Abstract
Background.
We aimed to determine if pre-existing immunocompromising conditions (ICCs) were associated with the presentation or outcome of patients with acute coronavirus disease 2019 (COVID-19) admitted for pediatric intensive care.
Methods.
Fifty-five hospitals in 30 US states reported cases through the Overcoming COVID-19 public health surveillance registry. Patients <21 years admitted 12 March 2020–30 December 2021 to the pediatric intensive care unit (PICU) or high-acuity unit for acute COVID-19 were included.
Results.
Of 1274 patients, 105 (8.2%) had an ICC, including 33 (31.4%) hematologic malignancies, 24 (22.9%) primary immunodeficiencies and disorders of hematopoietic cells, 19 (18.1%) nonmalignant organ failure with solid-organ transplantation, 16 (15.2%) solid tumors, and 13 (12.4%) autoimmune disorders. Patients with ICCs were older, had more underlying renal conditions, and had lower white blood cell and platelet counts than those without ICCs, but had similar clinical disease severity upon admission. In-hospital mortality from COVID-19 was higher (11.4% vs 4.6%, P = .005) and hospitalization was longer (P = .01) in patients with ICCs. New major morbidities upon discharge were not different between those with and without ICC (10.5% vs 13.9%, P = .40). In patients with ICCs, bacterial coinfection was more common in those with life-threatening COVID-19.
Conclusions.
In this national case series of patients <21 years of age with acute COVID-19 admitted for intensive care, existence of a prior ICCs were associated with worse clinical outcomes. Reassuringly, most patients with ICCs hospitalized in the PICU for severe acute COVID-19 survived and were discharged home without new severe morbidities.
Keywords: COVID-19, immunocompromised host, pediatrics, critical care, bacterial coinfection
Children with pre-existing medical conditions are vulnerable to developing life-threatening disease from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1]. Published studies reported conflicting findings on the severity and outcomes of SARS-CoV-2 infections in children with immunocompromising conditions (ICCs). Although some reports show that children with malignancies, transplants, or other ICCs have similar or improved outcomes compared with the general pediatric population [2–7], data from other studies suggest that they are at higher risk for poor outcomes [8, 9]. Published studies analyzed small or single-center cohorts of children with ICCs, and critical illness was uncommon. In general, children with ICCs have worse critical care outcomes and are at higher risk for severe respiratory failure [10]. However, several gaps in the field remain. Among children admitted to the pediatric intensive care unit (PICU), it is unclear if SARS-CoV-2 confers the same increased risk in children with ICCs compared with those without ICCs. There are also limited and conflicting data on whether specific types of ICCs or other clinical factors may increase the risk of life-threatening and fatal illness among children hospitalized for acute coronavirus disease 2019 (COVID-19) [2–6, 8, 9, 11, 12].
Risk assessment affects clinical management, since distinct therapeutic pathways have been proposed based on the risk of severe disease as well as the patient’s clinical presentation [13]. The relative rarity of ICCs in children, who may be on immunomodulatory and/or immunosuppressive medications, adds to the complexity and challenges in the care of patients [14]. Prior studies of solid-organ transplant recipients have indicated that outcomes may also be infection specific [15–17], as these patients have better outcomes from sepsis than those without transplant, but more susceptibility to viral infections [18]. Thus, a better understanding of the clinical presentation and associated outcomes can improve resource allocation for children with ICCs and COVID-19. To address this gap in knowledge, we compared the clinical characteristics and hospital course of children with or without ICCs in a large, national, multicenter registry of US children admitted for pediatric intensive care in the setting of acute COVID-19.
METHODS
Study Design
Case patients in this investigation were enrolled in the Overcoming COVID-19 public health surveillance registry from sentinel surveillance of children, adolescents, and young adults hospitalized with SARS-CoV-2–related illness at 55 hospitals in 30 US states from 12 March 2020 through 30 December 2021 [19, 20]. We followed reporting guidelines for uncontrolled case series [21]. The Boston Children’s Hospital (BCH) Institutional Review Board (IRB) reviewed and approved the protocol and served as the single IRB for 43 sites, and 11 sites had site-specific IRB approval. This activity was reviewed by the Centers for Disease Control and Prevention (CDC) and determined to meet the requirements of public health surveillance per 45 CFR §46.101(b)(4).
Data Collection and Definitions
As reported previously [22], enrolling centers employed trained study personnel who abstracted data from patient electronic medical records into standardized case report forms using REDCap (Vanderbilt University) hosted at BCH. The standardized data collection included demographics, underlying medical conditions, clinical course, laboratory values, diagnostic imaging, therapeutics, and outcomes. Patients included in this analysis were younger than 21 years of age on admission and were diagnosed with acute COVID-19 via positive reverse-transcription–polymerase chain reaction (RT-PCR) or antigen test. Those diagnosed with multisystem inflammatory syndrome in children were excluded. All patients included in the analysis were admitted to the PICU or high-acuity unit. Obesity was classified for patients aged 2 years or older as greater than or equal to the 95th body mass index (BMI) percentile for age and sex based on CDC national reference standards [23]. The social vulnerability index (SVI) [24] was determined using the first 4 digits of a patient’s home address zip code for patients admitted during 2020 and by home address for patients admitted during 2021, in order to obtain more accurate geolocated SVI estimates. Organ system involvement was defined using previously published criteria [20, 25], as shown in Supplementary Table 1. Left ventricular dysfunction was defined as a measurement of left ventricular ejection fraction of less than 55% on at least 1 echocardiogram during hospitalization. Bacterial coinfections, defined as a bacterial infection detected within 72 hours of admission assumed to be community acquired, were adjudicated by pediatric critical care and infectious disease experts [26]. Life-threatening COVID-19 illness was defined as receipt of invasive mechanical ventilation for 7 days or more, receipt of extracorporeal membrane oxygenation (ECMO), or in-hospital death. Additional new morbidity outcomes at discharge included discharge to a rehabilitation center and a new neurologic deficit, supplemental oxygen requirement, tracheostomy, or mechanical ventilation requirement.
Adjudication of Immunocompromising Conditions
The underlying conditions of eligible patients were adjudicated for immunocompromising conditions by experts in immunology (J. C. and B. L.) and critical care (C. M. R. and A. G. R.). Patients were classified as having 1 of the following types of ICCs [27]: (1) hematopoietic malignancy, (2) solid tumor, (3) nonmalignant organ failure with history of solid-organ transplantation, (4) autoimmune disorder, and (5) primary immunodeficiency and other disorders of hematopoietic cells. Primary immunodeficiencies were further subcategorized as innate immune defects, humoral immunodeficiencies due to impaired B-cell function, combined immunodeficiencies due to defective T-cell function, and primary immunoregulatory disorders characterized by a combination of autoimmunity and immunodeficiency [28].
Statistical Analysis
Categorical variables are summarized as counts and percentages, while continuous variables are expressed as median and interquartile range. Descriptive statistics were performed to compare differences between patients with and without immunocompromising conditions. Differences in categorical variables were compared using the chi-square test or Fisher’s exact test for expected counts less than 5, and differences in continuous variables were determined using a Wilcoxon rank-sum test. A 2-tailed P value less than .05 was considered statistically significant. All analyses were performed in R version 4.2.2 (R Project for Statistical Computing).
RESULTS
Of the 1274 patients younger than 21 years in the Overcoming COVID-19 registry with acute COVID-19 admitted for pediatric intensive care, 105 (8.2%) had an ICC. There were 33 (31.4%) patients with hematologic malignancies, 24 (22.9%) with primary immunodeficiencies and disorders of hematopoietic cells, 19 (18.1%) with nonmalignant organ failure post–solid-organ transplantation, 16 (15.2%) with solid tumors, and 13 (12.4%) with autoimmune disorders. The subtypes within each ICC category are shown in Figure 1.
Figure 1.
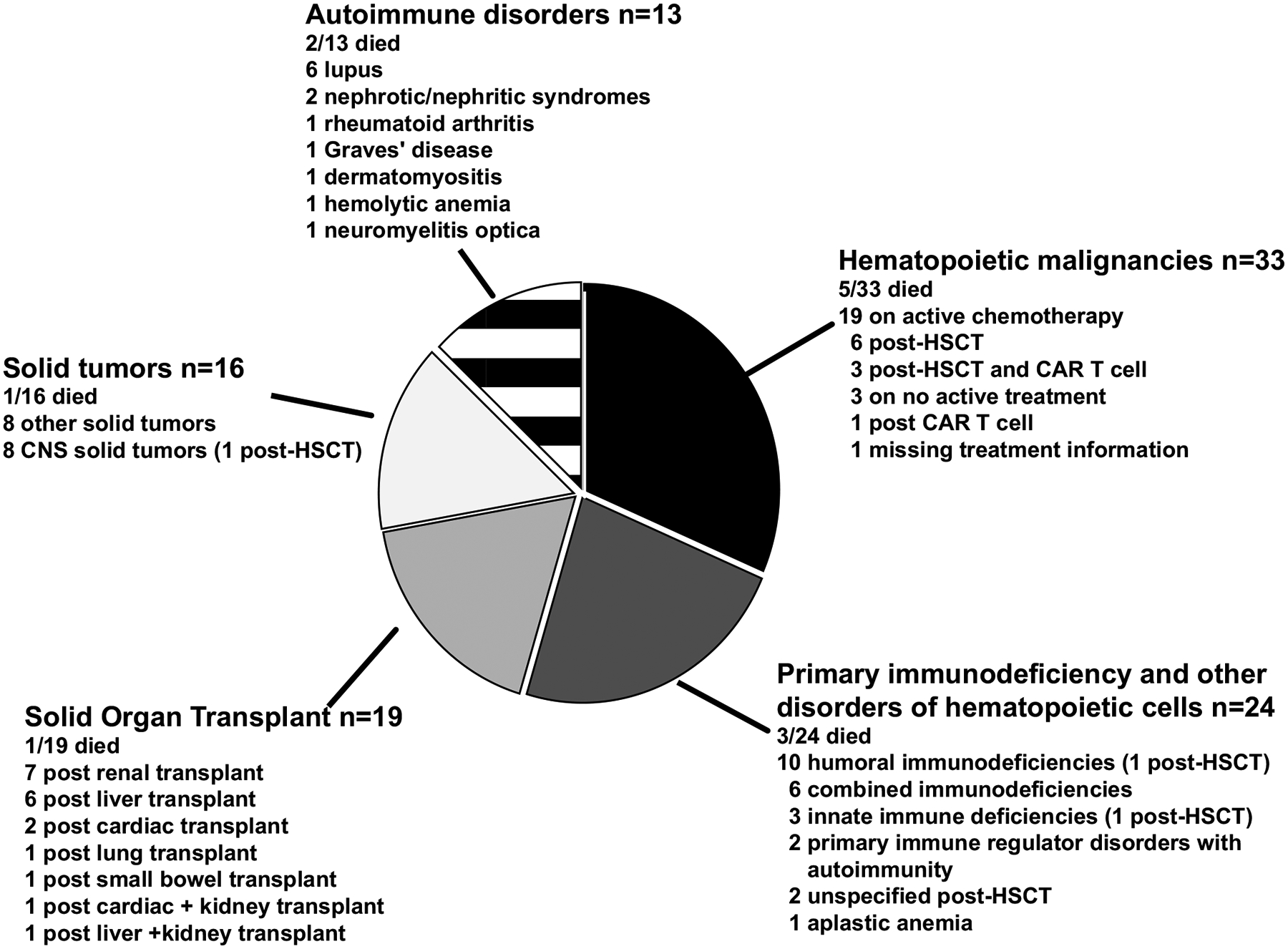
The distribution of specific type of ICCs. Mortality rates are described within each subcategory, along with the rates of HSCT. Abbreviations: CAR T, chimeric antigen receptor T-cell therapy; CNS, central nervous system; HSCT, hematopoietic stem cell transplantation; ICC, immunocompromising condition.
Overall, compared with patients in the PICU for acute COVID-19 without an ICC, patients with ICCs were older and had more underlying cardiovascular and renal conditions but were less frequently obese (all P < .05) (Table 1). The average pediatric sequential organ failure assessment (pSOFA) scores (on admission and maximum) were comparable between the 2 groups. The median Pediatric Logistic Organ Dysfunction–version 2 (PELOD-2) scores (on admission and maximum) were higher in patients with an ICC (P = .001). Individuals with ICCs had lower white blood cell counts, with reduced numbers of neutrophils, lymphocytes, and platelets. Levels of inflammatory markers, including C-reactive protein (CRP), procalcitonin, and ferritin, were comparable between those with and without ICCs in those with measurements at admission (Table 2). The frequency of neurologic and gastrointestinal involvement did not differ between ICC and non-ICC cohorts, but hematologic involvement was significantly more frequent in the ICC cohort (Figure 2A). Furthermore, there was no difference in cardiovascular involvement, including the occurrence of left ventricular dysfunction, coronary artery aneurysms, or receipt of vasoactive infusions between the 2 groups (Figure 2). Bacterial coinfections and viral co-detections within 72 hours of admission were reported with comparable frequencies in the ICC and non-ICC groups (Table 3 and Supplementary Table 2).
Table 1.
Baseline Characteristics of Patients Admitted to the Pediatric Intensive Care Unit for Acute COVID-19 Stratified by the Presence of an Immunocompromising Condition
| COVID-19 With ICCs (n = 105) | COVID-19 No ICCs (n = 1169) | P | |
|---|---|---|---|
| Age, median (IQR), y | 15.3 (7.7, 17.8) | 13.5 (4.3, 16.6) | <.001*** |
| Male sex, n (%) | 60 (57.1) | 615 (52.6) | .43 |
| Social vulnerability index, median (IQR) | 0.52 (0.38, 0.66) | 0.56 (0.37, 0.75) | .73 |
| Race and ethnicity, n (%) | |||
| White, non-Hispanic | 35 (33.3) | 430 (36.8) | .14 |
| Black, non-Hispanic | 21 (20) | 307 (26.3) | |
| Hispanic or Latino | 35 (33.3) | 313 (26.8) | |
| Asian | 7 (6.7) | 30 (2.6) | |
| Mixed/other race, non-Hispanic | 3 (2.9) | 40 (3.4) | |
| Unknown | 4 (3.8) | 49 (4.2) | |
| Non-immunocompromising underlying conditions, n (%) | |||
| Any non-immunocompromising condition | 82 (78.1) | 805 (68.9) | .06 |
| Respiratory | 38 (36.2) | 400 (34.2) | .76 |
| Asthma | 21 (20.0) | 241 (20.6) | .98 |
| Chronic lung diseasea | 13 (12.4) | 92 (7.9) | .15 |
| Upper airway disorder | 5 (4.8) | 38 (3.3) | .59 |
| Cardiovascular | 20 (19.0) | 138 (11.8) | .045* |
| Neuromuscular | 31 (29.5) | 244 (20.9) | .05 |
| Renal | 27 (25.7) | 66 (5.6) | <.001*** |
| Obesity | 34/100 (34.0) | 473/938 (50.4) | .003** |
| Acuity scores, median (IQR) | |||
| Admission PELOD-2 | 2 (0, 3) | 0 (0, 3) | .02** |
| Admission pSOFA | 2 (0, 4) | 3 (0, 4) | .65 |
| Maximum PELOD-2 | 2 (0, 4) | 1 (0, 3) | .001** |
| Maximum pSOFA | 4 (1, 6) | 4 (1, 5) | .66 |
| Outcomes | |||
| Died, n (%) | 12 (11.4%) | 54 (4.6%) | .005** |
| Length of IMV,b median (IQR) | 5.5 (1, 8.5) | 8 (2, 17.5) | .12 |
| Ventilator-free days, median (IQR) | 12 (7, 16.5) | 12 (7, 22) | .79 |
| Length of stay in PICU, median (IQR) | 5 (3, 9) | 4 (2, 9) | .11 |
| Length of stay in hospital, median (IQR) | 9 (5, 15) | 7 (4, 14) | .01** |
Abbreviations: COVID-19, coronavirus disease 2019; ICC, immunocompromising condition; IMV, invasive mechanical ventilation; IQR, interquartile range; PELOD-2, pediatric logistic organ dysfunction score version 2; pSOFA, pediatric sequential organ failure assessment.
Non-asthma chronic lung disease includes chronic restrictive lung disease, bronchopulmonary dysplasia/chronic lung disease of prematurity, cystic fibrosis, recurrent aspiration into lungs/chronic aspiration pneumonia, and pulmonary hypertension.
Among those receiving IMV.
P < .05;
P < .01;
P < .001.
Table 2.
Initial Laboratory Values on Admission of Patients Admitted to the Pediatric Intensive Care Unit for Acute COVID-19, Stratified by the Presence of an Immunocompromising Condition
| Initial Laboratory Values, Median (IQR) | COVID-19 With ICCs (n = 105) | COVID-19 No ICCs (n = 1169) | P |
|---|---|---|---|
| ANC (103/μL), n = 83 and 838 | 4.29 (1.66, 8.09) | 5.07 (2.95, 8.36) | .02 |
| Neutropenia (ANC <500/μL) | 11/83 (13.3) | 7/838 (0.8) | <.001 |
| ALC (103/μL), n = 86 and 861 | 0.83 (0.42, 1.38) | 1.24 (0.73, 2.28) | <.001 |
| Lymphopenia (ALC <1000/μL) | 53/86 (61.6) | 333/861 (38.7) | <.001 |
| NLR (103/μL), n = 82 and 838 | 5.00 (1.62, 8.94) | 3.95 (2.02, 7.91) | .72 |
| Platelet (103/μL) | 201 (120, 276) | 226 (168, 316) | .003 |
| Thrombocytopenia (<100 103/μL) | 19/93 (20.4) | 62/908 (6.8) | <.001 |
| CRP (mg/dL), n = 56 and 550 | 4.7 (2.2, 8.5) | 4.0 (1.4, 8.8) | .31 |
| Procalcitonin (ng/mL), n = 28 and 323 | 0.22 (0.14, 0.38) | 0.24 (0.10, 1.15) | .48 |
| Ferritin (ng/mL), n = 34 and 317 | 434 (117, 1507) | 255 (112, 593) | .14 |
Results presented are medians with interquartile ranges or counts with frequencies.
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ICC, immunocompromising condition; IQR, interquartile range; NLR, neutrophil-to-lymphocyte ratio.
Figure 2.
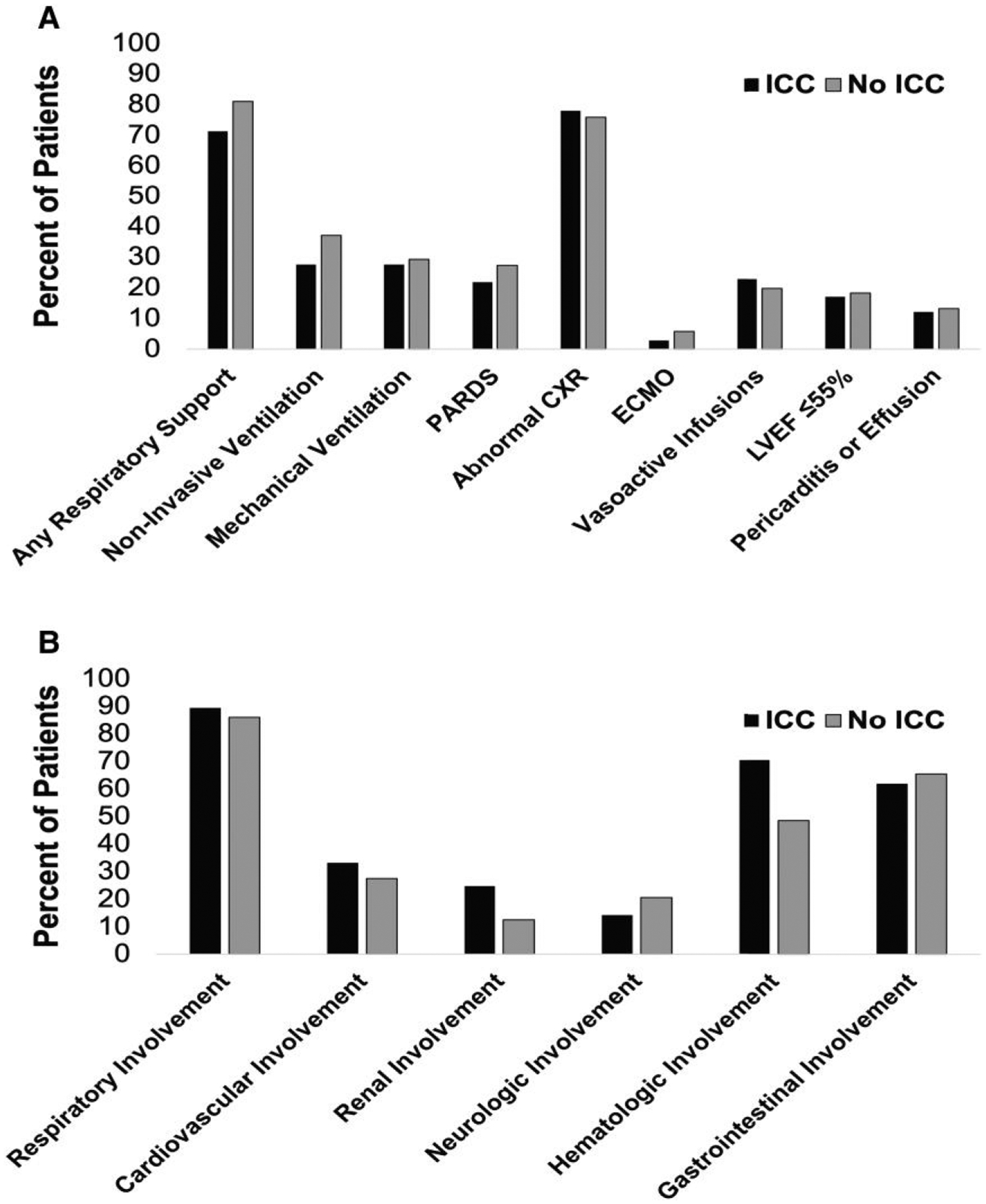
Organ involvement and support in children and adolescents admitted to the pediatric intensive care unit with acute COVID-19 stratified by those with and without an ICC. A, Details of outcomes and organ support. B, Distribution of organ involvement in pediatric acute COVID-19 comparing those with immunocompromising conditions, displayed with black bars, to those without ICC, displayed in gray bars. Denominators for ejection fraction and pericarditis or pericardial effusion are the number of patients undergoing echocardiography (n = 41 for ICCs and n = 417 for no ICCs, respectively). Abbreviations: COVID-19, coronavirus disease 2019; CXR, chest radiograph; ECMO, extracorporeal membrane oxygenation; ICC, immunocompromising condition; LVEF, left ventricular ejection fraction; PARDS, pediatric acute respiratory distress syndrome.
Table 3.
Respiratory Viral Co-Detection and Bacterial Coinfections Within 72 Hours of Admission in Patients Admitted to the Pediatric Intensive Care Unit for Acute COVID-19, Stratified by the Presence of an Immunocompromising Condition
| COVID-19 With ICCs (n = 105) | COVID-19 No ICCs (n = 1169) | |
|---|---|---|
| Respiratory viral co-detectiona | 10 (9.5%) | 113 (9.7%) |
| Adenovirus | 3 (2.9%) | 8 (0.7%) |
| Coronavirus (non–SARS-CoV-2) | 0 | 4 (0.3%) |
| Human metapneumovirus | 0 | 4 (0.3%) |
| Influenza | 0 | 2 (0.2%) |
| Parainfluenza | 0 | 12 (1.0%) |
| Rhinovirus/enterovirus | 3 (2.8%) | 67 (5.7%) |
| Respiratory syncytial virus | 1 (1.0%) | 35 (3.0%) |
| Other virusb | 4 (3.8%) | 4 (0.3%) |
| Bacterial coinfectionc,d | 10 (9.5%) | 85 (7.3%) |
| Bloodstream | 8 | 28 |
| Gram-positive bacteria | 3 | 17 |
| Gram-negative bacteria | 5 | 10 |
| Fungal | 0 | 1 |
| Central nervous system | 0 | 5 |
| Lower respiratory tract | 0 | 47 |
| Gram-positive bacteria | 0 | 25 |
| Staphylococcus aureus methicillin-resistant | 0 | 5 |
| Staphylococcus aureus methicillin-sensitive | 0 | 14 |
| Streptococcus pneumoniae | 0 | 4 |
| Gram-negative bacteria | 0 | 22 |
| Pseudomonas species | 0 | 12 |
| Urine | 2 | 28 |
| Gram-positive bacteria | 1 | 7 |
| Gram-negative bacteria | 1 | 19 |
| Escherichia coli | 1 | 10 |
| Enterococcus faecalis | 0 | 7 |
| Other | 0 | 2 |
| Fungal | 0 | 2 |
Abbreviations: COVID-19, coronavirus disease 2019; ICC, immunocompromising condition; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Among patients with ICCs, 1 patient had 2 viral co-detections. In patients without ICCs, 94 (83.2%) patients had 1 viral co-detection, 15 (13.3%) patients had 2 viral co-detections, and 4 (3.5%) patients had 3 viral co-detections.
In patients with ICCs: 2 patients had Epstein-Barr coinfection, 1 had cytomegalovirus coinfection, and 1 had cytomegalovirus, Epstein-Barr virus, and BK (human polyomavirus) virus coinfection. In patients without ICCs: 3 patients had Epstein-Barr virus coinfection and 1 patient had HHV-6 coinfection.
Patients with a positive bacterial culture from any site adjudicated by the site and study staff to be a true infection.
No patients with ICCs were adjudicated to have >1 bacterial coinfection, but in patients without ICCs, 64 (75.3%) had 1 bacterial coinfection, 18 (21.2%) had 2 coinfections, and 3 (3.5%) had 3 coinfections. A list of the organisms is included in Supplementary Table 2.
Specific therapies for COVID-19 were frequently used in both groups (Supplementary Table 3). Among all patients with COVID-19, corticosteroids were used in 64.8%, remdesivir in 52.0%, and tocilizumab in 5.9% of patients, with comparable frequencies in patients with or without ICCs. More patients with ICCs received intravenous immunoglobulin than those without ICCs (15/105 [14.3%] vs 48/1169 [4.1%], respectively; P < .001), likely due to its common use in patients with humoral and combined immunodeficiencies.
Patients with ICCs had similar clinical disease severity upon admission to those without ICCs, with similar proportions of children receiving mechanical ventilation, vasoactive infusions, or ECMO (Figure 2A). Despite similarities in clinical presentation, the outcomes were worse for those with ICCs (Table 1). In-hospital mortality from COVID-19 was higher in those with ICCs compared with those without ICCs (11.4% vs 4.6%; P = .005). Additionally, those with ICCs had longer hospitalizations compared with those without ICCs (P = .01). No association was identified between ICCs and any of the following individual or composite outcomes: new neurologic disability, supplemental oxygen, tracheostomy, or mechanical ventilation in survivors (Supplementary Table 4).
Within the ICC cohort, we investigated the clinical and laboratory characteristics of patients with or without life-threatening acute COVID-19, defined as 7 or more days of invasive mechanical ventilation, use of ECMO, or in-hospital death (Table 4). Among the 105 patients with ICCs, 17 (16.2%) had life-threatening COVID-19. Patients with life-threatening COVID-19 were older than those with non–life-threatening COVID-19, but there were no statistically significant differences in biological sex, race, ethnicity, or comorbidities associated with life-threatening COVID-19 in patients with ICCs (Table 4 and Supplementary Table 5). Furthermore, no specific type of ICC was more frequently associated with life-threatening COVID-19. Bacterial coinfections were more common among patients with ICCs who developed life-threatening COVID-19, as was involvement of the pulmonary, cardiac, and neurologic systems. Details of the bacterial and fungal coinfections of those patients with ICCs and life-threatening COVID-19 are described in Supplementary Table 6. Briefly, 5 patients had bacterial coinfections. Of the patients with ICCs, those who developed life-threatening COVID-19 had a higher initial CRP level but comparable white blood cell counts, neutrophil-to-lymphocyte ratio, and ferritin at the time of presentation (Supplementary Table 5).
Table 4.
Comparison of the Characteristics of Patients With Immunocompromising Conditions With Life-Threatening (≥7 Days of Invasive Mechanical Ventilation, Receipt of Extracorporeal Membrane Oxygenation Support, or Death) Versus Non–Life-Threatening COVID-19
| Patients With Immunocompromising Conditions and Acute COVID-19 | Life-Threatening Illness (n = 17) | Non–Life-Threatening Illness (n = 88) | P |
|---|---|---|---|
| Age, median (IQR), y | 16.9 (15.8, 18.8) | 14.8 (6.8, 17.5) | .03 |
| Male sex, n (%) | 10 (58.8%) | 50 (56.8%) | 1.00 |
| Race and ethnicity, n (%) | |||
| White, non-Hispanic | 4 (23.5%) | 31 (35.2%) | .21 |
| Black, non-Hispanic | 5 (29.4%) | 16 (18.2%) | |
| Hispanic or Latino | 4 (23.5%) | 31 (35.2%) | |
| Asian | 2 (11.8%) | 5 (5.7%) | |
| Mixed/other race, non-Hispanic | 0 (0%) | 3 (3.4%) | |
| Unknown | 2 (11.8%) | 2 (2.3%) | |
| Type of immunocompromising disorder, n (%) | |||
| Hematologic malignancy | 5 (29.4%) | 28 (31.8%) | 1.00 |
| Solid tumor | 4 (23.5%) | 12 (13.6%) | .29 |
| Primary immunodeficiency and other disorders of hematopoietic cells | 4 (23.5%) | 20 (22.7%) | 1.00 |
| Autoimmune disorders | 3 (17.6%) | 10 (11.4%) | .44 |
| Nonmalignant organ failure with history of solid-organ transplant | 1 (5.9%) | 18 (20.5%) | .30 |
| Immunomodulatory therapies, n (%) | |||
| On chemo/immunosuppressive medication at the time of infection | 11 (64.7%) | 53 (60.2%) | .79 |
| History of HSCT <100 d | 2 (11.8%) | 1 (1.1%) | .07 |
| History of HSCT ≥100 d | 3 (17.6%) | 9 (10.2%) | .41 |
| Clinical characteristics | |||
| Any viral coinfection | 2 (17.6%) | 8 (9.1%) | .66 |
| Any bacterial coinfectiona | 5 (29.4%) | 5 (5.7%) | .009 |
| Any respiratory support | 17 (100.0%) | 58 (65.9%) | .003 |
| Any cardiovascular involvement | 13 (76.5%) | 22 (25.0%) | <.001 |
| Receipt of vasoactive infusions | 13 (76.5%) | 11 (12.5%) | <.001 |
| Any neurologic involvement | 6 (35.3%) | 9 (10.2%) | .02 |
| Any hematologic involvement | 15 (88.2%) | 59 (67.0%) | .09 |
| Any gastrointestinal involvement | 11 (64.7%) | 54 (61.4%) | 1.00 |
Abbreviations: COVID-19, coronavirus disease 2019; HSCT, hematopoietic stem cell transplantation; IQR, interquartile range.
Patients with a positive bacterial culture from any site within 72 hours of admission that was then adjudicated by study staff.
Twelve patients with ICCs died of COVID-19: 5 of the 14 patients (35.7%) post–hematopoietic stem cell transplantation (HSCT) and 7 of the 91 (7.7%) patients with ICCs who did not undergo HSCT (P = .009). In the HSCT patients, the deaths occurred in 2 patients who underwent transplantation less than 100 days prior to admission and 3 patients at 100 days or more. Three of these patients had coinfections. One patient who underwent transplantation less than 100 days prior to onset of COVID-19 had adenovirus, Epstein-Barr virus, BK virus (human polyomavirus), and cytomegalovirus detected via blood PCR. Two patients, both of whom developed COVID-19 more than 100 days after their HSCT, had Escherichia coli bacteremia. Those with a history of HSCT had a higher rate of receipt of vasoactive infusions (HSCT: 6/14, 42.9%; other ICCs: 18/91, 19.8%; and no ICCs: 231/1169, 19.8%).
DISCUSSION
This large, multicenter case series demonstrates that immunocompromised patients younger than 21 years old admitted for intensive care for acute COVID-19 had worse clinical outcomes despite presenting with similar disease severity to non-immunocompromised children. Those with ICCs had a higher mortality, especially for patients post-HSCT. Within the ICC group, those with life-threatening COVID-19 had more frequent bacterial coinfections and involvement of the pulmonary, cardiac, and/or neurologic systems compared with patients with severe but not life-threatening illness. Reassuringly, most patients with ICCs who were hospitalized in the PICU for severe acute COVID-19 survived and were discharged home without documented severe morbidities.
Patients with a history of HSCT were overrepresented in those with fatal disease in our cohort of children admitted to the PICU for acute COVID-19. Patients with a history of HSCT represented only 1% of the overall cohort with acute COVID-19 but comprised almost half of the fatalities. These findings are consistent with the high mortality of children with post-HSCT admitted to the PICU for other indications [29, 30]. Although our numbers are small, those patients who developed COVID-19 before or after post-transplantation day 100 had comparable rates of life-threatening COVID-19, similar to what has been reported previously [31]. Other studies have shown increased risk for severe COVID-19 in patients who received HSCT less than 1 year prior to infection [32, 33]. During this time period, there is ongoing engraftment of myeloid cells and T cells, which may be delayed if patients develop graft-versus-host disease (GVHD), and thus increased susceptibility to severe COVID-19. The timing of chemotherapy also influences serologic immunity to SARS-CoV-2, which is lower in patients receiving glucocorticoids in the last 2 weeks or chemotherapy within 3 months prior to infection [34]. Our work complements other studies, which investigated inpatient and outpatient outcomes in patients with HSCT. A Center for International Blood and Marrow Transplant Research cohort study of 167 children with a history of HSCT and COVID-19 found that disease severity was mild in 87% of study participants [12], similar to outcomes reported in smaller case series [35, 36]. Thus, while a history of HSCT is not associated with severe COVID-19 in most cases, it may be associated with more severe disease in patients admitted to the PICU for COVID-19. The poor outcomes of those post-HSCT are likely multifactorial and related to conditioning, immunosuppression, and alloreactivity.
Among children with ICCs, patients diagnosed with bacterial coinfection were more likely to have life-threatening COVID-19. Prior studies have not consistently investigated the frequency of concomitant infections in children with inborn errors of immunity [37–42]. While bacterial coinfections in the general adult and pediatric populations [26] have been associated with severe COVID-19, few studies have reported on bacterial coinfections in adults with ICCs. One study of 451 adults with hematologic malignancies found that 187 (41%) had coinfections, but details on the type of infection were not reported [43]. In a cohort of 77 adults who had received HSCT or cellular therapies, such as chimeric antigen receptor T cells, 10 (13%) had a coinfection, the majority of which were bacterial [44]. Finally, a study of 684 adults with cancer identified bacterial coinfection with their COVID-19 diagnosis in 19% of patients [42]. Similar to our study, they found that the presence of a superimposed infection was associated with worse outcomes, including increased intensive care unit admissions, higher rates of acute respiratory distress syndrome, higher rates of mechanical ventilation, and higher mortality. Elevated CRP levels were noted in adults with cancer who presented with acute COVID-19 and a bacterial/fungal coinfection at the time of admission [42]. Additionally, increased mortality was associated with bacterial coinfection, regardless of whether the bacterial infection occurred before or after the SARS-CoV-2 infection [42]. The increased inflammatory markers and worse clinical outcomes have been attributed, at least in part, to cross-talk among innate immune receptors, such as Toll-like receptors capable of recognizing bacterial and viral proteins [45]. These proinflammatory cytokines disrupt the integrity of the pulmonary epithelium, creating an environment permissive for bacterial coinfection [45]. Thus, the underlying increased risk of infections in individuals with ICCs contributes to increased risk of bacterial coinfection and likely more severe COVID-19.
In this national case series of patients with acute COVID-19 admitted to the PICU, no single type of ICC was associated with the development of life-threatening COVID-19, although receipt of HSCT was associated with increased mortality. Although some studies have found increased risk of severe disease in patients with disorders of T-cell function, multi-systemic autoimmune disorders, and innate immunity [46, 47], others have shown no increased risk of severe COVID-19 attributable to any single type of immunodeficiency [41, 46, 48, 49]. These variable outcomes likely reflect the small numbers of patients with these rare diseases and variability in disease phenotypes and severity. Among patients with ICCs, a history of chemotherapy or other immunosuppressant medications was not predictive of worse outcomes in our investigation, consistent with previously published pediatric cohorts [50, 51]. Patients with a history of solid-organ transplantation in our study had no increased severity of disease, supporting previous studies that showed no difference in outcomes despite ongoing immunosuppression [52].
This study has limitations. The public health registry was restricted to patients admitted to the PICU or high-acuity unit. This limitation is especially relevant when considering the burden of SARS-CoV-2 infections in the immunocompromised population, as we excluded cases of mild to moderate disease. We were also limited by the lack of details regarding the degree of immunosuppression, including engraftment status and GVHD prophylaxis or treatment for children who were post-HSCT. While standard measures of acuity were not different between those with ICCs and those without, these standard acuity measures may not be as accurate in those with ICCs. We do not have specific information regarding the decision to evaluate patients for bacterial coinfection and the bacterial co-infection workup was not standardized. We also lack information on empiric antibiotic use. The number of those with life-threatening COVID-19 was small and groups were unmatched, limiting the ability to adjust for possible confounders. There may also be differences in those with ICCs and life-threatening COVID-19 compared with those without life-threatening COVID-19 that the study was not powered to detect. We also did not have detailed information on cause of death. Differences in new morbidities at discharge may be underappreciated, including for new renal dysfunction, which we did not collect. Finally, enrollment for this study ended in 2021. Since then, the Omicron variant emerged as the predominant variant, followed by surges of infections and updated COVID-19 vaccines. The continued emergence of circulating subvariants and changes in population immunity will continue to influence the infection risk to individuals with ICCs. Thus, it is important to acknowledge that future studies will be needed to assess for changes in risk factors associated with life-threatening COVID-19 in patients with ICCs.
In this large case series of children who received intensive care for COVID-19, children with ICCs had similar clinical presentations of COVID-19 to those without ICCs but overall worse outcomes. Bacterial coinfection occurred more commonly in patients with underlying ICCs who developed life-threatening or fatal complications. Future studies are needed to determine if earlier identification and treatment of bacterial coinfection in patients with ICCs and COVID-19 improves outcomes. Reassuringly, most patients with ICCs who were hospitalized in the PICU for severe acute COVID-19 survived and were discharged home without new severe morbidities.
Supplementary Material
Acknowledgments.
The authors appreciate and thank the many research coordinators at the Overcoming COVID-19 hospitals who assisted in data collection for this investigation. They also thank the leadership of the Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network for their ongoing support.
Financial support.
This work was supported through contracts (numbers 75D30120C07725 and 75D30121C10297) from the US Centers for Disease Control and Prevention paid to Boston Children’s Hospital. C. M. R. is supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL150244).
Potential conflicts of interest.
B. M. C. reports grants from NHLBI, American Lung Association, Doris Duke Foundation/Walder Foundation, and American Thoracic Society; payment for expert testimony from Tripplett Woolf Garretson; and participation on a multidisciplinary team for Sobi. N. B. H. reports grants from Sanofi, Quidel, and Merck. C. V. H. reports royalties, consulting fees, for Reviewer for Up To Date and Dynamed clinical databases; payment for presentations from Biofire; and expert consultation for the AstraZeneca FluMist Board. A. G. R. reports licenses as a Section Editor for Pediatric Critical Care Medicine, UpToDate, Inc; consulting fees from ThermoFisher, Inotrem; honoraria from a Grand Rounds presentation at St. Jude; support for meetings and/or travel from International Sepsis Forum, Institut Merieux, ThermoFisher; participation on an advisory board for the NIH Grace Study, REMAP-CAP, NIH-PREVENT-VILI; a medical advisory board member for Families Fighting Flu; chair member for International Sepsis Forum; and received reagents from Illumina, Inc. M. K. reports support for meetings and/or travel from the National Institute of Health (NIH) and a role on an advisory board for KultureCity. H. D. reports honoraria from Delex Pharma International, Inc. N. Z. C. reports grants from Cincinnati Children’s Hospital Medical Center. J. C. F. reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK119463 and P50DK114786), and Pennsylvania CURE Grant. M. W. H. reports licenses from Kiadis, sub-board service for the American Board of Pediatrics, participation on a Data and Safety Monitoring Board (DSMB) for Abbvie, and receipt of drugs from Partner Therapeutics and Sobi. J. C. reports receipt of equipment from Illumina. B. L. reports receipt of an Immune Deficiency Foundation Research Grant. G. E. M. reports payment for expert testimony from Orlando Health, Bush Ross, Hall, Schiefflin & Smith, PA, Poole Brooks and Plumlee, PA, Hilltop Specialty Insurance, Smith, Hulsey, and Busey, PA. J. E. S. reports grants from the Food and Drug Administration (FDA), consulting fees from the Association for Professionals in Infection Control and Epidemiology (APIC), payment for presentations from the American Academy of Pediatrics, and participation on an advisory board for the American Association of Medical Colleges. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Overcoming COVID-19 Study Group Investigators (listed in PubMed, and ordered by US state).
The following study group members were all closely involved with the design, implementation, and oversight of the Overcoming COVID-19 study. Alabama: Children’s of Alabama, Birmingham— Michele Kong, MD; Arizona: University of Arizona, Tucson—Mary Glas Gaspers, MD; Katri V. Typpo, MD; Arkansas: Arkansas Children’s Hospital, Little Rock—Ronald C. Sanders Jr, MD, MS; Katherine Irby, MD; California: Children’s Hospital of Orange County, Orange—Adam J. Schwarz, MD; Miller Children’s & Women’s Hospital Long Beach, Long Beach—Christopher J. Babbitt, MD; Children’s Hospital Los Angeles, Los Angeles—Pia S. Pannaraj, MD, MPH; Rady Children’s Hospital, San Diego—Helen Harvey, MD, MS; UCSF Benioff Children’s Hospital Oakland, Oakland—Natalie Z. Cvijanovich, MD; UCSF Benioff Children’s Hospital, San Francisco—Matt S. Zinter, MD; Colorado: Children’s Hospital Colorado, Aurora—Aline B. Maddux, MD, MSCS; Emily Port, BA, PMP; Sara Shankman, DNP, CPNC-AC; Rachel Mansour, BSN, RN, CPN; Connecticut: Connecticut Children’s, Hartford—Christopher L. Carroll, MD, MS; Yale–New Haven Children’s Hospital, New Haven—John S. Giuliano, Jr, MD; Florida: Holtz Children’s Hospital, Miami—Gwenn E. McLaughlin, MD, MSPH; Nicklaus Children’s Hospital, Miami—Paula S. Espinal, MD, MPH; Georgia: Children’s Healthcare of Atlanta at Egleston, Atlanta—Keiko M. Tarquinio, MD; Illinois: Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago—Kelly N. Michelson, MD, MPH; Bria M. Coates, MD; Indiana: Riley Hospital for Children, Indianapolis—Courtney M. Rowan, MD, MS; Iowa: University of Iowa Stead Family Children’s Hospital, Iowa City—Kari Wellnitz, MD; Guru Bhoojhawon, MBBS, MD; Kentucky: University of Louisville and Norton Children’s Hospital, Louisville—Janice E. Sullivan, MD; Vicki L. Montgomery, MD; Kevin M. Havlin, MD; Louisiana: Children’s Hospital of New Orleans, New Orleans—Tamara T. Bradford, MD; Maryland: Johns Hopkins Children’s Center, Baltimore—Melania M. Bembea, MD, MPH, PhD; University of Maryland Children’s Hospital, Baltimore—Ana Lia Graciano, MD; Massachusetts: Boston Children’s Hospital, Boston—Adrienne G. Randolph, MD; Margaret M. Newhams, MPH; Sabrina R. Chen, BA; Cameron C. Young, BS; Suden Kucukak, MD; Mary Beth F. Son, MD; Janet S. Chou, MD, Brenna LaBere, MD; Mass General Hospital for Children, Boston—Ryan W. Carroll, MD, MPH; Phoebe H. Yager, MD; Neil D. Fernandes, MBBS; Michigan: University of Michigan CS Mott Children’s Hospital, Ann Arbor—Heidi R. Flori, MD, FAAP; Minnesota: University of Minnesota Masonic Children’s Hospital, Minneapolis—Janet R. Hume, MD, PhD; Mayo Clinic, Rochester—Emily R. Levy, MD; Brandi A. Johnson; Noelle M. Drapeau, BA; Supriya Behl, MSc; Mississippi: Children’s Hospital of Mississippi, Jackson—Charlotte V. Hobbs, MD; Lacy Malloch, BS; Lora Martin, MSN; Sarah McGraw, NP; Missouri: Children’s Mercy Hospital, Kansas City—Jennifer E. Schuster, MD; Washington University in St. Louis—Philip C. Spinella, MD; Amanda R. Kolmar, MD; Nebraska: Children’s Hospital & Medical Center, Omaha—Melissa L. Cullimore, MD, PhD; Russell J. McCulloh, MD; New Jersey: Hackensack University Medical Center, Hackensack—Katharine N. Clouser, MD; Cooperman Barnabas Medical Center, Livingston—Shira J. Gertz, MD; Bristol-Myers Squibb Children’s Hospital, New Brunswick—Lawrence C. Kleinman, MD, MPH, FAAP; Simon Li, MD, MPH; Steven M. Horwitz, MD; New York: Golisano Children’s Hospital, Rochester—Joseph Kuebler, MD; Maria Fareri Children’s Hospital, Valhalla—Aalok R. Singh, MD; Hassenfeld Children’s Hospital at NYU Langone, New York—Adam J. Ratner, MD, MPH; Heda Dapul, MD; Vijaya L. Soma, MD; Stony Brook University Hospital, Stony Brook—Katherine V. Biagas, MD; SUNY Downstate Medical Center University Hospital, Brooklyn—Sule Doymaz, MD; North Carolina: University of North Carolina at Chapel Hill, Chapel Hill—Stephanie P. Schwartz, MD; Tracie C. Walker, MD; Ohio: University Hospitals Rainbow Babies and Children’s Hospital, Cleveland—Steven L. Shein, MD, FCCM; Amanda N. Lansell, MD; Nationwide Children’s Hospital, Columbus—Mark W. Hall MD, FCCM; Akron Children’s Hospital, Akron—Ryan A. Nofziger, MD, MBA; Cincinnati Children’s Hospital, Cincinnati—Mary Allen Staat, MD, MPH; Pennsylvania: Children’s Hospital of Philadelphia, Philadelphia—Julie C. Fitzgerald, MD, PhD, MSCE; Ryan H. Burnett, BS; Jenny L. Bush, RNC, BSN; Penn State Children’s Hospital, Hershey—Neal J. Thomas, MD, MSc; UPMC Children’s Hospital of Pittsburgh—Ericka L. Fink, MD, MS; Joseph A. Carcillo, MD; St. Christopher’s Hospital for Children, Philadelphia—Andrew Butler, MD; South Carolina: MUSC Children’s Health, Charleston—Elizabeth H. Mack, MD, MS; Nelson Reed, MD; Tennessee: Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville—Natasha B. Halasa, MD, MPH; Laura Stewart, PhD; Meena Golchha, MD; Texas: Texas Children’s Hospital, Houston—Laura L. Loftis, MD; Marian Samperio MD; University of Texas Health Science Center, Houston—Alvaro Coronado Munoz, MD; Jacob Qurashi, RN; University of Texas Southwestern, Children’s Medical Center Dallas, Dallas—Cindy Bowens, MD, MSCS; Mia Maamari, MD; Utah: Primary Children’s Hospital, Salt Lake City—Hillary Crandall, MD, PhD; Washington: Seattle Children’s Hospital, Seattle—Lincoln S. Smith, MD; John K. McGuire, MD.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- 1.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis 2021; 103:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minotti C, Tirelli F, Barbieri E, Giaquinto C, Dona D. How is immunosuppressive status affecting children and adults in SARS-CoV-2 infection? A systematic review. J Infect 2020; 81:e61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazzaniga M, Testa S, Brambilla M, Vergori A, Viganoni M, Montini G. Incidence and outcome of SARS-CoV-2 infection in a pediatric kidney transplant recipient cohort from a single center in Northern Italy. Pediatr Transplant 2022; 26:e14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chappell H, Patel R, Driessens C, et al. Immunocompromised children and young people are at no increased risk of severe COVID-19. J Infect 2022; 84:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J 2021; 40:e137–45. [DOI] [PubMed] [Google Scholar]
- 6.Kuczborska K, Ksiazyk J. Prevalence and course of SARS-CoV-2 infection among immunocompromised children hospitalised in the tertiary referral hospital in Poland. J Clin Med 2021; 10:4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman AG, Danziger-Isakov LA. The impact of COVID-19 on the pediatric solid organ transplant population. Semin Pediatr Surg 2022; 31:151178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JI, Dubois MM, Savage TJ, et al. Comorbidities associated with hospitalization and progression among adolescents with symptomatic coronavirus disease 2019. J Pediatr 2022; 245:102–10, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JH, Choi SH, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci 2022; 37:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005; 171:995–1001. [DOI] [PubMed] [Google Scholar]
- 11.Hall VG, Solera JT, Al-Alahmadi G, et al. Severity of COVID-19 among solid organ transplant recipients in Canada, 2020–2021: a prospective, multicentre cohort study. CMAJ 2022; 194:E1155–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt NS, Sharma A, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in pediatric and early adolescent and young adult hematopoietic stem cell transplant recipients: a cohort study. Transplant Cell Ther 2022; 28:696 e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. A guide to immunotherapy for COVID-19. Nat Med 2022; 28:39–50. [DOI] [PubMed] [Google Scholar]
- 14.Janssen M, Endeman H, Bos LDJ. Targeted immunomodulation: a primer for intensivists. Intensive Care Med 2023; 49:462–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil AC, Syed A, Rupp ME, et al. Is bacteremic sepsis associated with higher mortality in transplant recipients than in nontransplant patients? A matched case-control propensity-adjusted study. Clin Infect Dis 2015; 60:216–22. [DOI] [PubMed] [Google Scholar]
- 16.Donnelly JP, Locke JE, MacLennan PA, et al. Inpatient mortality among solid organ transplant recipients hospitalized for sepsis and severe sepsis. Clin Infect Dis 2016; 63:186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackerman KS, Hoffman KL, Diaz I, et al. Effect of sepsis on death as modified by solid organ transplantation. Open Forum Infect Dis 2023; 10:ofad148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Delden C, Stampf S, Hirsch HH, et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss transplant cohort study. Clin Infect Dis 2020; 71:e159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol 2011; 151:7–10e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son MBF, Murray N, Friedman K, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med 2021; 385:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Defining child BMI categories. Available at: https://www.cdc.gov/obesity/basics/childhood-defining.html. Accessed 24 October 2023.
- 24.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the centers for disease control and prevention’s social vulnerability index. J Environ Health 2018; 80:34–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Maddux AB, Berbert L, Young CC, et al. Health impairments in children and adolescents after hospitalization for acute COVID-19 or MIS-C. Pediatrics 2022; 150:e2022057798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffitt KL, Nakamura MM, Young CC, et al. Community-onset bacterial coinfection in children critically ill with severe acute respiratory syndrome coronavirus 2 infection. Open Forum Infectious Diseases 2023; 10:ofad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Society of Hematology. Treatment of COVID-19 in immunocompromised patients with hematologic conditions. Available at: https://www.hematology.org/covid-19/treatment-of-covid-19-in-immunocompromised-patients-with-hematologic-conditions. Accessed 6 April 2023.
- 28.Tangye SG, Al-Herz W, Bousfiha A, et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2022; 42:1473–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowan CM, Gertz SJ, McArthur J, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: a multicenter study. Pediatr Crit Care Med 2016; 17:294–302. [DOI] [PubMed] [Google Scholar]
- 30.Duncan CN, Lehmann LE, Cheifetz IM, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med 2013; 14:261–7. [DOI] [PubMed] [Google Scholar]
- 31.Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia 2021; 35:2885–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agrawal N, Singh R, Sharma SK, et al. Outcomes of COVID-19 in hematopoietic stem cell transplant recipients: multicenter retrospective analysis. Indian J Hematol Blood Transfus 2022; 38:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busca A, Salmanton-Garcia J, Marchesi F, et al. Outcome of COVID-19 in allogeneic stem cell transplant recipients: results from the EPICOVIDEHA registry. Front Immunol 2023; 14:1125030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shanap MA, Sughayer M, Alsmadi O, et al. Factors that predict severity of infection and seroconversion in immunocompromised children and adolescents with COVID-19 infection. Front Immunol 2022; 13:919762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucchini G, Furness C, Lawson S, et al. COVID-19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience. Br J Haematol 2021; 194:e74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer 2020; 67:e28514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milota T, Sobotkova M, Smetanova J, et al. Risk factors for severe COVID-19 and hospital admission in patients with inborn errors of immunity—results from a multicenter nationwide study. Front Immunol 2022; 13:835770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koltan S, Zietkiewicz M, Grzesk E, et al. COVID-19 in unvaccinated patients with inborn errors of immunity-Polish experience. Front Immunol 2022; 13:953700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castano-Jaramillo LM, Yamazaki-Nakashimada MA, O’Farrill-Romanillos PM, et al. COVID-19 in the context of inborn errors of immunity: a case series of 31 patients from Mexico. J Clin Immunol 2021; 41:1463–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyts I, Bucciol G, Quinti I, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol 2021; 147:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goudouris ES, Pinto-Mariz F, Mendonca LO, et al. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: a cross-sectional study. J Clin Immunol 2021; 41:1479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gudiol C, Dura-Miralles X, Aguilar-Company J, et al. Co-infections and superinfections complicating COVID-19 in cancer patients: a multicentre, international study. J Infect 2021; 83:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020; 7: e737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah GL, DeWolf S, Lee YJ, et al. Favorable outcomes of COVID-19 in recipients of hematopoietic cell transplantation. J Clin Invest 2020; 130:6656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter P. Co-infection: when whole can be greater than the sum: the complex reaction to co-infection of different pathogens can generate variable symptoms. EMBO Rep 2018; 19:e46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bucciol G, Tangye SG, Meyts I. Coronavirus disease 2019 in patients with inborn errors of immunity: lessons learned. Curr Opin Pediatr 2021; 33:648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puel A, Bastard P, Bustamante J, Casanova JL. Human autoantibodies underlying infectious diseases. J Exp Med 2022; 219:e20211387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shields AM, Anantharachagan A, Arumugakani G, et al. Outcomes following SARS-CoV-2 infection in patients with primary and secondary immunodeficiency in the UK. Clin Exp Immunol 2022; 209:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marcus N, Frizinsky S, Hagin D, et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol 2020; 11:614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parker RS, Le J, Doan A, et al. COVID-19 outcomes in children, adolescents and young adults with cancer. Int J Cancer 2022; 151:1913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicastro E, Verdoni L, Bettini LR, et al. COVID-19 in immunosuppressed children. Front Pediatr 2021; 9:629240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goss MB, Galvan NTN, Ruan W, et al. The pediatric solid organ transplant experience with COVID-19: an initial multi-center, multi-organ case series. Pediatr Transplant 2021; 25:e13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


