptive statistics were used to summarize the patients’ demographic and clinical characteristics. Continuous variables were reported as median (interquartile range [IQR]), while categorical variables were reported as frequencies and percentages. All percentages were raw and provided for descriptive purposes only. Results were presented as event rates calculated using the Kaplan-Meier estimator. Event rates were estimated by dividing the number of events by the total number of individuals at risk at each time point for each treatment group from the randomization date until D29 or until the occurrence of a CAE prior to D29, whichever came first. Confidence intervals (CIs) around the event rates were also calculated to provide a measure of precision.
Censored data were encountered when patients either dropped out of the study before experiencing a CAE or remained in the study until the end of the follow-up period without experiencing an event. Patients lost to follow-up were censored at their last known contact. Patients who were still alive and did not experience the CAE of interest at the time of data cutoff were right-censored at D29. Participants who died from causes unrelated to the CAE of interest were censored at the date of death. Of note, in this analysis, deaths were not considered as events, as also recently proposed by reliable estimates using this approach [20, 21].
To account for censored data, we used the Kaplan-Meier method to estimate the survival curves of CAEs over time. We adjusted for potential confounders, such as the COVID-19 severity at baseline, given the randomization was stratified based on this variable. This adjustment was performed using a Cox proportional hazards model. A sensitivity analysis using the Fine and Gray model was performed with death as competing risk. All statistical analyses were performed with SAS software, version 9.4.
RESULTS
Overview of Baseline Characteristics
The DisCoVeRy trial (between 22 March 2020 and 21 January 2021) enrolled 857 participants randomized to a remdesivir group (n = 429) or control group (n = 428) in France (n = 724), Belgium (n = 51), Portugal (n = 36), Austria (n = 31), and Luxemburg (n = 15).
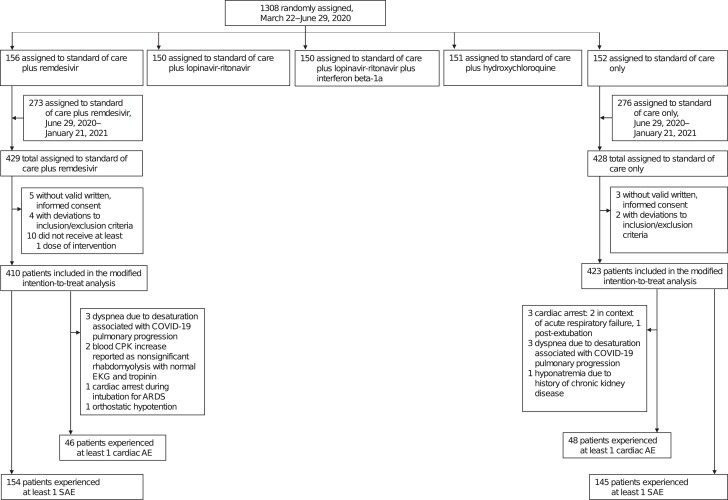
In total, 833 participants were included in the safety analysis (remdesivir, n = 410; control, n = 423), and the demographic and clinical characteristics of the participants were balanced at baseline in both randomized groups, consistently with the previously published final results (Figure 1, Table 1). Among participants in the remdesivir group, the median duration of treatment was 9 days (IQR, 5–10).
Figure 1.
Flow chart of the safety analysis showing the distribution of the 1308 randomly assigned participants in the DisCoVeRy randomized, controlled trial per arm, first randomized in a 1:1:1:1:1 ratio, then 1:1 for remdesivir and control groups once the other 3 arms were stopped for futility. In this safety analysis, 410 and 423 patients were included in the remdesivir and control groups, respectively. Abbreviations: AE, adverse event; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CPK, creatine phosphokinase; EKG, electrocardiogram; SAE, serious adverse event.
Table 1.
Baseline Characteristics of Participants in the Modified Intention-to-Treat Population, Allocated to Remdesivir Group or Control Group
| Characteristic at Baseline | Remdesivir (n = 410) | Control (n = 423) |
|---|---|---|
| Median age, (interquartile range), y | 63 (55–73) | 64 (54–72) |
| Sex, n (%) | ||
| Male | 287 (70.0) | 292 (69.0) |
| Female | 123 (30.0) | 131 (31.0) |
| Coexisting comorbidity, n (%) | ||
| Hypertension | 108 (26.3) | 114 (27.0) |
| Cardiac historya | 132 (32.2) | 154 (36.4) |
| Rhythm, including bradycardia and conduction disorders at random assignmentb | 94 (22.9) | 115 (27.2) |
| Normal electrocardiogram | 256 (78.5) | 242 (72.2) |
| Other cardiovascular risk factorsc | 35 (8.5) | 41 (9.7) |
| Respiratory disease | 88 (21.5) | 101 (23.9) |
| Diabetes mellitus | 109 (26.6) | 118 (27.9) |
| Obesity | 181 (39.7) | 170 (40.5) |
| Renal disease | 36 (8.8) | 45 (10.6) |
| Dyslipidemia | 27 (6.6) | 33 (7.8) |
| Cancerd | 39 (9.5) | 47 (11.1) |
| Severe coronavirus disease 2019 at random assignment, n (%) | 159 (38.8) | 167 (39.6) |
| Ventilatory support at random assignment, n (%) | ||
| Room air | 6 (1.4) | 6 (1.4) |
| Oxygen support with nasal cannula or face mask | 245 (59.8) | 249 (59.0) |
| High-flow oxygen device | 14 (3.4) | 17 (4.0) |
| Noninvasive ventilation | 74 (18.0) | 71 (16.8) |
| Invasive mechanical ventilation | 71 (17.3) | 77 (18.2) |
| Extracorporeal membrane oxygenation | 0 (0.0) | 2 (0.5) |
aCardiac history included documented diseases known to increase the cardiovascular risk: cardiac diseases including chronic (eg, myocardial infarction, acute coronary syndrome, atrial fibrillation, arrhythmias, stent or pacemaker holder), peripheral vascular diseases (eg, peripheral obliterative arteriopathy, aortic aneurysm), and cerebrovascular diseases.
bBradycardia was defined as heart rate <60 beats per minute (remdesivir, n = 40; control, n = 47). Other cardiac disorders at random assignment included rhythm excluding bradycardia and conduction disorders (eg, tachycardia, left ventricular hypertrophy, atrioventricular block, atrial fibrillation, bundle branch block, ventricular extrasystoles, and repolarization disorders).
cOther cardiovascular risk factors included solid organ transplantation, active infection (tuberculosis, AIDS/human immunodeficiency virus), and autoinflammatory diseases.
dCancer included active malignant neoplasm and malignant hemopathy.
Primary Endpoint
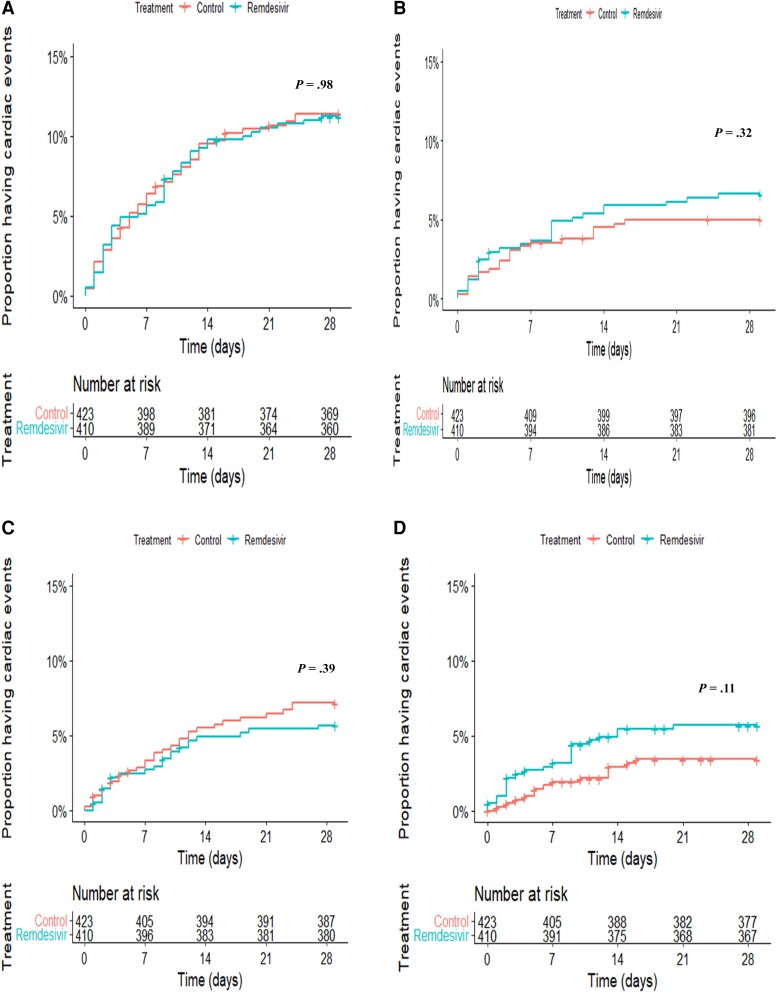
Kaplan-Meier survival curves of the times to CAEs are displayed in Figure 2.
Figure 2.
Kaplan-Meier estimates showing the times to cardiac adverse events from the modified intention-to-treat population in the remdesivir and control groups: overall cardiac adverse events (A), serious cardiac adverse events (B), any nonserious adverse cardiac events (C), and serious arrhythmic events (D). Blue lines show the remdesivir group. Orange lines show the control group. A number-at-risk table is provided for each plot by treatment group.
By D29, 46 (11.2%) of 410 and 48 (11.3%) of 423 participants experienced at least 1 CAE in the remdesivir and control groups, respectively, which was not statistically different after adjustment for the severity of COVID-19 at baseline (hazard ratio [HR], 1.0; 95% CI, .7–1.5; P = .98; Table 2).
Table 2.
Cardiac and Serious Adverse Events in the Modified Intention-to-Treat Population Between Remdesivir Group and Control Group
| Remdesivir (n = 410) | Control (n = 423) | HR (95% CI) [P Value] | HRa (95% CI) [P Value] | |||
|---|---|---|---|---|---|---|
| N Patients (%) | Event Rate (95% CI) | N Patients (%) | Event Rate (95% CI) | |||
| Any cardiac adverse events | 46 (11.2) | 0.076 (.062–.089) | 48 (11.3) | 0.075 (.061–.089) | 1.0 (.6–1.5) [.95] | 1.0 (.7–1.5) [.98] |
| Any serious cardiac adverse eventsb | 27 (6.6) | 0.043 (.036–.050) | 21 (5.0) | 0.042 (.034–.049) | 1.3 (.7–2.3) [.30] | 1.3 (.8–2.4) [.32] |
| Any nonserious cardiac adverse eventsc | 23 (5.6) | 0.039 (.031–.049) | 30 (7.1) | 0.040 (.031–.049) | 0.8 (.5–1.3) [.38] | 0.7 (.4–1.4) [.39] |
| Grades 1–2 | 14 (3.4) | 0.031 (.025–.039) | 25 (5.9) | 0.032 (.025–.039) | 0.6 (.3–1.1) [.10] | 0.6 (.3–1.1) [.10] |
| Grades 3–4 | 8 (2.0) | 0.010 (.007–.013) | 6 (1.4) | 0.009 (.007–.013) | 1.8 (.6–5.5) [.26] | 1.9 (.6–5.6) [.25] |
| Subclasses of cardiac events | ||||||
| Any arrhythmic events | 39 (9.5) | 0.062 (.050–.075) | 40 (9.5) | 0.062 (.049–.074) | 1.1 (.6–1.6) [.74] | 1.1 (.7–1.7) [.68] |
| Any serious arrhythmic events | 23 (5.6) | 0.033 (.026–.041) | 14 (3.3) | 0.032 (.024–.039) | 1.7 (.8–3.3) [.10] | 1.7 (.9–3.3) [.11] |
| Atrial fibrillation | 9 (2.2) | 6 (1.4) | ||||
| Bradycardia | 3 (0.7) | 2 (0.5) | ||||
| Tachycardia | 4 (1.0) | 2 (0.5) | ||||
| Ventricular tachycardia | 0 (0.0) | 2 (0.5) | ||||
| Arrhythmia not specified | 7 (1.7) | 2 (0.5) | ||||
| Any nonserious arrhythmic events | ||||||
| Grades 1–2 | 13 (3.2) | 0.027 (.022–.033) | 24 (5.7) | 0.028 (.022–.034) | 0.6 (.3–1.2) [.16] | 0.6 (.3–1.2) [.17] |
| Grades 3–4 | 7 (1.7) | 0.009 (.007–.012) | 5 (1.2) | 0.009 (.007–.012) | 1.4 (.4–4.5) [.52] | 1.5 (.5–4.6) [.51] |
| Any non-arrhythmic events | 10 (2.4) | 0.018 (.014–.021) | 12 (2.8) | 0.018 (.014–.021) | 0.8 (.3–1.9) [.70] | 0.9 (.4–2.0) [.71] |
| Any serious adverse events | 154 (37.6) | 0.380 (.342–.408) | 145 (34.3) | 0.372 (.339–.405) | 1.2 (.9–1.4) [.20] | 1.2 (.9–1.5) [.21] |
Abbreviations: CI, confidence interval; HR, hazard ratio.
aHRs adjusted for the severity of coronavirus disease 2019 at baseline based on the Cox model.
bPer protocol, a serious adverse event was defined as an adverse event that results in death, is life threatening, requires prolongation of existing hospitalization, results in persistent or significant disability or incapacity, consists of a congenital anomaly/birth defect, or is an important medical event.
cPer protocol, the severity (intensity) of all adverse events was graded using the Division of AIDS table, corrected version 2.1 (July 2017). Some patients experienced different grades of adverse events.
There were no significant differences between groups regarding serious CAEs (HR, 1.3; 95% CI, .8–2.4; P = .32) and nonserious CAEs (HR, 0.7; 95% CI, .4–1.4; P = .39), including when focusing on grades 1–2 (HR, 0.6; 95% CI, .3–1.1; P = .10) and grades 3–4 (HR, 1.9; 95% CI, .6–5.6; P = .25) separately. The sensitivity analysis using the Fine and Gray model revealed similar results (data shown in Supplementary Table 1).
Secondary Endpoints
Cardiac AE Subclasses
Arrhythmic CAEs, including bradycardia, were observed in 39 (84.8%) of 46 and 40 (83.3%) of 48 participants who experienced at least 1 CAE in the remdesivir and control groups, respectively, and no significant difference between both groups was observed (HR, 1.1; 95% CI, .7–1.7; P = .68), including nonarrhythmic events (HR, 0.9; 95% CI, .4–2.0; P = .71).
There were no significant differences between the 2 groups regarding serious arrhythmic AEs (HR, 1.7; 95% CI, .9–3.3; P = .11) and nonserious arrhythmic AEs, including when focusing on grades 1–2 (HR, 0.6; 95% CI, .3–1.2; P = .17) and grades 3–4 (HR, 1.5; 95% CI, .5–4.6; P = .51) separately.
Most serious arrhythmic events occurred either concomitantly or within a few days following respiratory-, renal-, sepsis-, or infection-related complications (remdesivir, 82.1%; control, 87.5%), suggesting COVID-19–induced cardiac events. Four patients in the remdesivir group experienced serious arrhythmic AEs apart from COVID-19 complications, but all had preexisting conditions such as a history of rhythm disorder.
During the trial, reports of bradycardia were rare (remdesivir, n = 8; control, n = 8), and most were considered nonserious and occurred within 2 days after randomization, with a favorable outcome in all.
Of note, no QT prolongation nor torsade de pointes were reported during the trial, and all ventricular tachycardia events (n = 3) were reported in the control group (cumulative CAEs in Supplementary Table 2).
Potential Influence of Risk Factors or Preexisting Cardiac Condition at Baseline
In both groups, cardiovascular risk factors associated with older age and hypertension, COVID-19 severity at enrollment, and invasive ventilation mode were the most common parameters in patients with a reported CAE (Table 3). Among those who experienced a CAE, the frequency of patients with preexisting cardiac history was higher in the control group, and bradycardia and abnormal EKG at baseline were more frequent in the remdesivir group. However, in the latter group, 21% of patients had missing EKG results.
Table 3.
Potential Influence of Risk Factors or Preexisting Cardiac Condition at Baseline on Cardiac Adverse Event Occurrence in Remdesivir and Control Groups
| Parameter | Remdesivir (N = 410) | Control (N = 423) | ||
|---|---|---|---|---|
| Cardiac AEs (n = 46) n (%) |
Other (n = 364) n (%) |
Cardiac AEs (n = 48) n (%) |
Other (n = 375) n (%) |
|
| Median age, (IQR), y | 69.5 (58.0–75.0) | 62.0 (54.0–72.0) | 69.0 (61.0–75.0) | 64.0 (53.0–72.0) |
| Sex, n (%) | ||||
| Male | 38 (82.6) | 249 (68.4) | 35 (72.9) | 257 (68.5) |
| Female | 8 (17.4) | 115 (31.6) | 13 (27.1) | 118 (31.5) |
| Coexisting comorbidity, n (%) | ||||
| Hypertension | 18 (39.1) | 90 (24.7) | 23 (49.7) | 91 (24.3) |
| Cardiac historya | 18 (39.1) | 114 (31.3) | 29 (60.4) | 125 (33.3) |
| Rhythm, including bradycardia and conduction disorders at random assignmentb | 19 (41.3) | 75 (20.6) | 14 (29.2) | 101 (26.9) |
| Normal electrocardiogram | 24 (61.5) | 232 (80.8) | 20 (58.8) | 222 (73.7) |
| Other cardiovascular risk factorsc | 5 (10.9) | 30 (8.2) | 5 (10.4) | 36 (9.6) |
| Respiratory disease | 7 (15.2) | 81 (22.3) | 17 (35.4) | 84 (22.4) |
| Diabetes mellitus | 14 (30.4) | 95 (26.1) | 14 (29.2) | 104 (27.7) |
| Obesity | 22 (47.8) | 139 (38.7) | 22 (45.8) | 148 (39.8) |
| Renal disease | 5 (10.9) | 31 (8.5) | 9 (18.7) | 36 (9.6) |
| Dyslipidemia | 5 (10.9) | 22 (6.0) | 4 (8.3) | 29 (7.7) |
| Cancerd | 4 (8.7) | 35 (9.6) | 8 (16.7) | 39 (10.4) |
| Severe coronavirus disease 2019 at random assignment, n (%) | 27 (58.7) | 132 (36.3) | 35 (72.9) | 132 (35.3) |
| Ventilatory support at random assignment, n (%) | ||||
| Room air | 0 (0.0) | 6 (1.6) | 1 (2.0) | 5 (1.3) |
| Oxygen support with nasal cannula or face mask | 19 (41.3) | 226 (62.1) | 12 (25.0) | 237 (63.3) |
| High-flow oxygen device | 9 (19.6) | 62 (17.0) | 10 (20.8) | 67 (17.9) |
| Noninvasive ventilation | 3 (6.5) | 11 (3.0) | 2 (4.1) | 15 (4.0) |
| Invasive mechanical ventilation | 15 (32.6) | 59 (16.2) | 22 (45.8) | 49 (13.1) |
| Extracorporeal membrane oxygenation | 0 (0.0) | 0 (0.0) | 1 (2.1) | 1 (0.3) |
Abbreviation: AE, adverse event.
aCardiac history included documented diseases known to increase the cardiovascular risk: cardiac diseases including chronic (eg, myocardial infarction, acute coronary syndrome, atrial fibrillation, arrhythmias, stent or pacemaker holder), peripheral vascular diseases (eg, peripheral obliterative arteriopathy, aortic aneurysm) and cerebrovascular diseases.
bBradycardia was defined as heart rate <60 beats per minute (remdesivir: cardiac events, n = 6; other, n = 34; control: cardiac events, n = 5; other, n = 42). Other cardiac disorders at random assignment included rhythm excluding bradycardia and conduction disorders (eg, tachycardia, left ventricular hypertrophy, atrioventricular block, atrial fibrillation, bundle branch block, ventricular extrasystoles, and repolarization disorders).
cOther cardiovascular risk factors included solid organ transplantation, active infection (tuberculosis, AIDS/human immunodeficiency virus), and autoinflammatory diseases.
dCancer included active malignant neoplasm and malignant hemopathy.
Overall SAEs
Of the 833 patients included in the safety analysis, 299 (35.9%) experienced at least 1 SAE, which was slightly higher in both groups than in the previously published DisCoVeRy final results (remdesivir, n = 154 vs 147; control, n = 145 vs 138, respectively; Figure 1). However, the overall rate of SAEs remained not statistically different between both groups, with an HR of 1.2 (95% CI; .9–1.5; P = .21; Table 2).
Other Safety Data
Median Time to Onset of Cardiac AE From Randomization
In addition to the survival curves shown in Figure 2, the median time to onset of CAEs (whether serious or not) was approximately 7.5 days (IQR, 0–27) vs 6.5 days (IQR, 0–24) in the remdesivir and control groups, respectively. Supraventricular arrhythmias (remdesivir, n = 14; control, n = 14) occurred respectively within 3.0 days (IQR, 1–12) and 9.0 days (IQR, 2–24).
Of note, CAEs, mostly arrhythmic, were reported within 5 days in 20 (43.5%) of 46 and 22 (45.8%) of 48 participants in the remdesivir and control group, respectively.
Outcomes of Cardiac AEs, Including Death
Among the 39 deaths in the remdesivir group and 46 in the control group (pre-D29: remdesivir, n = 33; control, n = 38), 9 patients in each group experienced a CAE. Notably, no CAE was identified as a cause of death in the remdesivir group and only 1 in the control group (fatal cardiac failure at D1 in a 92-year-old female patient with a history of pacemaker insertion, atrial fibrillation, and thyroidectomy). Two male patients in the remdesivir group experienced a serious CAE associated with a fatal respiratory complication, including a cardiogenic shock following pulmonary embolism and atrial fibrillation preceded by acute respiratory failure.
A favorable outcome was reported for most CAEs in both groups (remdesivir, 87.0%; control, 81.3%). The patients who experienced a CAE with a nonfavorable outcome experienced acute or chronic CAEs such as atrial fibrillation, tachycardia, and heart failure. These outcomes were not documented and were reported as unresolved or unknown.
Action Taken With Remdesivir Due to Cardiac AE
Remdesivir was discontinued in 3 (6.5%) patients due to CAEs, all assessed as serious: atrioventricular block on D7 and bradycardia on D2, both resolved within 24 hours, and the previously described cardiogenic shock on D1 after pulmonary embolism, which was not resolved at the time of death.
Of note, remdesivir was continued in 25 (54.4%) participants after the occurrence of CAEs, with a favorable outcome in 22. These events were mostly arrhythmic (n = 23), including 6 bradycardia. Unresolved events concerned 2 nonserious atrial fibrillation and 1 congestive cardiac failure.
In 18 (39.1%) participants, the CAEs occurred after study drug discontinuation per protocol (up to D10) and were thus associated with no intervention. These events were mostly arrhythmic (n = 14), including 1 bradycardia.
Concomitant Cardiotoxic Drugs
Among patients with a reported CAE, 44 (95.7%) of 46 and 47 (97.9%) of 48 were concomitantly exposed to cardiotoxic drugs in the remdesivir and control group, respectively. The main drug classes were part of the COVID-19 management, that is, SoC: systemic antibiotic and corticosteroid therapies and antithrombotic agents.
DISCUSSION
In this safety analysis, we reviewed all serious and nonserious AEs reported in the remdesivir and control groups of the DisCoVeRy RCT designed for hospitalized patients with moderate or severe COVID-19, providing an overview of the CAEs and a focus on arrhythmic events.
Based on our results, the use of remdesivir was not associated with an increased risk of CAEs, whether serious or not and regardless of AE severity, compared with the control group. As previously reported [2–4, 17, 22], no significant difference was evidenced in the occurrence of SAEs between both groups.
While bradycardia seems to be associated with remdesivir in some cohort studies [11, 12, 14] with mostly mild to moderate manifestations, we found no significant differences in the arrhythmic event rates between the 2 groups, regardless of severity. In the reported cases of bradycardia, time to onset was mostly consistent with several case reports and published studies, that is, on the first days of remdesivir initiation, including during or within 24 hours of infusion. Importantly, however, the same observations were made in the control group, which was lacking in these observational studies. The majority of reported bradycardic episodes resolved while the patient was still exposed to remdesivir, while some arrhythmic events occurred after remdesivir was discontinued.
As almost half of the CAEs in both groups occurred within 5 days of randomization, which is the currently recommended duration of remdesivir treatment for hospitalized patients, we examined some parameters at baseline that might influence the occurrence of such events. However, these observations should be interpreted with caution given the small sample size and the fact that these parameters were explored at baseline, as some may be different at the time of CAE occurrence, depending on COVID-19 progression and clinical status.
Some cohort studies suggest the higher incidence of arrhythmic events in patients receiving remdesivir to be due to its close structural resemblance with adenosine [23]. A molecular model suggests possible binding to adenosine receptors via GS-441524 bioaccumulation [24], remdesivir's main extracellular metabolite [25]. However, in vitro studies that demonstrate arrhythmogenic activity should not be overinterpreted as the concentrations of remdesivir might differ from actual plasma concentrations in the clinical setting [26–30]. DisCoVeRy's exploratory endpoints of the post-infusion plasma concentrations did not show remdesivir or GS-441524 bioaccumulation [17].
To date, preclinical data have shown no clinically relevant effect on the cardiovascular system [31], although prior cardiac safety concerns were observed in other RdRp inhibitors [32–34].
Our study has several limitations. First, the sample size was relatively small, as the trial was stopped before reaching the planned sample size (n = 1308 vs n = 3100 planned, that is, 620 per arm), and the study was not powered to detect differences in specific subgroups of patients, especially those with prior cardiac conditions or those who presented with hypoxemia at baseline. In the subanalyses that assessed some types of CAEs such as bradycardia, the small number of CAE reports impedes any relevant interpretation. Second, there was possible bias in notification induced by the open-label design, although the AEs were collected at the onset of the pandemic with no particular a priori on remdesivir's safety profile. Finally, this safety analysis focused on CAEs associated with remdesivir; however, confounding factors may have contributed to these events. Cardiac events can be caused by COVID-19 as SARS-CoV-2 is believed to cause direct cardiac injury and can induce severe hypoxia, ARDS, and hyperinflammation, resulting in cardiac impairment such as arrhythmias, myocardial injury, or cardiac arrest [16]. Other confounding factors involve the presence of cardiovascular risk factors, including diabetes mellitus and obesity, and underlying cardiac disease; these are some of the comorbidities associated with the worst outcomes for COVID-19. Furthermore, the concomitant polypharmacy context often implies cardiotoxic treatments.
To date, although some cohort studies and disproportionality analyses have noted an association between remdesivir and cardiac events, no safety concern of cardiotoxicity was identified in meta-analyses of RCTs [35–38], consistent with the results of our safety analysis. These findings, which are considered robust compared with case reports and observational cohorts given they are based on an RCT, contribute to the growing body of evidence that supports the safety of remdesivir use in patients with COVID-19. As medical knowledge is constantly evolving, new research, especially on larger sample sizes, may provide further insights on the relationship between remdesivir and cardiac events. In the meantime, regardless of whether remdesivir is administered or not, the identification of hospitalized patients with COVID-19 at high risk for cardiac events, such as those with preexisting cardiovascular disease, is important to establish preventive interventions and comprehensive monitoring strategies, if needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Vida Terzić, Clinical Trial Safety and Public Health, ANRS|Emerging Infectious Diseases, Paris, France; Clinical Research Safety Department, INSERM, Paris, France.
Joe Miantezila Basilua, Clinical Trial Safety and Public Health, ANRS|Emerging Infectious Diseases, Paris, France; Clinical Research Safety Department, INSERM, Paris, France.
Nicolas Billard, Department of Epidemiology, Biostatistics and Clinical Research, Hospital Bichat, APHP, Paris, France.
Lucie de Gastines, Clinical Trial Safety and Public Health, ANRS|Emerging Infectious Diseases, Paris, France; Clinical Research Safety Department, INSERM, Paris, France.
Drifa Belhadi, Department of Epidemiology, Biostatistics and Clinical Research, Hospital Bichat, APHP, Paris, France; Université Paris Cité, IAME, INSERM, Paris, France.
Claire Fougerou-Leurent, Pharmacology Unit, University Hospital Rennes, CIC Inserm 1414, University Hospital Rennes, Rennes, France.
Nathan Peiffer-Smadja, Infectious Diseases Department, Hôpital Bichat—Claude-Bernard, APHP, Paris, France; Université Paris Cité, IAME, INSERM, Paris, France.
Noémie Mercier, Clinical Trial Safety and Public Health, ANRS|Emerging Infectious Diseases, Paris, France; Clinical Research Safety Department, INSERM, Paris, France.
Christelle Delmas, Pôle de Recherche Clinique, INSERM, Paris, France.
Assia Ferrane, Pôle de Recherche Clinique, INSERM, Paris, France.
Aline Dechanet, Department of Epidemiology, Biostatistics and Clinical Research, Hospital Bichat, APHP, Paris, France.
Julien Poissy, UMR 8576—UGSF—Unité de Glycobiologie Structurale et Fonctionnelle, Université de Lille, Inserm U1285, CHU Lille, Pôle de réanimation, CNRS, Lille, France.
Hélène Espérou, Pôle de Recherche Clinique, INSERM, Paris, France.
Florence Ader, Département des Maladies infectieuses et tropicales, Hospices Civils de Lyon, Lyon, France; Centre International de Recherche en Infectiologie (CIRI), Inserm 1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, École Normale Supérieure de Lyon, Univ Lyon, Lyon, France.
Maya Hites, Clinic of Infectious Diseases, Hôpital Universitaire de Bruxelles (HUB)-Erasme, Brussels, Belgium.
Claire Andrejak, Pulmonolgy Unit, University Hospital Amiens-Picardie, UR 4294 AGIR, Université Picardie Jules Verne, Amiens, France.
Richard Greil, IIIrd Medical Department, Paracelsus Medical University Salzburg, Salzburg Cancer Research Institute-Center for clinical Cancer and Immunology Trials (SCRI-CCCIT), Cancer Cluster Salzburg, Austrian Group for Medical Tumor Therapy (AGMT), Salzburg, Austria.
José-Artur Paiva, Serviço de Medicina Intensiva, Centro Hospitalar Universitário São João, Porto, Portugal.
Thérèse Staub, Infectious Diseases Department, Centre Hospitalier de Luxembourg, Luxembourg, Luxembourg.
Evelina Tacconelli, Infectious Diseases, Dept. Diagnostic and Public Health, University of Verona, Verona, Italy.
Charles Burdet, Department of Epidemiology, Biostatistics and Clinical Research, Hospital Bichat, APHP, Paris, France.
Dominique Costagliola, Sorbonne Université, INSERM, Institut Pierre Louis d’Épidémiologie et de Santé Publique, Paris, France.
France Mentré, Department of Epidemiology, Biostatistics and Clinical Research, Hospital Bichat, APHP, Paris, France; Université Paris Cité, IAME, INSERM, Paris, France.
Yazdan Yazdanpanah, Université Paris Cité, IAME, INSERM, Paris, France; Infectious Diseases Department, Hôpital Bichat—Claude-Bernard, APHP, Paris, France; ANRS|Emerging Infectious Diseases, Paris, France.
Alpha Diallo, Clinical Trial Safety and Public Health, ANRS|Emerging Infectious Diseases, Paris, France; Clinical Research Safety Department, INSERM, Paris, France.
DisCoVeRy Study Group:
Sandrine Couffin-Cadièrgues, Hélène Esperou, Bernd Lamprecht, Michael Joannidis, Alexander Egle, Richard Greil, Antoine Altdorfer, Vincent Fraipont, Leila Belkhir, Maya Hites, Gil Verschelden, Violaine Tolsma, David Bougon, Agathe Delbove, Marie Gousseff, Nadia Saidani, Guilhem Wattecamps, Félix Djossou, Loïc Epelboin, Jean-Philippe Lanoix, Pierre-Alexandre Roger, Claire Andrejak, Yoann Zerbib, Kevin Bouiller, Catherine Chirouze, Jean-Christophe Navellou, Alexandre Boyer, Charles Cazanave, Alexandre Duvignaud, Didier Gruson, Denis Malvy, Henry Lessire, Martin Martinot, Pascal Andreu, Mathieu Blot, Lionel Piroth, Jean Pierre Quenot, Olivier Epaulard, Nicolas Terzi, Karine Faure, Emmanuel Faure, Julien Poissy, Saad Nseir, Florence Ader, Laurent Argaud, Tristan Ferry, Thomas Perpoint, Vincent Piriou, Jean-Christophe Richard, Julien Textoris, Florent Valour, Florent Wallet, André Cabié, Jean-Marie Turmel, Cyrille Chabartier, Rostane Gaci, Céline Robert, Alain Makinson, Vincent Le Moing, Kada Klouche, Olivier Hinschberger, Joy Mootien, Sébastien Gibot, François Goehringer, Antoine Kimmoun, Benjamin Lefevre, David Boutoille, Emmanuel Canet, Benjamin Gaborit, Paul Le Turnier, François Raffi, Jean Reignier, Johan Courjon, Jean Dellamonica, Sylvie Leroy, Charles-Hugo Marquette, Paul Loubet, Claire Roger, Albert Sotto, Cédric Bruel, Benoît Pilmis, Guillaume Geri, Elisabeth Rouveix-Nordon, Olivier Bouchaud, Samy Figueiredo, Stéphane Jaureguiberry, Xavier Monnet, Lila Bouadma, François-Xavier Lescure, Nathan Peiffer-Smadja, Jean-François Timsit, Yazdan Yazdanpanah, Solen Kerneis, Marie Lachâtre, Odile Launay, Jean-Paul Mira, Julien Mayaux, Valérie Pourcher, Jérôme Aboab, Flora Crockett, Naomi Sayre, Clément Dubost, Cécile Ficko, David Lebeaux, Sébastien Gallien, Armand Mekontso-Dessap, Jérôme Le Pavec, Francois Stefan, Hafid Ait-Oufella, Karine Lacombe, Jean-Michel Molina, Murielle Fartoukh, Gilles Pialoux, Firouzé Bani-Sadr, Bruno Mourvillier, François Benezit, Fabrice Laine, Bruno Laviolle, Yves Le Tulzo, Matthieu Revest, Elisabeth Botelho-Nevers, Amandine Gagneux-Brunon, Guillaume Thiery, François Danion, Yves Hansmann, Ferhat Meziani, Walid Oulehri, Charles Tacquard, Fanny Bounes-Vardon, Guillaume Martin-Blondel, Marlène Murris-Espin, Béatrice Riu-Poulenc, Vanessa Jeanmichel, Eric Senneville, Louis Bernard, Denis Garot, Jean Reuter, Thérèse Staub, Marc Berna, Sandra Braz, Joao Miguel Ferreira Ribeiro, José-Artur Paiva, Roberto Roncon-Albuquerque, and Benjamin Leveau
Notes
Author contributions. V. T., J. M. B. and A. D. drafted the manuscript. A. D., Y. Y., F. A., F. M., M. H., H. E., and D. C. primarily reviewed the manuscript. C. D., D. B., C. A., C. B., C. F. L., N. P. S., F. A., M. H., H. E., and J. P. contributed to refinement of the prefinal version, and all authors reviewed and approved the final version of the manuscript. V. T., J. M. B., F. M., and D. C. contributed equally to writing of the article.
Acknowledgments. The authors acknowledge the patients enrolled in the study and the healthcare workers involved in patient management. They acknowledge the members of the trial Data and Safety Monitoring Board (DSMB): Stefano Vella (chair), Karen Barnes, Phaik Yeong Cheah, Guanhua Du, Mike Jacobs, Donata Medaglini, Stuart Pocock, Sylvie Van Der Werf, Patrick Yéni, and the independent statistician Tim Collier. They acknowledge the support of Gilead Sciences, which provided the study drug. They acknowledge the Working Group of the DisCoVeRy Trial involved in the safety matters (by alphabetical order): Drifa Belhadi, Aline Dechanet, Christelle Delmas, Alpha Diallo, Joe Miantezila-Basilua, Noémie Mercier, Laurence Moachon, Nathan Peiffer-Smadja, and Vida Terzić. They acknowledge the data and monitoring team (monitoring project managers, clinical research associates, data managers) for ensuring data quality.
This study has been labeled as a national research priority by the National Orientation Committee for Therapeutic Trials and Other Research on Coronavirus Disease 2019 (COVID-19). The authors acknowledge ANRS–Emerging Infectious Diseases for their scientific support, the French Ministry of Health and Prevention, and the French Ministry of Higher Education, Research, and Innovation for their funding and support.
Data sharing statement. With publication, deidentified, individual participant data that underlie this article, along with a data dictionary describing variables in the dataset, will be made available to researchers whose proposed purpose of use is approved by the DisCoVeRy Steering Committee. To request the dataset, please address the request directly to the corresponding author (alpha.diallo@anrs.fr) and to the sponsor's representative (helene.esperou@inserm.fr) to obtain a data access form. All requests will be evaluated by the Trial Management Team and the DisCoVeRy Steering Committee.
For accepted requests, data will be shared after signing a data transfer agreement with the study sponsor. Data will be shared directly or through access on the INSERM repository. Related documents, such as the study protocol, statistical analysis plan, and informed consent form, will be made available (with publication) on request to the corresponding author or to the sponsor's representative. The data will be open access for the informed consent form, protocol, and statistical analysis plan.
Financial support. This work used the data collected during the DisCoVeRy clinical trial, part of the European Union (EU)-Response consortium that received funding from the following sources: EU's Horizon 2020 Societal Challenges (Research and Innovation Program Europe N°101015736); Austrian Group Medical Tumor Therapy (Austria); Belgian Health Care Knowledge Centre (Belgium); Fonds Erasme-COVID-Université Libre de Bruxelles (Belgium); REACTing, a French multidisciplinary collaborative network working on emerging infectious diseases (France); Ministry of Health France (France PHRC 20-0351); Domaine d’intérêt majeur One Health Île-de-France (France); European Regional Development Fund (Luxembourg); Ministry of Health (Portugal); and Agency for Clinical Research and Biomedical Innovation (Portugal). All authors are associated with the EU-Response project because their employers are Beneficiaries of the EU-Response Consortium.
References
- 1. Warren T, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016; 531:381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324:1048–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldman JD, Lye DC, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 2020; 383:1827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta AK, Parker BM, Priyadarshi V, Parker J. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus 2020; 12:e11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barkas F, Styla CP, Bechlioulis A, et al. Sinus bradycardia associated with remdesivir treatment in COVID-19: a case report and literature review. J Cardiovasc Dev Dis 2021; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donepudi B, Agarwal S, Thakur L. Severe bradycardia leading to hemodynamic instability associated with remdesivir use in a patient with COVID-19 pneumonia. Case Rep Crit Care 2022; 2022:8807957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hajimoradi M, Sharif Kashani B, Dastan F, et al. Remdesivir associated sinus bradycardia in patients with COVID-19: a prospective longitudinal study. Front Pharmacol 2023; 13:1107198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alsowaida YS, Shehadeh F, Kalligeros M, Mylonakis E. Incidence and potential risk factors for remdesivir-associated bradycardia in hospitalized patients with COVID-19: a retrospective cohort study. Front Pharmacol 2023; 14:1106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devgun JM, Zhang R, Brent J, et al. Identification of bradycardia following remdesivir administration through the US Food and Drug Administration American College of Medical Toxicology COVID-19 Toxic Pharmacovigilance Project. JAMA Netw Open 2023; 6:e2255815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rafaniello C, Ferrajolo C, Sullo MG, et al. Cardiac events potentially associated to remdesivir: an analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2021; 14:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung SY, Kim MS, Li H, et al. Cardiovascular events and safety outcomes associated with remdesivir using a World Health Organization international pharmacovigilance database. Clin Transl Sci 2022; 15:501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Touafchia A, Bagheri H, Carrié D, et al. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clin Microbiol Infect 2021; 27:791.e5–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Medicines Agency. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 7–10 June 2021. Available at: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-7-10-june-2021. Accessed 7 December 2023.
- 16. Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID-19: a review. JACC Clinical Electrophysiology 2020; 6:1193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ader F, Bouscambert-Duchamp M, Hites M, et al. Final results of the DisCoVeRy trial of remdesivir for patients admitted to hospital with COVID-19. Lancet Infect Dis 2022; 22:764–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amstutz A, Speich B, Mentré F, et al. Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir Med 2023; 11:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, corrected version 2.1. [July 2017]. Available at: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf. Accessed 7 December 2023.
- 20. Ghebremichael-Weldeselassie Y, Jabagi MJ, Botton J, et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med 2022; 41:1735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Botton J, Jabagi MJ, Bertrand M, et al. Risk for myocardial infarction, stroke, and pulmonary embolism following COVID-19 vaccines in adults younger than 75 years in France. Ann Intern Med 2022; 175:1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized COVID-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol 2021; 31:e2187. [DOI] [PubMed] [Google Scholar]
- 23. Pantely GA, Bristow JD. Adenosine: renewed interest in an old drug. Circulation 1990; 82:1854–6. [DOI] [PubMed] [Google Scholar]
- 24. Kingsley R, Rohlman C, Otto A, et al. Remdesivir-induced conduction abnormalities: a molecular model-based explanation. J Pharm Pharm Sci 2023; 26:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci 2020; 6:672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi SW, Shin JS, Park SJ, et al. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res 2020; 184:104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwok M, Lee C, Li HS, et al. Remdesivir induces persistent mitochondrial and structural damage in human induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc Res 2022; 118:2652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szendrey M, Guo J, Li W, et al. COVID-19 drugs chloroquine and hydroxychloroquine, but not azithromycin and remdesivir, block hERG potassium channels. J Pharmacol Exp Ther 2021; 377:265–72. [DOI] [PubMed] [Google Scholar]
- 29. Cubeddu LX, de la Rosa D, Ameruoso M. Antiviral and anti-inflammatory drugs to combat COVID-19: effects on cardiac ion channels and risk of ventricular arrhythmias. BioImpacts 2022; 12:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haghjoo M, Golipra R, Kheirkhah J, et al. Effect of COVID-19 medications on corrected QT interval and induction of torsade de pointes: results of a multicenter national survey. Int J Clin Pract 2021; 75:e14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Medicines Agency. EPAR—EU risk management plan for Veklury. Last updated 23 September 2022. Table SII.1. Table of key safety findings from non-clinical studies. Available at: https://www.ema.europa.eu/en/documents/rmp-summary/veklury-epar-risk-management-plan_en.pdf. Accessed 7 December 2023.
- 32.Bristol-Myers Squibb press release 23/08/2012. Bristol-Myers Squibb discontinues development of BMS-986094, an investigational NS5B nucleotide for the treatment of hepatitis C. Available at: https://news.bms.com/news/details/2012/Bristol-Myers-Squibb-Discontinues-Development-of-BMS-986094-an-Investigational-NS5B-Nucleotide-for-the-Treatment-of-Hepatitis-C/default.aspx. Accessed 7 December 2023.
- 33. Ahmad T, Yin P, Saffitz J, et al. Cardiac dysfunction associated with a nucleotide polymerase inhibitor for treatment of hepatitis C. Hepatology 2015; 62:409–16. [DOI] [PubMed] [Google Scholar]
- 34.European Medicines Agency press release 24/04/2015. EMA recommends avoidance of certain hepatitis C medicines and amiodarone together. Available at: https://www.ema.europa.eu/en/news/ema-recommends-avoidance-certain-hepatitis-c-medicines-amiodarone-together. Accessed 7 December 2023.
- 35. Lai CC, Chen CH, Wang CY, et al. Clinical efficacy and safety of remdesivir in patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. J Antimicrob Chemother 2021; 76:1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh S, Khera D, Chugh A, et al. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open 2021; 11:e048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lai CC, Wang YH, Chen KH, et al. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials. Viruses 2022; 14:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng Q, Zhao G, Chen J, et al. Comparative efficacy and safety of pharmacological interventions for severe COVID-19 patients: an updated network meta-analysis of 48 randomized controlled trials. Medicine (Baltimore) 2022; 101:e30998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.