Abstract
Background
Evidence about the clinical impact of rapid diagnostic tests (RDTs) for the diagnosis of bloodstream infections is limited, and whether RDT are superior to conventional blood cultures (BCs) embedded within antimicrobial stewardship programs (ASPs) is unknown.
Methods
We performed network meta-analyses using results from studies of patients with bloodstream infection with the aim of comparing the clinical impact of RDT (applied on positive BC broth or whole blood) to conventional BC, both assessed with and without ASP with respect to mortality, length of stay (LOS), and time to optimal therapy.
Results
Eighty-eight papers were selected, including 25 682 patient encounters. There was an appreciable amount of statistical heterogeneity within each meta-analysis. The network meta-analyses showed a significant reduction in mortality associated with the use of RDT + ASP versus BC alone (odds ratio [OR], 0.72; 95% confidence interval [CI], .59–.87) and with the use of RDT + ASP versus BC + ASP (OR, 0.78; 95% CI, .63–.96). No benefit in survival was found associated with the use of RDT alone nor with BC + ASP compared to BC alone. A reduction in LOS was associated with RDT + ASP versus BC alone (OR, 0.91; 95% CI, .84–.98) whereas no difference in LOS was shown between any other groups. A reduced time to optimal therapy was shown when RDT + ASP was compared to BC alone (−29 hours; 95% CI, −35 to −23), BC + ASP (−18 hours; 95% CI, −27 to −10), and to RDT alone (−12 hours; 95% CI, −20 to −3).
Conclusions
The use of RDT + ASP may lead to a survival benefit even when introduced in settings already adopting effective ASP in association with conventional BC.
Keywords: bloodstream infection, blood culture, rapid diagnostic tests, antimicrobial stewardship, network meta-analysis
This network meta-analysis of 88 studies found that using rapid diagnostic tests in conjunction with antimicrobial stewardship programs may reduce mortality for patients with bloodstream infections compared to conventional blood culture systems, even in settings already using antimicrobial stewardship programs.
Management of bloodstream infection (BSI) requires prompt initiation of effective treatment to improve patient outcomes [1–3]. The gold standard for the diagnosis of BSI is the blood culture (BC), which suffers from several limitations, including long turnaround times and limited sensitivity [4]. The implementation of matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS) in routine microbiology in the past 2 decades has offered significant advance with respect to shortening time to results. Nonetheless, MALDI-TOF MS is still mainly a culture-dependent system, most frequently applied on isolates from cultures or early subcultures from positive BC bottles [5] and thus still has substantial limitations in terms of effective turnaround time, which should be taken into account when evaluating its clinical utility [6].
Several further novel diagnostic tests have emerged in the last years with the aim of shortening time to results and improving sensitivity of conventional BC methods [7]. These are mainly molecular tests, often based on the detection of microbial DNA from BC broth or directly from whole blood and are commonly referred to as rapid diagnostic tests (RDT) [8].
The diagnostic accuracy of RDT is mostly adequate to meet criteria for clinical implementation according to the main international regulatory agencies [8, 9]. However, data about the impact of RDT on clinical outcomes are scarce, and the best way to implement RDT in clinical practice is yet to be defined.
A meta-analysis published in 2017 showed a decreased mortality and length of stay (LOS) associated with the use of RDT when these were used in association with antimicrobial stewardship program (ASP), but not in their absence [10]. This meta-analysis represented an extremely valuable contribution to the literature and encouraged further research in this field with several new studies published since then. However, it left the unanswered question about whether the implementation of RDT in association to ASP is superior to the use of ASP alone for the implementation of conventional BC results, and if introducing RDT in a setting where effective ASPs are already in place in association with conventional systems may be beneficial. Furthermore, this meta-analysis classified MALDI-TOF MS as an RDT. Nonetheless, MALDI-TOF MS on isolated colonies growing on solid media is now a standard practice in high-income and many middle-income countries. In these same settings, RDTs may not yet be standard practice and their introduction requires further demonstration of benefits in patient outcome.
Network meta-analysis (NMA) allows to compare multiple interventions in a single analysis by combining both direct and indirect evidence across a network of studies. It yields more precise estimates than a direct comparison and estimates the ranking of interventions [11]. We performed NMA to compare the clinical impact of RDT and conventional BC, both assessed with and without ASP (4 interventions), in patients with BSI. The impact of the different strategies has been assessed with respect to (1) time to optimal treatment (TOT), (2) LOS, and (3) mortality. Our focus is on culture-independent tests; therefore, MALDI-TOF MS has been considered as an RDT when directly applied on BC broth/pellet and as a conventional test when applied on isolates growing on solid media.
METHODS
Our systematic review and meta-analyses are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Network Meta-Analyses [12] (Supplementary Table 1).
Search Strategy
The literature search, built by an experienced librarian, was performed in Medline, Embase, Web of Science, and the Cochrane Library in June 2022, and was updated in November 2023. The search terms included “bloodstream infection,” “rapid diagnostic,” “molecular diagnostic techniques,” “blood culture,” “length of stay,” “mortality,” “time to treatment,” and the brand names of several RDT. No restriction in publication date or language was introduced. Supplementary Table 2 reports the detailed search.
Inclusion Criteria
We included studies comparing any of the 4 following different strategies for the diagnosis of BSI with respect to TOT, LOS, and mortality: (1) BC (in the absence of ASP); (2) BC + ASP; (3) RDT (in the absence of ASP); and (4) RDT + ASP. RDT are defined as culture-independent tests applied on positive BC broth or whole blood for the identification of single or multiple pathogens (±antimicrobial resistance markers). MALDI-TOF MS was also eligible as RDT when applied directly on positive BC (broth/pellet). Conventional methods for the identification of bloodstream pathogens include biochemical BC-based techniques for phenotypic profiling (manual and automated) and the use of MALDI-TOF MS on isolates growing on solid media. Conventional methods for antimicrobial resistance included phenotypic testing, manual (eg, disk diffusion, Etest) or automated (eg, automated broth microdilution), as well as immunochromatographic lateral flow tests (eg, PBP2a detection in Staphylococcus aureus). ASP was defined as any antimicrobial stewardship intervention aimed at actively implementing the RDT or BC results, by means of recommendations from the ASP teams. Our definition of ASP specific to active implementation of RDT or BC results did not include broad stewardship interventions (ie, weekly audit and feedback or preauthorization strategies).
To reduce the risk of confounding by indication associated with observational studies, only randomized controlled trials (RCTs) and quasi-experimental studies were included [13]. Case-control studies were excluded. For studies comparing BC versus BC + ASP or RDT versus RDT + ASP, it was required that the BC methods or RDT used in the 2 groups be the same. For studies comparing BC + ASP versus RDT + ASP, it was required that the ASP methods in the 2 groups be the same.
Studies were excluded if rapid tests were culture dependent (ie, applied on colonies on solid media), if they reported theoretical outcomes, or were performed on non-human subjects. Studies including contaminated BC only were also excluded.
Screening and Selection
Papers identified during the main database search strategy were exported into Endnote [14] and subsequently in Rayyan [15]. Duplicates were deleted by both software. The screening of the papers was performed in Rayyan by 2 independent reviewers (A. M. P., W. L.) who resolved discrepancies upon discussion.
Data Extraction and Outcome Definitions
Once confirmed for inclusion, data were extracted from studies according to the 4 groups forming the network (RDT; RDT + ASP; BC; BC + ASP) (Figure 1). Data extracted included year of publication, the country where the study was performed, study design, patient population, type of RDT, and conventional tests used and outcomes.
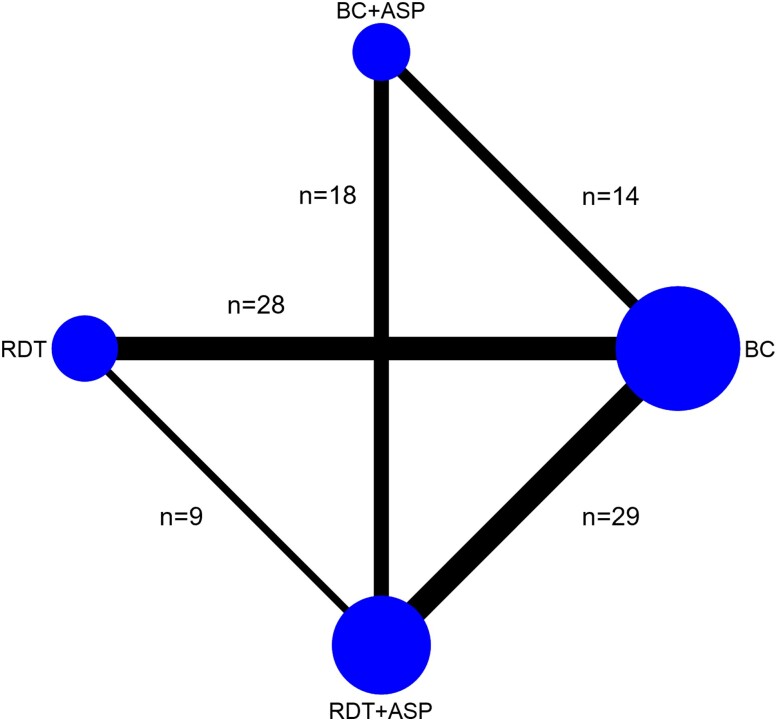
Figure 1.
Network plot reporting the number of studies assessing each of the comparisons included in the NMA. It was expected that a paucity of studies would be found comparing BC + ASP to RDT alone as in the common context of a pre/postinterventional study, the implementation of RDT in a setting already using ASP with conventional BC would unlikely involve ceasing the use of ASP. Abbreviations: ASP, antimicrobial stewardship program; BC, blood culture; NMA, network meta-analysis; RDT, rapid diagnostic test.
TOT was defined as the time from either blood specimen collection or from positive Gram stain/BC positivity (in the case of studies evaluating RDT applied on positive BC broth) to initiation of the antimicrobial treatment targeted on the final isolate. LOS was defined as total hospital LOS or post-BSI LOS. For TOT, when means and standard deviation (SDs) were not available, but the quartiles were available, we estimated the mean by (q1 + median + q3)/3, and the SD by (q3-q1)/1.35. For LOS, medians were used as an estimate of the geometric mean, and geometric SDs [16] were estimated by GSD = exp[(ln(q3) – ln(q1))/1.35]. Otherwise, calculations assumed a lognormal distribution to convert arithmetic means and SDs to geometric means and GSDs. If different summary statistics were reported (eg, median 95% confidence interval [CI]) authors were contacted for further information. If available, infection related-mortality was extracted; otherwise, 30-day and in-hospital mortality were chosen next, respectively. If available, post-BSI LOS was extracted; otherwise, total LOS was used.
Quality Assessment
The latest Cochrane risk-of-bias tool was used to assess the risk of bias in RCTs [17]. The Risk of Bias in non-Randomized Studies of Interventions [18] was used for quasi-experimental studies.
Data Analysis
As well as performing NMA for each outcome, we also performed conventional random-effects meta-analyses to share the summary data we used and visualize the added value of the NMA. For TOT we report mean differences with 95% CIs; for LOS, ratios of geometric means with 95% CI; for mortality, odds ratios (ORs) with 95% CI.
The statistical heterogeneity parameter τ within each conventional meta-analysis and for the NMA was reported and 95% prediction intervals were calculated. A global test of consistency was performed for each NMA [19]. Publication bias was assessed using comparison-adjusted funnel plots [20]. Data analysis was performed using Stata 18 [21] and the following user-written packages: network [19], intervalplot, and netfunnel [22].
RESULTS
Yield of Strategy and Study Characteristics
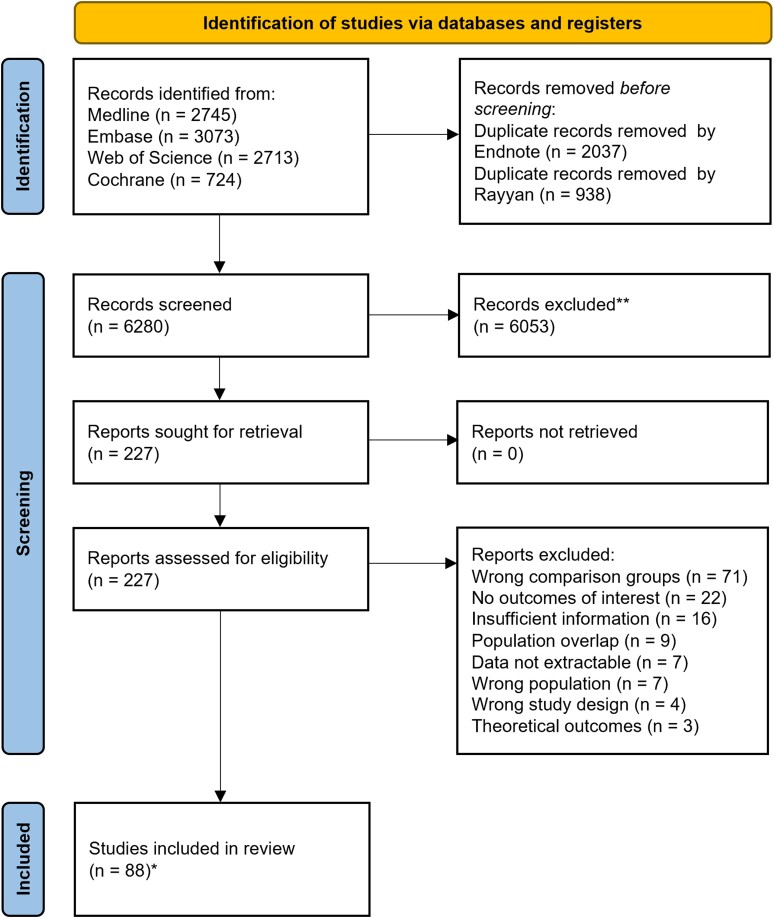
A total of 9255 records were identified at the primary databases search, of which 227 were screened as full-text. The screening process is summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart (Figure 2). In the analyses, 88 studies were included, representing 25 682 patients [23–110]. Forty-two studies (48%) reported information about TOT, 71 (81%) about LOS (of which 20/71, 28% reported post-BSI LOS), and 76 (86%) about mortality. Seventy-eight studies (89%) had a quasi-experimental design; 10 (11%) were RCTs. Five studies (6%) had more than 2 comparison groups. Twelve (14%) focused on children/newborns, 4 (5%) on intensive care unit patients. Thirty-two studies (36%) included Gram-positive BSI (of which 11 focused exclusively on S. aureus) and 15 (17%) Gram-negative BSI, 7 (8%) focused on candidemia and 34 (39%) included mixed infections. Among studies focusing on RDT (n = 75), 56 (75%) included RDT applied on positive BC broth, 12 (16%) MALDI-TOF MS applied on positive BC broth/pellet, 3 (4%) a combination of the former methods and 4 (5%) RDT applied on whole blood. Among studies including conventional methods (n = 83), 23 (28%) included MALDI-TOF MS applied on isolates grown on solid media. Characteristics of commercial RDT used by the included studies are summarized in Supplementary Table 3. Table 1 summarizes the study characteristics.
Figure 2.
PRISMA flow diagram for studies selection. *Studies coincided with reports.
Table 1.
Study Characteristics
| Author, y | Country | Study Design | Patients | BSI Type | Group | Conventional Systems | RDT | Outcomes | Sample Size | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC | BC + ASP | RDT | RDT + ASP | TOT | LOS | Mort | |||||||||
| AlQahtani, 2021 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | VITEK-2 | Xpert MRSA/SA BC assay | Yes | Yes | Yes | 25/14 | [23] | ||
| Alvarez, 2012 | Spain | Quasi-exp | ICU | GP/GN/Y | ✓ | ✓ | Conventional culture methods | LightCycler SeptiFast | No | Yes | Yes | 54/48 | [24] | ||
| Antworth, 2013 | U.S. | Quasi-exp | Adults and children | Candida | ✓ | ✓ | VITEK-2 | NA | No | Yes | No | 37/41 | [25] | ||
| Avdic, 2017 | U.S. | Quasi-exp | Adults | GP | ✓ | ✓ | NA | Verigene GP-BC | Yes | Yes | Yes | 136/137 | [26] | ||
| Bandy, 2023 | U.S. | Quasi-exp | Adults | VRE | ✓ | ✓ | VITEK-2 | Verigene GP-BC | Yes | Yes | Yes | 50/54 | [27] | ||
| Banerjee, 2015 | U.S. | RCT | Adults and children | GP/GN/Y | ✓ | ✓ | ✓ | MALDI-TOF MS, PBP2 immuno-chromatographic test for MRSA | BCID | No | Yes | Yes | 207/198/212 | [28] | |
| Banerjee, 2021 | U.S. | RCT | … | GN | ✓ | ✓ | MALDI-TOF MS, BMD, agar dilution | Accelerate Pheno Test | No | Yes | Yes | 22/222 | [29] | ||
| Bauer, 2010 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | MicroScan WalkAway System, cefoxitin disk testing | Xpert MRSA/SA BC assay | No | Yes | Yes | 74/82 | [30] | ||
| Beal, 2015 | U.S. | Quasi-exp | … | GP | ✓ | ✓ | Conventional culture methods, VITEK-2 | Verigene GP-BC | Yes | Yes | Yes | 80/67 | [31] | ||
| Beganovic, 2017 | U.S. | Quasi-exp | Adults and children | GP/GN | ✓ | ✓ | MALDI-TOF MS | NA | Yes | Yes | Yes | 126/126 | [32] | ||
| Benoist, 2018 | France | Quasi-exp | Adults and children | Candida | ✓ | ✓ | MALDI-TOF MS, E-test | NA | No | No | Yes | 33/37 | [33] | ||
| Ben-Zvi, 2019 | Israel | Quasi-exp | Adults | S. aureus | ✓ | ✓ | Conventional culture methods, chromogenic test, disk-diffusion, E-test | Xpert MRSA/SA BC assay | No | Yes | Yes | 125/129 | [34] | ||
| Beuving, 2015 | The NLD | RCT | Adults | GP/GN | ✓ | ✓ | BD Phoenix System | Multiplex PCR + semi-molecular AST | Yes | Yes | Yes | 109/114 | [35] | ||
| Bhat, 2016 | India | RCT | NICU | GP/GN | ✓ | ✓ | VITEK-2 | Multiplex PCR | No | Yes | Yes | 183/185 | [36] | ||
| Bhavsar, 2018 | U.S. | Quasi-exp | Children | GP/GN | ✓ | ✓ | VITEK-2, API Identification System | MALDI-TOF MS | No | Yes | Yes | 210/137 | [37] | ||
| Bouza, 2004 | Spain | RCT | … | GP/GN/Y | ✓ | ✓ | Conventional culture methods, BMD | NA | No | No | Yes | 208/89 | [38] | ||
| Bowman, 2021 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | Conventional culture methods | Verigene GN-BC | Yes | Yes | No | 77/80 | [39] | ||
| Box, 2015 | U.S. | Quasi-exp | Adults | GP | ✓ | ✓ | BD Phoenix System | Verigene GP-BC | No | Yes | Yes | 103/64 | [40] | ||
| Brock, 2019 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | Conventional culture methods | NA | No | Yes | No | 243/259 | [41] | ||
| Brosh-Nissimov, 2023 | Israel | Quasi-exp | … | GN | ✓ | ✓ | MALDI-TOF MS, VITEK-2 | Accelerate Pheno Test | No | Yes | Yes | 46/57 | [42] | ||
| Bukowski, 2018 | U.S. | Quasi-exp | Adults | S. aureus and CONS | ✓ | ✓ | VITEK-2, latex agglutination test, PBP2 immuno-chromatographic test for MRSA | Xpert MRSA/SA BC assay | Yes | Yes | Yes | 143/109 | [43] | ||
| Buss, 2018 | U.S. | Quasi-exp | Oncology | GP/GN/Y | ✓ | ✓ | ✓ | MALDI-TOF MS, BD Phoenix System | BCID | Yes | No | Yes | 52/43 | [44] | |
| Cairns, 2016 | Australia | RCT | Adults | GP/GN | ✓ | ✓ | MALDI-TOF MS | NA | Yes | No | Yes | 81/79 | [45] | ||
| Campos, 2022 | Brazil | Quasi-exp | ICU | GN | ✓ | ✓ | MALDI-TOF MS, VITEK-2, E-test, disk diffusion, BMD | MALDI-TOF MS + Gen Multi Sepsis Flow Chip | No | Yes | Yes | 114/102 | [46] | ||
| Chiasson, 2022 | U.S. | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | Conventional culture methods and MicroScan WalkAway System | BCID | Yes | Yes | Yes | 82/98 | [47] | ||
| Claeys, 2020 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | ✓ | VITEK-2 | Verigene GN-BC | Yes | Yes | Yes | 237/308/287 | [48] | |
| Cosgrove, 2016 | U.S. | RCT | Adults | Enterococci | ✓ | ✓ | MALDI-TOF MS, BD Phoenix System | E. faecalis/OE PNA-FISH | Yes | Yes | Yes | 79/77 | [49] | ||
| Dare, 2021 | U.S. | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, VITEK-2, PBP2A latex agglutination test for MRSA | Accelerate PhenoTest | Yes | Yes | Yes | 188/155 | [50] | ||
| Dow, 2022 | Canada | Quasi-exp | … | GP/GN/Y | ✓ | ✓ | Conventional culture methods, VITEK-2 | NA | No | Yes | Yes | 226/195 | [51] | ||
| Dwriega, 2019a | U.S. | Quasi-exp | Children | S. aureus | ✓ | ✓ | MALDI-TOF MS, VITEK-2, E-test | S. aureus/CNS PNA-FISH + Xpert MRSA/SA BC assay | Yes | Yes | No | 50/32 | [52] | ||
| Dwriega, 2019b | U.S. | Quasi-exp | Children | CoNS | ✓ | ✓ | MALDI-TOF MS, VITEK-2, E-test | S. aureus/CNS Quick FISH | No | Yes | No | 152/59 | [53] | ||
| Emonet, 2016 | Switzerland | RCT | Adults | S. aureus & CONS | ✓ | ✓ | MALDI-TOF MS, disk diffusion test | real-time PCR | Yes | Yes | Yes | 41/48 | [54] | ||
| Erickson, 2019 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | NA | BCID | No | Yes | Yes | 51/86 | [55] | ||
| Faugno, 2021 | Australia | Quasi-exp | Children | GP/GN/Y | ✓ | ✓ | Conventional culture methods, disc susceptibility testing, VITEK-2 |
MALDI-TOF MS from positive BC + GeneXpert MRSA/SA | Yes | Yes | Yes | 129/126 | [56] | ||
| Felsenstein, 2016 | U.S. | Quasi-exp | Children | GP | ✓ | ✓ | Conventional culture methods, VITEK-2, E-test + cefoxitin disk diffusion | Verigene GP-BC | Yes | Yes | Yes | 194/189 | [57] | ||
| Forrest 2008 | U.S. | Quasi-exp | Adults | Enterococci | ✓ | ✓ | Conventional culture methods, catalase detection, VITEK-2, disc diffusion | E. faecalis/OE PNA-FISH | No | No | Yes | 132/95 | [58] | ||
| Frye 2012 | U.S. | Quasi-exp | Adults | S. aureus and CoNS | ✓ | ✓ | Conventional culture methods, catalase and latex coagulase test, PBP2 latex agglutination test for MRSA | BD GeneOhm StaphSR PCR assay | No | Yes | Yes | 134/110 | [59] | ||
| Gawrys, 2020 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | VITEK-2 | Verigene GN-BC | Yes | Yes | Yes | 68/73 | [60] | ||
| Goshorn, 2023 | U.S. | Quasi-exp | … | CoNS | ✓ | ✓ | ✓ | MALDI-TOF MS, Microscan WalkAway system | ePlex System | No | Yes | Yes | 65/60/57 | [61] | |
| Gritte, 2021 | U.S. | Quasi-exp | Adults | S. aureus and CONS | ✓ | ✓ | VITEK-2 | GeneXpert MRSA/SA BC | Yes | Yes | Yes | 113/73 | [62] | ||
| Heil, 2012 | U.S. | Quasi-exp | … | Candida | ✓ | ✓ | CHROMagar and API 20C | Candida PNA-FISH | Yes | Yes | Yes | 61/21 | [63] | ||
| Hogan, 2020 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | Microscan WalkAway system | MALDI-TOF MS + VITEK-2 on pos BC | No | Yes | Yes | 336/335 | [64] | ||
| Karpen, 2023 | U.S. | Quasi-exp | Adults, noncritically ill | GP/GN | ✓ | ✓ | NA | Verigene GP-BC and GN-BC | No | Yes | Yes | 100/100 | [65] | ||
| Koh, 2018 | Ireland | Quasi-exp | NICU | S. aureus and CONS | ✓ | ✓ | VITEK-2 | GeneXpert MRSA/SA | No | Yes | No | 42/45 | [66] | ||
| Kremer, 2023 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | MALDI-TOF MS | BCID | Yes | Yes | Yes | 120/120 | [67] | ||
| Lockwood, 2015 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | Conventional culture methods | MALDI-TOF MS and BD-Phoenix system on positive BC | Yes | Yes | Yes | 149/241 | [68] | ||
| Lopez-Pintor, 2021 | Spain | Quasi-exp | … | GN | ✓ | ✓ | MALDI-TOF MS, semiautomatic AST | MALDI-TOF MS and semiautomatic AST on positive BC | No | Yes | Yes | 125/188 | [69] | ||
| MacGowan, 2020 | UK | RCT | Adults | GP/GN/Y | ✓ | ✓ | Conventional biochemical culture methods | MALDI-TOF MS on positive BC | No | Yes | Yes | 2810/2740 | [70] | ||
| MacVane, 2016 | U.S. | Quasi-exp | Adults | GP/GN | ✓ | ✓ | ✓ | Traditional phenotypic methods, MicroScan WalkAway System | BCID + direct coagulase test for S. aureus | No | Yes | Yes | 115/104/145 | [71] | |
| Magarifuchi, 2018 | Japan | Quasi-exp | … | GP/GN | ✓ | ✓ | Conventional culture methods, BMD | MALDI-TOF MS + direct disk diffusion | No | No | Yes | 129/119 | [72] | ||
| Mahrous, 2020 | Saudi Arabia | Quasi-exp | Adults | GP/GN | ✓ | ✓ | VITEK-2 | Verigene GP-BC and GN-BC | No | Yes | Yes | 164/148 | [73] | ||
| Malcolmson, 2017 | Canada | Quasi-exp | Children | GP/GN/Y | ✓ | ✓ | Conventional culture methods, BD Phoenix System, E-test, disc-diffusion | MALDI-TOF MS on positive BC | Yes | Yes | Yes | 100/121 | [74] | ||
| Mancini, 2014 | Italy | Quasi-exp | Hematology | GP/GN/Y | ✓ | ✓ | VITEK-2 | LightCycler SeptiFast | No | No | Yes | 101/101 | [75] | ||
| Mazzillo-Vega, 2020 | Spain | Quasi-exp | Children | GP/GN/Y | ✓ | ✓ | BD Phoenix System | BCID | Yes | No | No | 50/50 | [76] | ||
| McCarthy, 2022 | U.S. | Quasi-exp | … | GP/GN | ✓ | ✓ | NA | Verigene GP-BC and GN-BC | No | Yes | Yes | 67/57 | [77] | ||
| Messacar, 2017 | U.S. | Quasi-exp | Children | GP/GN/Y | ✓ | ✓ | Microscan panel, chromogenic methods, API20. PBP2 latex agglutination for MRSA | BCID | Yes | Yes | Yes | 200/100 | [78] | ||
| Mohayya, 2023 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | MALDI-TOF MS, BD Phoenix System | Acelerate Pheno Test | No | Yes | Yes | 93/131 | [79] | ||
| Moni, 2022 | India | Quasi-exp | Adults | Candida | ✓ | ✓ | VITEK-2 | NA | No | No | Yes | 103/72 | [80] | ||
| Nakagawa, 2018 | U.S. | Quasi-exp | Adults | VRE | ✓ | ✓ | Sensititre | Verigene GP-BC, direct disk diffusion | Yes | Yes | Yes | 44/20 | [81] | ||
| Nasef, 2020 | UAE | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | VITEK-2 | BCID | Yes | Yes | Yes | 86/120 | [82] | ||
| Niwa, 2018 | Japan | Quasi-exp | … | GP/GN/Y | ✓ | ✓ | Automated system for identification and AST (RAISUS system) | MALDI-TOF MS on positive BC | Yes | Yes | Yes | 180/186 | [83] | ||
| Ohashi, 2018 | Japan | Quasi-exp | Adults | MRSA | ✓ | ✓ | Conventional culture methods | NA | No | Yes | Yes | 43/21 | [84] | ||
| Osthoff, 2017 | Switzerland | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | Conventional culture methods, MALDI-TOF MS, VITEK-2, E-test | MALDI-TOF MS on positive BC | No | Yes | Yes | 200/168 | [85] | ||
| Page, 2017 | Ireland | Quasi-exp | Obstetric | S. aureus & CONS | ✓ | ✓ | VITEK-2 | Xpert MRSA/SA BC assay | No | Yes | No | 25/15 | [86] | ||
| Pardo, 2016 | U.S. | Quasi-exp | Adults | GP/GN | ✓ | ✓ | VITEK-2. For yeasts: API ID strips, Sensititre YeastOne | BCID | No | Yes | Yes | 252/84 | [87] | ||
| Patch, 2018 | U.S. | Quasi-exp | Adults | Candida | ✓ | ✓ | Conventional culture methods | T2Candida | Yes | Yes | Yes | 19/20 | [88] | ||
| Perez, 2014 | U.S. | Quasi-exp | Adults | GN | ✓ | ✓ | BD Phoenix system | MALDI-TOF MS on positive BC | Yes | Yes | Yes | 157/112 | [89] | ||
| Perez-Lazo, 2023 | Peru | Quasi-exp | Hematology | GP/GN/Y | ✓ | ✓ | Conventional culture methods, VITEK-2. | BCID2 | No | Yes | Yes | 62/31 | [90] | ||
| Pettit, 2019 | U.S. | Quasi-exp | Adults | Candida | ✓ | ✓ | MALDI-TOF MS | NA | No | Yes | Yes | 42/42 | [91] | ||
| Puckett, 2021 | U.S. | Quasi-exp | Children | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, BD Phoenix system; E-test, disk diffusion | MALDI-TOF MS on positive BC | Yes | No | No | 65/66 | [92] | ||
| Reed, 2014 | U.S. | Quasi-exp | Adults | Candida | ✓ | ✓ | Conventional culture methods | NA | No | Yes | Yes | 85/88 | [93] | ||
| Rivard, 2017 | U.S. | Quasi-exp | … | GN | ✓ | ✓ | MALDI-TOF MS, VITEK-2, disk diffusion, Sensititre, Etest | Verigene GN-BC | No | Yes | Yes | 456/421 | [94] | ||
| Rodrigues, 2019 | Brazil | RCT | Adults | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, VITEK-2, disk diffusion and or MIC detection according to the laboratory protocol. | LightCycler SeptiFast | Yes | Yes | Yes | 100/100 | [95] | ||
| Romero-Gomez, 2017 | Spain | Quasi-exp | Adult and children | S. aureus | ✓ | ✓ | Conventional culture methods, VITEK-2 | MALDI-TOF MS on positive BC + PCR | No | Yes | No | 133/94 | [96] | ||
| Rosa, 2018 | U. S. | Quasi-exp | … | S. aureus | ✓ | ✓ | Latex agglutination test, VITEK-2 | NA | No | No | Yes | 132/117 | [97] | ||
| Roshdy, 2015 | U.S. | Quasi-exp | … | GP | ✓ | ✓ | Conventional culture methods, MALDI-TOF MS, VITEK-2, disk diffusion, Etests | Verigene GP-BC | Yes | No | No | 65/74 | [98] | ||
| Sango, 2013 | U.S. | Quasi-exp | … | Enterococci | ✓ | ✓ | Conventional culture methods, VITEK-2 | Verigene GP-BC | Yes | Yes | Yes | 46/28 | [99] | ||
| Schuman, 2021 | Germany | Quasi-exp | ICU | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, BD Phoenix System, disc diffusion | BCID | Yes | No | No | 149/178 | [100] | ||
| Senda, 2011 | Japan | Quasi-exp | … | MRSA | ✓ | ✓ | Conventional culture methods | MALDI-TOF MS on positive BC | No | No | Yes | 40/25 | [101] | ||
| Senok, 2023 | UAE | Quasi-exp | ICU | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, VITEK-2 | BCID2 | No | No | Yes | 99/86 | [102] | ||
| Smith, 2018 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | NA | NA | Yes | No | Yes | 86/172 | [103] | ||
| Tritle, 2022 | U.S. | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | Microscan Walkaway system with ESBL confirmatory testing | BCID | Yes | Yes | Yes | 94/172 | [104] | ||
| Tseng, 2018 | U.S. | Quasi-exp | Adults | GP/GN/Y | ✓ | ✓ | MALDI-TOF MS, BD Phoenix System | BCID | No | No | Yes | 103/100 | [105] | ||
| Turner, 2017 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | VITEK-2; E-test (for daptomycin) | Xpert MRSA/SA BC assay | Yes | Yes | Yes | 343/130 | [106] | ||
| Walker, 2016 | U.S. | Quasi-exp | Cancer | GN | ✓ | ✓ | VITEK-2, e-test (ESBL), modified Hodge test (carbapenemases) | Verigene GN-BC | Yes | Yes | Yes | 98/97 | [107] | ||
| Welch, 2020 | U.S. | Quasi-exp | Children | S. aureus | ✓ | ✓ | MicroScan WalkAway system | BCID | Yes | Yes | Yes | 32/36 | [108] | ||
| Wenzler, 2017 | U.S. | Quasi-exp | Adults | S. aureus | ✓ | ✓ | NA | Verigene GP-BC | No | Yes | Yes | 45/39 | [109] | ||
| Yamada, 2023 | Japan | Quasi-exp | … | S. aureus and CONS | ✓ | ✓ | MicroScan WalkAway system | Xpert MRSA/SA BC assay | Yes | Yes | Yes | 98/97 | [110] | ||
Abbreviations: ASP, antimicrobial stewardship program; BSI, bloodstream infection; BC, blood culture; BCID, BioFire FilmArray blood culture identification panel; CNS, central nervous system; CoNS, coagulase negative Staphylococcus spp.; GN, Gram-negative; GP, Gram-positive; ICU, intensive care unit; LOS, length of stay; MALDI-TOF MS, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry; Mort, mortality; MRSA/SA, methicillin-resistant S. aureus/S. aureus; NA, not applicable; NICU, neonatal intensive care unit; NLD, Netherlands; OE, other enterococci; PBP2, penicillin-binding protein; PCR, polymerase chain reaction; PNA-FISH, peptide nucleic acid fluorescent in situ hybridization; quasi-exp, quasi-experimental; RCT, randomized controlled trial; RDT, rapid diagnostic test; TOT, time to optimal therapy; UAE, United Arab Emirates; VRE, vancomycin-resistant Enterococcus spp.; Y, yeast.
Network Characteristics
The most common comparisons assessed by the selected studies were between RDT, either alone or with ASP, and conventional BC (RDT vs BC, n = 28; RDT + ASP vs BC, n = 29), as well as between RDT and BC both embedded with ASP (RDT + ASP vs BC + ASP, n = 18). The comparisons BC + ASP versus BC (n = 14) and RDT + ASP versus RDT (n = 9) were less common. No study compared RDT alone to BC with ASP (Figure 1). Most studies included any hospitalized patients with BSI, ensuring balance in the distribution of the main effect modifiers (transitivity) as patient characteristics across the studies were not expected to vary based on the diagnostic methods used.
Quality Assessment
The study’s quality assessment is reported in Supplementary Tables 4a-b and Supplementary Figure 1. Approximately 30% of quasi-experimental studies had serious risk of bias due to confounding with respect to LOS and mortality, 20% with respect to TOT. All quasi-experimental studies had moderate risk of bias in the selection of reported results as a study protocol was never available. Similarly, most RCTs did not report adhering to a predefined protocol. In contrast, most of the other domains were scored as low risk of bias.
Time to Optimal Treatment
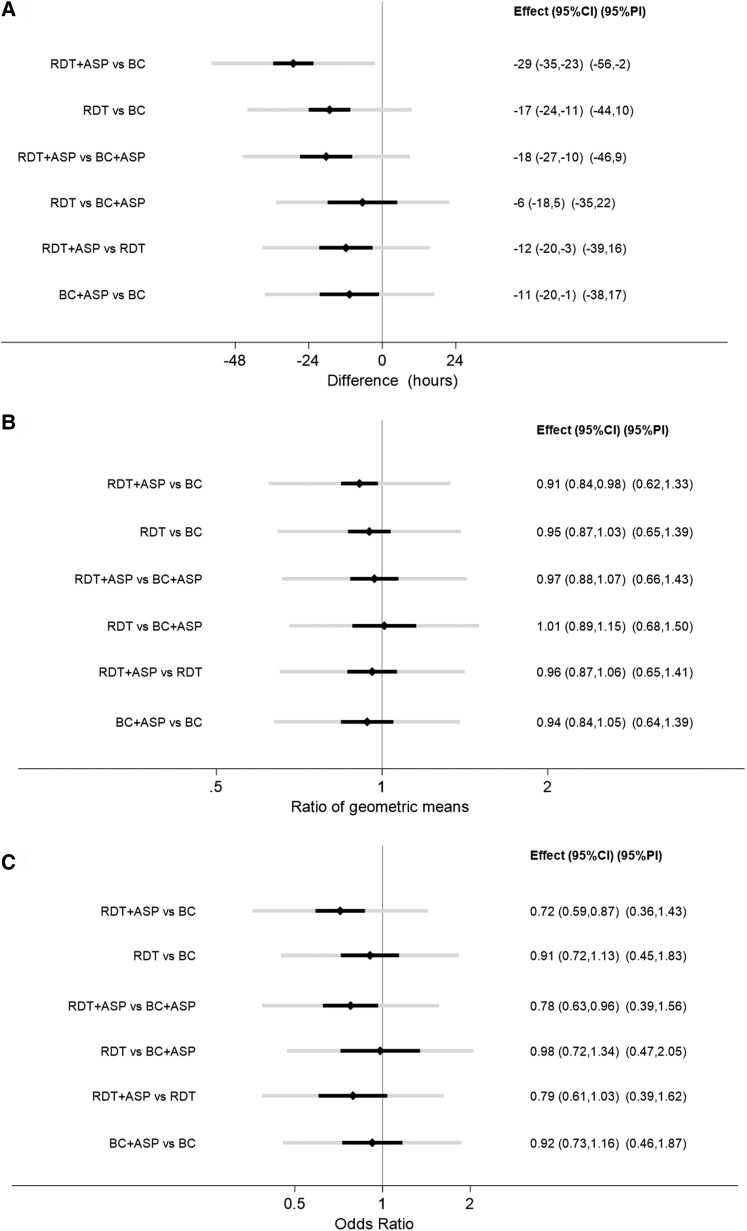
The NMA showed a significant reduction in TOT associated with the use of RDT. The difference was most pronounced when RDT + ASP was compared to BC alone (−29 hours; 95% CI, −35 to −23), whereas it was reduced to −18 hours (95% CI, −27 to −10) when RDT + ASP was compared to BC + ASP, and to −12 hours (95% CI, −20 to −3) when compared to RDT alone. A significant reduction of TOT was also observed when comparing BC + ASP to BC alone (−11 hour; 95% CI, −20 to −1) as well as when comparing RDT to BC in the absence of ASP (−17 hours; 95% CI, −24 to −11). Differently, no significant difference was found in TOT between the use of RDT alone and BC + ASP (−6 hours; 95% CI, −18 to 5). Pooled estimates from conventional and NMA for TOT are shown in Table 2 and Figure 3A and forest plots are shown in Supplementary Figures 2a–2f.
Table 2.
Pooled Estimates From Conventional and Network Meta-analyses for the Outcomes
| Comparison | Direct Comparison, N of Studies | Conventional Meta-analysis | Network Meta-Analysis | |
|---|---|---|---|---|
| Pooled mean differences for TOT | ||||
| RDT + ASP | BC (no ASP) | 16 | −28 (−36 to −21) | −29 (−35 to −23) |
| RDT (no ASP) | BC (no ASP) | 15 | −15 (−22 to −8) | −17 (−24 to −11) |
| RDT + ASP | BC + ASP | 9 | −22 (−30 to −14) | −18 (−27 to −10) |
| RDT (no ASP) | BC + ASP | 0 | … | −6 (−18 to 5) |
| RDT + ASP | RDT (no ASP) | 3 | 1 (−3 to 5) | −12 (−20 to −3) |
| BC + ASP | BC (no ASP) | 3 | −22 (−36 to −8) | −11 (−20 to −1) |
| Pooled ratios of geometric means for LOS | ||||
| RDT + ASP | BC (no ASP) | 26 | 0.92 (0.82–1.03) | 0.91 (0.84–0.98) |
| RDT (no ASP) | BC (no ASP) | 23 | 0.97 (0.92–1.03) | 0.95 (0.87–1.03) |
| RDT + ASP | BC + ASP | 14 | 0.97 (0.89–1.07) | 0.97 (0.88–1.07) |
| RDT (no ASP) | BC + ASP | 0 | … | 1.01 (0.89–1.15) |
| RDT + ASP | RDT (no ASP) | 8 | 1.02 (0.83–1.25) | 0.96 (0.87–1.06) |
| BC + ASP | BC (no ASP) | 8 | 0.92 (0.78–1.08) | 0.94 (0.84–1.05) |
| Pooled odds ratios for mortality | ||||
| RDT + ASP | BC (no ASP) | 28 | 0.71 (0.55–0.92) | 0.72 (0.59–0.87) |
| RDT (no ASP) | BC (no ASP) | 21 | 0.89 (0.69–1.14) | 0.91 (0.72–1.13) |
| RDT + ASP | BC + ASP | 16 | 0.81 (0.64–1.02) | 0.78 (0.63–0.96) |
| RDT (no ASP) | BC + ASP | 0 | … | 0.98 (0.72–1.34) |
| RDT + ASP | RDT (no ASP) | 9 | 0.66 (0.38–1.15) | 0.79 (0.61–1.03) |
| BC + ASP | BC (no ASP) | 12 | 0.97 (0.75–1.26) | 0.92 (0.73–1.16) |
Abbreviations: ASP, antimicrobial stewardship program; BC, blood culture; LOS, length of stay; RDT, rapid diagnostic test; TOT, time to optimal therapy.
Figure 3.
Estimates, 95% confidence intervals and 95% prediction intervals for (A) TOT, (B) LOS, (C) mortality. Abbreviations: ASP, antimicrobial stewardship program; BC, blood culture; CI, confidence interval; LOS, length of stay; PI, prediction interval; RDT, rapid diagnostic test; TOT, time to optimal therapy.
There was appreciable statistical heterogeneity (τ = 13 hours in NMA) with all prediction intervals including 0 except for the RDT + ASP versus BC comparison. There was no suggestion of publication bias (Supplementary Figure 3).
Length of Stay
The NMA showed a reduction in LOS when comparing the use of RDT + ASP to BC alone (0.91; 95% CI, .84–.98), whereas it failed to show any difference in LOS between any of the other groups (Table 2, Figure 3B, Supplementary Figure 4F). Supplementary Figures 4A–F show forest plots from conventional and NMA. Findings were similar for the 27 studies including Gram-positive BSI only (data not shown). No difference in LOS between any of the groups was found when considering studies including Gram-negative BSI only (n = 15) or post-BSI LOS only (n = 20) (data not shown). There was appreciable statistical heterogeneity (τ = 0.19 in NMA) with all prediction intervals including 1. There was no suggestion of publication bias (Supplementary Figure 5).
Mortality
The NMA estimated a decreased mortality when RDT are used with ASP compared to conventional systems alone (OR, 0.72; 95% CI, .59–.87). Interestingly, the NMA also showed a reduced mortality associated with the use of RDT versus conventional systems when both are embedded within ASP (RDT + ASP vs BC + ASP; OR, 0.78; 95% CI, .63–.96), whereas no difference in mortality was observed between RDT and conventional systems in the absence of ASP (RDT vs BC; OR, 0.91; 95% CI, .72–1.13). The NMA also failed to show any differences in mortality when comparing RDT versus BC + ASP (OR, 0.98; 95% CI, .72–1.34), nor when comparing BC + ASP versus BC (OR, 0.92; 95% CI, .73–1.16) or RDT + ASP versus RDT (OR, 0.79; 95% CI, .61–1.03). Pooled estimates from conventional and NMA for mortality are reported in Table 2 and Figure 3C and forest plots in Supplementary Figures 6A–6F.
There was appreciable statistical heterogeneity (τ = 0.33 in NMA) and no suggestion of publication bias (Supplementary Figure 7).
When including RCTs only (n = 10), NMA failed to show any statistically significant results for the 3 outcomes when comparing any of the groups. The estimates resulting from NMA restricted to the 10 RCTs were associated with a large amount of imprecision (data not shown).
DISCUSSION
Our NMA confirmed what was expected according to previous evidence showing a decreased mortality associated with the use of RDT in combination with ASP compared to BC alone [8]. Importantly, the NMA also showed a survival benefit associated with RDT versus conventional systems when both are embedded within ASP. This is a novel finding as the relative contribution of RDT and ASP on improved survival was unknown, not having been assessed by previous meta-analyses. Timbrook et al acknowledge that most of the RDT + ASP studies included in their meta-analysis did not state whether ASP was also present in the conventional BC comparator group, and they could not perform any analysis to compare these 2 specific interventions (RDT + ASP vs BC + ASP) [10]. In contrast, we were able to include 18 studies assessing this comparison, with the NMA adding further evidence based on indirect comparisons. This observation has important clinical implications because it suggests how even centers with efficient ASP in place to implement conventional BC results may benefit from the introduction of RDT, whereas the impact of ASP alone on survival seems more limited. Nonetheless, several challenges remain with respect to clinical implementation in real-life scenarios as the most efficient ASP and RDT have not yet been defined and may not necessarily be universal to all institutions.
Another novel finding of our work is the impact of RDT + ASP versus conventional BC on TOT, whereas the previous meta-analysis had focused on time to effective therapy instead [10]. Transitioning from a broad-spectrum early effective therapy to a narrow-spectrum optimal/targeted treatment is sometimes delayed in clinical practice, despite microbiological results being available. Although time to effective therapy has been more widely associated with increased survival compared to TOT [1–3], shortening TOT is advisable to reduce the exposure to broad-spectrum antibiotics, possibly reducing antimicrobial resistance, improve safety, and reduce costs [111–113]. Early deescalation might also positively affect LOS [114].
We showed an overall limited impact of RDT or ASP on LOS. This is relevant not only from a clinical point of view but also when assessing costs associated with the implementation of RDT and ASP because the increased expenses derived from establishing such interventions may not always be balanced by a shorter patient LOS.
Strengths of our work include NMA, which allowed us to evaluate the use of RDT and BC both with and without ASP, as well as the inclusion of studies with more than 2 comparison groups. Another strength is the high number of studies included, for a total of 25 682 patients—more than 4 times those of Timbrook et al [10].
Limitations include the high heterogeneity among studies, likely reflecting the use of different tests, different study settings (both in terms of epidemiology and hospital facilities), different provider attitudes toward implementation of tests results and antimicrobial prescription, and different types of ASP and laboratory practices in place. Different definitions of TOT and LOS among studies could have also increased heterogeneity. It is possible that the validity of our results could be restricted to some specific settings or type of RDT or ASP only. In a quasi-experimental study involving 130 hospitals in the United States, Britt et al have shown how some specific component of the ASP (such as infectious diseases consultation or the frequency of patient review at days 1–2) had the most significant impact on early antimicrobial deescalation coupled with BioFire FilmArray blood culture identification panel implementation [115]. Therefore, studies evaluating the impact of specific RDT/ASP in specific settings may be warranted to define and support local practices. A second limitation refers to our classification of MALDI-TOF MS as an RDT when applied to BC broth/pellet and as a conventional system when applied on isolates growing on solid media. We chose this classification to focus on the impact of culture independent testing. Yet, we acknowledge that the application of MALDI-TOF MS on early subcultures may have a turnaround time closer to molecular testing applied of positive BC broth rather than to conventional culture methods, and that overall heterogeneity was present in the conventional BC group because of the inclusion of both MS and traditional phenotypic testing. The validity of our results is also limited by the risk of confounding of some of the quasi-experimental studies, which is however intrinsically due to their lack of randomization.
In conclusion, our NMA suggests how the implementation of RDT + ASP may confer a survival benefit even in institutions already implementing conventional culture results through effective ASP, overall supporting the recommendation of the Infectious Diseases Society of America to use RDT within ASP for the management of BSI [116].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Anna Maria Peri, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia.
Mark D Chatfield, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia.
Weiping Ling, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia.
Luis Furuya-Kanamori, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia.
Patrick N A Harris, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia; Herston Infectious Diseases Institute, Herston, Brisbane, Queensland, Australia; Central Microbiology, Pathology Queensland, Royal Brisbane and Women’s Hospital, Brisbane, Queensland, Australia.
David L Paterson, The University of Queensland, UQ Centre for Clinical Research, Brisbane, Queensland, Australia; ADVANCE-ID, Saw Swee Hock School of Public Health, National University of Singapore, Singapore, Singapore; Infectious Diseases Translational Research Programme, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore.
Notes
Author Contributions. A. M. P. designed the study and drafted the original version of the manuscript, A. M. P. and W. L. performed the screening of the papers, M. D. C. performed statistical analysis, and all authors significantly contributed to revising the manuscript for important intellectual content and approved its last version.
Acknowledgments . The authors thank Lars Eriksson for the support with building the literature search.
Financial support . No funding was received for writing this paper. A. M. P. is receiving a scholarship from the University of Queensland in support of her PhD candidature.
References
- 1. Ferrer R, Martin-Loeches I, Phillips G, et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med 2014; 42:1749–55. [DOI] [PubMed] [Google Scholar]
- 2. Van Heuverswyn J, Valik JK, Desiree van der Werff S, Hedberg P, Giske C, Naucler P. Association between time to appropriate antimicrobial treatment and 30-day mortality in patients with bloodstream infections: a retrospective cohort study. Clin Infect Dis 2023; 76:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohnuma T, Chihara S, Costin B, et al. Association of appropriate empirical antimicrobial therapy with in-hospital mortality in patients with bloodstream infections in the US. JAMA Netw Open 2023; 6:e2249353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sweeney TE, Liesenfeld O, May L. Diagnosis of bacterial sepsis: why are tests for bacteremia not sufficient? Expert Rev Mol Diagn 2019; 19:959–62. [DOI] [PubMed] [Google Scholar]
- 5. Lamy B, Sundqvist M, Idelevich EA; ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis (ESGBIES) . Bloodstream infections—standard and progress in pathogen diagnostics. Clin Microbiol Infect 2020; 26: 142–50. [DOI] [PubMed] [Google Scholar]
- 6. Apisarnthanarak A, Bin Kim H, Moore LSP, et al. Utility and applicability of rapid diagnostic testing in antimicrobial stewardship in the Asia-Pacific region: a Delphi Consensus. Clin Infect Dis 2022; 74:2067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peri AM, O'Callaghan K, Rafiei N, et al. Persistence of detectable pathogens by culture-independent systems (T2 magnetic resonance) in patients with bloodstream infection: prognostic role and possible clinical implications. Clin Infect Dis 2024; 78:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peker N, Couto N, Sinha B, Rossen JW. Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: recent developments in molecular approaches. Clin Microbiol Infect 2018; 24:944–55. [DOI] [PubMed] [Google Scholar]
- 9. Peri AM, Ling W, Furuya-Kanamori L, Harris PNA, Paterson DL. Performance of BioFire blood culture identification 2 panel (BCID2) for the detection of bloodstream pathogens and their associated resistance markers: a systematic review and meta-analysis of diagnostic test accuracy studies. BMC Infect Dis 2022; 22:794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:15–23. [DOI] [PubMed] [Google Scholar]
- 11. Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane handbook for systematic reviews of interventions version 6.4 (updated August 2023). Cochrane, 2023. [Google Scholar]
- 12. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162:777–84. [DOI] [PubMed] [Google Scholar]
- 13. Harris AD, Bradham DD, Baumgarten M, Zuckerman IH, Fink JC, Perencevich EN. The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004; 38:1586–91. [DOI] [PubMed] [Google Scholar]
- 14. Team TE . Endnote. In Endnote X9 ed. Philadelphia, PA: Clarivate; 2013. [Google Scholar]
- 15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirkwood TBL. Geometric means and measures of dispersion. Biometrics 1979; 35:908–9. [Google Scholar]
- 17. Sterne JAC, Savovic J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 18. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. White I. Network meta-analysis. Stata J 2015; 15:951–85. [Google Scholar]
- 20. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods 2012; 3:161–76. [DOI] [PubMed] [Google Scholar]
- 21. StataCorp . Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC, 2021. [Google Scholar]
- 22. Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata J 2015; 15:905–50. [Google Scholar]
- 23. AlQahtani H, Alqahtani FY, Aleanizy FS, Baloch S, Tabb D. Impact of rapid identification of Staphylococcus species in positive blood culture using GeneXpert methicillin-resistant Staphylococcus aureus/Staphylococcus aureus blood culture assay combined with antibiotic stewardship. Microb Drug Resist 2021; 27:1037–43. [DOI] [PubMed] [Google Scholar]
- 24. Alvarez J, Mar J, Varela-Ledo E, et al. Cost analysis of real-time polymerase chain reaction microbiological diagnosis in patients with septic shock. Anaesth Intensive Care 2012; 40:958–63. [DOI] [PubMed] [Google Scholar]
- 25. Antworth A, Collins CD, Kunapuli A, et al. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacotherapy 2013; 33:137–43. [DOI] [PubMed] [Google Scholar]
- 26. Avdic E, Wang R, Li DX, et al. Sustained impact of a rapid microarray-based assay with antimicrobial stewardship interventions on optimizing therapy in patients with gram-positive bacteraemia. J Antimicrob Chemother 2017; 72:3191–8. [DOI] [PubMed] [Google Scholar]
- 27. Bandy SM, Jackson CB, Black CA, Godinez W, Gawrys GW, Lee GC. Molecular rapid diagnostics improve time to effective therapy and survival in patients with vancomycin-resistant Enterococcus bloodstream infections. Antibiotics (Basel) 2023; 12:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid Multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banerjee R, Komarow L, Virk A, et al. Randomized trial evaluating clinical impact of RAPid IDentification and Susceptibility Testing for Gram-negative Bacteremia: RAPIDS-GN. Clin Infect Dis 2021; 73:e39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 2010; 51:1074–80. [DOI] [PubMed] [Google Scholar]
- 31. Beal SG, Thomas C, Dhiman N, et al. Antibiotic utilization improvement with the nanosphere verigene gram-positive blood culture assay. Proc (Bayl Univ Med Cent) 2015; 28:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beganovic M, Costello M, Wieczorkiewicz SM. Effect of matrix-assisted Laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) alone versus MALDI-TOF MS combined with real-time antimicrobial stewardship interventions on time to optimal antimicrobial therapy in patients with positive blood cultures. J Clin Microbiol 2017; 55:1437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benoist H, Rodier S, de La Blanchardiere A, et al. Appropriate use of antifungals: impact of an antifungal stewardship program on the clinical outcome of candidaemia in a French University Hospital. Infection 2019; 47:435–40. [DOI] [PubMed] [Google Scholar]
- 34. Ben-Zvi H, Drozdinsky G, Kushnir S, et al. Influence of GeneXpert MRSA/SA test implementation on clinical outcomes of Staphylococcus aureus bacteremia—a before-after retrospective study. Diagn Microbiol Infect Dis 2019; 93:120–4. [DOI] [PubMed] [Google Scholar]
- 35. Beuving J, Wolffs PF, Hansen WL, et al. Impact of same-day antibiotic susceptibility testing on time to appropriate antibiotic treatment of patients with bacteraemia: a randomised controlled trial. Eur J Clin Microbiol Infect Dis 2015; 34:831–8. [DOI] [PubMed] [Google Scholar]
- 36. Bhat BV, Prasad P, Ravi Kumar VB, et al. Syndrome Evaluation System (SES) versus blood culture (BACTEC) in the diagnosis and management of neonatal sepsis–A randomized controlled trial. Indian J Pediatr 2016; 83:370–9. [DOI] [PubMed] [Google Scholar]
- 37. Bhavsar SM, Dingle TC, Hamula CL. The impact of blood culture identification by MALDI-TOF MS on the antimicrobial management of pediatric patients. Diagn Microbiol Infect Dis 2018; 92:220–5. [DOI] [PubMed] [Google Scholar]
- 38. Bouza E, Sousa D, Munoz P, Rodriguez-Creixems M, Fron C, Lechuz JG. Bloodstream infections: a trial of the impact of different methods of reporting positive blood culture results. Clin Infect Dis 2004; 39:1161–9. [DOI] [PubMed] [Google Scholar]
- 39. Bowman C, Holloway M, Scott L, Russell C, Lott S, Amin R. Impact of pharmacist involvement on the utility of a gram-negative blood culture identification panel on antimicrobial usage. J Pharm Technol 2021; 37:304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Box MJ, Sullivan EL, Ortwine KN, et al. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 2015; 35:269–76. [DOI] [PubMed] [Google Scholar]
- 41. Brock JB, Cretella DA, Parham JJ. An antimicrobial stewardship intervention improves adherence to standard of care for Staphylococcus aureus bloodstream infection. J Healthc Qual 2019; 41:e83–9. [DOI] [PubMed] [Google Scholar]
- 42. Brosh-Nissimov T, Tzur A, Grupel D, et al. Clinical impact of the accelerate PhenoTest(R) BC system on patients with gram-negative bacteremia and high risk of antimicrobial resistance: a prospective before-after implementation study. Ann Clin Microbiol Antimicrob 2023; 22:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bukowski PM, Jacoby JS, Jameson AP, Dumkow LE. Implementation of rapid diagnostic testing without active stewardship team notification for gram-positive blood cultures in a community teaching hospital. Antimicrob Agents Chemother 2018; 62:e01334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buss BA, Baures TJ, Yoo M, et al. Impact of a multiplex PCR assay for bloodstream infections with and without antimicrobial stewardship intervention at a cancer hospital. Open Forum Infect Dis 2018; 5:ofy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cairns KA, Doyle JS, Trevillyan JM, et al. The impact of a multidisciplinary antimicrobial stewardship team on the timeliness of antimicrobial therapy in patients with positive blood cultures: a randomized controlled trial. J Antimicrob Chemother 2016; 71:3276–83. [DOI] [PubMed] [Google Scholar]
- 46. Campos AF, Arantes T, Cambiais A, et al. Impact of an antimicrobial stewardship program intervention associated with the rapid identification of microorganisms by MALDI-TOF and detection of resistance genes in ICU patients with gram-negative bacteremia. Antibiotics (Basel) 2022; 11:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chiasson JM, Smith WJ, Jodlowski TZ, Kouma MA, Cutrell JB. Impact of a rapid blood culture diagnostic panel on time to optimal antimicrobial therapy at a Veterans Affairs Medical Center. J Pharm Pract 2022; 35:722–9. [DOI] [PubMed] [Google Scholar]
- 48. Claeys KC, Heil EL, Hitchcock S, Johnson JK, Leekha S. Management of gram-negative bloodstream infections in the era of rapid diagnostic testing: impact with and without antibiotic stewardship. Open Forum Infect Dis 2020; 7:ofaa427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cosgrove SE, Li DX, Tamma PD, et al. Use of PNA FISH for blood cultures growing gram-positive cocci in chains without a concomitant antibiotic stewardship intervention does not improve time to appropriate antibiotic therapy. Diagn Microbiol Infect Dis 2016; 86:86–92. [DOI] [PubMed] [Google Scholar]
- 50. Dare RK, Lusardi K, Pearson C, et al. Clinical impact of accelerate pheno rapid blood culture detection system in bacteremic patients. Clin Infect Dis 2021; 73:e4616–26. [DOI] [PubMed] [Google Scholar]
- 51. Dow G, MacLaggan T, Allard J. Impact of a bloodstream infection stewardship program in hospitalized patients. J Assoc Med Microbiol Infect Dis Can 2022; 7:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drwiega EN, Nichols KR, Israel EN, Knoderer CA. Impact of rapid mecA polymerase chain reaction rapid diagnostic testing for Staphylococcus aureus in a pediatric setting. Infect Dis Clin Prac 2019; 27:268–72. [Google Scholar]
- 53. Drwiega EN, Nichols KR, Kaschak M, et al. Impact of peptide nucleic acid fluorescence in situ hybridization testing for coagulase-negative staphylococci in a pediatric setting. Infect Dis Clin Prac 2019; 27:334–8. [Google Scholar]
- 54. Emonet S, Charles PG, Harbarth S, et al. Rapid molecular determination of methicillin resistance in staphylococcal bacteraemia improves early targeted antibiotic prescribing: a randomized clinical trial. Clin Microbiol Infect 2016; 22:946.e9–e15. [DOI] [PubMed] [Google Scholar]
- 55. Erickson RM, Tritle BJ, Spivak ES, Timbrook TT. Impact of an antimicrobial stewardship bundle for uncomplicated gram-negative bacteremia. Open Forum Infect Dis 2019; 6:ofz490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faugno AK, Laidman AY, Perez Martinez JD, Campbell AJ, Blyth CC. Do rapid diagnostic methods improve antibiotic prescribing in paediatric bacteraemia? J Paediatr Child Health 2021; 57:574–80. [DOI] [PubMed] [Google Scholar]
- 57. Felsenstein S, Bender JM, Sposto R, Gentry M, Takemoto C, Bard JD. Impact of a rapid blood culture assay for gram-positive identification and detection of resistance markers in a pediatric hospital. Arch Pathol Lab Med 2016; 140:267–75. [DOI] [PubMed] [Google Scholar]
- 58. Forrest GN, Roghmann MC, Toombs LS, et al. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 2008; 52:3558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Frye AM, Baker CA, Rustvold DL, et al. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol 2012; 50:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gawrys GW, Tun K, Jackson CB, et al. The impact of rapid diagnostic testing, surveillance software, and clinical pharmacist staffing at a large community hospital in the management of gram-negative bloodstream infections. Diagn Microbiol Infect Dis 2020; 98:115084. [DOI] [PubMed] [Google Scholar]
- 61. Goshorn ES, Viehman JA, Bariola JR, Khadem T, Potoski BA, Shields RK. Impact of rapid identification and stewardship intervention on coagulase-negative Staphylococcus bloodstream infection. Open Forum Infect Dis 2023; 10:ofad416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gritte AS, Morneau KM, Frei CR, Cadena-Zuluaga JA, Walter EA, Hopkins TL. Clinical impact of implementation of rapid diagnostic testing of blood cultures with Staphylococcus aureus on patient outcomes. Diagn Microbiol Infect Dis 2021; 101:115474. [DOI] [PubMed] [Google Scholar]
- 63. Heil EL, Daniels LM, Long DM, Rodino KG, Weber DJ, Miller MB. Impact of a rapid peptide nucleic acid fluorescence in situ hybridization assay on treatment of Candida infections. Am J Health Syst Pharm 2012; 69:1910–4. [DOI] [PubMed] [Google Scholar]
- 64. Hogan CA, Ebunji B, Watz N, et al. Impact of rapid antimicrobial susceptibility testing in gram-negative rod bacteremia: a quasi-experimental study. J Clin Microbiol 2020; 58:e00360-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karpen R, Murphy C, Reed E, et al. Evaluation of an automated, pharmacist-driven, antimicrobial patient acuity scoring system for hospitalized bacteremic patients. Hospital Pharmacy 2024; 59:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koh LL, O'Rourke S, Brennan M, et al. Impact of a rapid molecular test for positive blood cultures from neonatal intensive care patients on clinical management: a retrospective audit. Ir J Med Sci 2018; 187:423–7. [DOI] [PubMed] [Google Scholar]
- 67. Kremer AM, Bouchard JL, Orvin AI. Impact of gram-negative rod bacteremia rapid diagnostic testing and real-time clinical pharmacist intervention. J Pharm Pract 2023; doi: 10.1177/08971900231200900 [DOI] [PubMed] [Google Scholar]
- 68. Lockwood AM, Perez KK, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship in two community hospitals improved process measures and antibiotic adjustment time. Infect Control Hosp Epidemiol 2016; 37:425–32. [DOI] [PubMed] [Google Scholar]
- 69. Lopez-Pintor JM, Sanchez-Lopez J, Navarro-San Francisco C, Sanchez-Diaz AM, Loza E, Canton R. Real life clinical impact of antimicrobial stewardship actions on the blood culture workflow from a microbiology laboratory. Antibiotics (Basel) 2021; 10:1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. MacGowan A, Grier S, Stoddart M, et al. Impact of rapid microbial identification on clinical outcomes in bloodstream infection: the RAPIDO randomized trial. Clin Microbiol Infect 2020; 26:1347–54. [DOI] [PubMed] [Google Scholar]
- 71. MacVane SH, Nolte FS. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J Clin Microbiol 2016; 54:2455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Magarifuchi H, Hamada Y, Oho M, Kusaba K, Urakami T, Aoki Y. Clinical utility of direct application of matrix-assisted laser desorption ionization time-of-flight mass spectrometry and rapid disk diffusion test in presumptive antimicrobial therapy for bacteremia. J Infect Chemother 2018; 24:881–6. [DOI] [PubMed] [Google Scholar]
- 73. Mahrous AJ, Thabit AK, Elarabi S, Fleisher J. Clinical impact of pharmacist-directed antimicrobial stewardship guidance following blood culture rapid diagnostic testing. J Hosp Infect 2020; 106:436–46. [DOI] [PubMed] [Google Scholar]
- 74. Malcolmson C, Ng K, Hughes S, et al. Impact of matrix-assisted Laser desorption and ionization time-of-flight and antimicrobial stewardship intervention on treatment of bloodstream infections in hospitalized children. J Pediatric Infect Dis Soc 2017; 6:178–86. [DOI] [PubMed] [Google Scholar]
- 75. Mancini N, Sambri V, Corti C, et al. Cost-effectiveness of blood culture and a multiplex real-time PCR in hematological patients with suspected sepsis: an observational propensity score-matched study. Expert Rev Mol Diagn 2014; 14:623–32. [DOI] [PubMed] [Google Scholar]
- 76. Mazzillo Vega L, Cabrera Bravo N. [Rational use of antibiotics and FilmArray technology for rapid identification of bacteremias in a pediatric intensive care unit]. Rev Chil Pediatr 2020; 91:553–60. [DOI] [PubMed] [Google Scholar]
- 77. McCarthy L, Colley P, Nguyen HL, Berhe M. Impact of pharmacist intervention in response to automated molecular diagnostic tests of blood culture results. J Pharm Pract 2022; 35:47–53. [DOI] [PubMed] [Google Scholar]
- 78. Messacar K, Hurst AL, Child J, et al. Clinical impact and provider acceptability of real-time antimicrobial stewardship decision support for rapid diagnostics in children with positive blood culture results. J Pediatric Infect Dis Soc 2017; 6:267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mohayya SM, Arsalan M, Narayanan N, et al. Impact of phenotypic rapid diagnostic assay on duration of empiric antibiotics for gram-negative bacteremia. Antimicrob Steward Healthc Epidemiol 2023; 3:e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moni M, Sidharthan N, Sudhir S, et al. A quality improvement initiative to improve the appropriateness of candidemia management by the implementation of a comprehensive candidemia care bundle at a tertiary care hospital in South India: results of a quasi-experimental study. Medicine (Baltimore) 2022; 101:e28906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakagawa R, Jain R, Bryan AB, Chan JD. Optimization of antimicrobial therapy in vancomycin-resistant enterococcal bacteraemia using a rapid detection gram-positive blood culture assay. J Hosp Infect 2018; 99:153–7. [DOI] [PubMed] [Google Scholar]
- 82. Nasef R, El Lababidi R, Alatoom A, Krishnaprasad S, Bonilla F. The impact of integrating rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program in the United Arab of Emirates. Int J Infect Dis 2020; 91:124–8. [DOI] [PubMed] [Google Scholar]
- 83. Niwa T, Yonetamari J, Hayama N, et al. Clinical impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry combined with antimicrobial stewardship interventions in patients with bloodstream infections in a Japanese tertiary hospital. Int J Clin Pract 2019; 73:e13332. [DOI] [PubMed] [Google Scholar]
- 84. Ohashi K, Matsuoka T, Shinoda Y, et al. Evaluation of treatment outcomes of patients with MRSA bacteremia following antimicrobial stewardship programs with pharmacist intervention. Int J Clin Pract 2018; 72:e13065. [DOI] [PubMed] [Google Scholar]
- 85. Osthoff M, Gurtler N, Bassetti S, et al. Impact of MALDI-TOF-MS-based identification directly from positive blood cultures on patient management: a controlled clinical trial. Clin Microbiol Infect 2017; 23:78–85. [DOI] [PubMed] [Google Scholar]
- 86. Page A, O'Rourke S, Brennan M, et al. Impact of Xpert MRSA/SA blood culture PCR assay on management of positive blood cultures in obstetric patients: a retrospective audit. Ir J Med Sci 2017; 186:995–8. [DOI] [PubMed] [Google Scholar]
- 87. Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis 2016; 84:159–64. [DOI] [PubMed] [Google Scholar]
- 88. Patch ME, Weisz E, Cubillos A, Estrada SJ, Pfaller MA. Impact of rapid, culture-independent diagnosis of candidaemia and invasive candidiasis in a community health system. J Antimicrob Chemother 2018; 73:iv27–30. [DOI] [PubMed] [Google Scholar]
- 89. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant gram-negative bacteremia. J Infect 2014; 69:216–25. [DOI] [PubMed] [Google Scholar]
- 90. Perez-Lazo G, Del Valle-Mendoza J, Sandoval-Ahumada R, et al. Impact of adding a rapid PCR-based blood culture identification panel to the antimicrobial stewardship program of patients with febrile neutropenia in a Peruvian referral hospital. Antibiotics (Basel) 2023; 12:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pettit NN, Han Z, Nguyen CT, et al. Antimicrobial stewardship review of automated candidemia alerts using the epic stewardship module improves bundle-of-care adherence. Open Forum Infect Dis 2019; 6:ofz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Puckett LM, Rajkotia P, Coppola L, et al. Impact of direct from blood culture identification of pathogens paired with antimicrobial stewardship interventions in a pediatric hospital. J Pediatr Pharmacol Ther 2021; 26:802–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Reed EE, West JE, Keating EA, et al. Improving the management of candidemia through antimicrobial stewardship interventions. Diagn Microbiol Infect Dis 2014; 78:157–61. [DOI] [PubMed] [Google Scholar]
- 94. Rivard KR, Athans V, Lam SW, et al. Impact of antimicrobial stewardship and rapid microarray testing on patients with gram-negative bacteremia. Eur J Clin Microbiol Infect Dis 2017; 36:1879–87. [DOI] [PubMed] [Google Scholar]
- 95. Rodrigues C, Siciliano RF, Filho HC, et al. The effect of a rapid molecular blood test on the use of antibiotics for nosocomial sepsis: a randomized clinical trial. J Intensive Care 2019; 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Romero-Gomez MP, Cendejas-Bueno E, Garcia Rodriguez J, Mingorance J. Impact of rapid diagnosis of Staphylococcus aureus bacteremia from positive blood cultures on patient management. Eur J Clin Microbiol Infect Dis 2017; 36:2469–73. [DOI] [PubMed] [Google Scholar]
- 97. Rosa R, Zavala B, Cain N, Anjan S, Aragon L, Abbo LM. Antimicrobial stewardship program implementation of a quality improvement intervention using real-time feedback and an electronic order set for the management of Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 2018; 39:346–9. [DOI] [PubMed] [Google Scholar]
- 98. Roshdy DG, Tran A, LeCroy N, et al. Impact of a rapid microarray-based assay for identification of positive blood cultures for treatment optimization for patients with streptococcal and enterococcal bacteremia. J Clin Microbiol 2015; 53:1411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 2013; 51:4008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schumann J, Johanns U, Ahmad-Nejad P, Ghebremedhin B, Woebker G. The impact of the FilmArray-based detection of microbial pathogens from positive blood culture vials on the time to optimal antimicrobial regimen in intensive care units of the Helios University Clinic Wuppertal, Germany. J Clin Med 2021; 10:5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Senda Y, Fujita S, Sakai Y, Wada T. [Clinical evaluation of rapid identification of bacteria from positive-testing blood culture bottles by internal transcribed spacer PCR]. Rinsho Byori 2011; 59:439–45. [PubMed] [Google Scholar]
- 102. Senok A, Dabal LA, Alfaresi M, et al. Clinical impact of the BIOFIRE blood culture identification 2 panel in adult patients with bloodstream infection: a multicentre observational study in the United Arab Emirates. Diagnostics (Basel) 2023; 13:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Smith JR, Frens JJ, Snider CB, Claeys KC. Impact of a pharmacist-driven care package on Staphylococcus aureus bacteremia management in a large community healthcare network: a propensity score-matched, quasi-experimental study. Diagn Microbiol Infect Dis 2018; 90:50–4. [DOI] [PubMed] [Google Scholar]
- 104. Tritle BJ, Watteyne R, Hickman A, et al. No implementation without representation: real-time pharmacist intervention optimizes rapid diagnostic tests for bacteremia at a small community hospital. Hosp Pharm 2022; 57:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tseng AS, Kasule SN, Rice F, et al. Is it actionable? An evaluation of the rapid PCR-based blood culture identification panel on the management of gram-positive and gram-negative blood stream infections. Open Forum Infect Dis 2018; 5:ofy308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Turner RB, Lalikian K, Fry M, Schwartz J, Chan D, Won R. Impact of rapid identification of Staphylococcus aureus bloodstream infection without antimicrobial stewardship intervention on antibiotic optimization and clinical outcomes. Diagn Microbiol Infect Dis 2017; 89:125–30. [DOI] [PubMed] [Google Scholar]
- 107. Walker T, Dumadag S, Lee CJ, et al. Clinical impact of laboratory implementation of Verigene BC-GN microarray-based assay for detection of gram-negative Bacteria in positive blood cultures. J Clin Microbiol 2016; 54:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Welch SN, Patel RM, Morris LE, Dassner AM, Rozario NL, Forrester JB. Impact of antimicrobial stewardship and rapid diagnostics in children with Staphylococcus aureus bacteremia. J Am Coll Clin Pharm 2020; 3:1304–11. [Google Scholar]
- 109. Wenzler E, Goff DA, Mangino JE, Reed EE, Wehr A, Bauer KA. Impact of rapid identification of Acinetobacter baumannii via matrix-assisted laser desorption ionization time-of-flight mass spectrometry combined with antimicrobial stewardship in patients with pneumonia and/or bacteremia. Diagn Microbiol Infect Dis 2016; 84:63–8. [DOI] [PubMed] [Google Scholar]
- 110. Yamada K, Imoto W, Shibata W, et al. Impact of antimicrobial stewardship with the Xpert MRSA/SA BC assay at a tertiary hospital in Japan. J Infect Chemother 2023; 29:693–9. [DOI] [PubMed] [Google Scholar]
- 111. Canton R, Morosini MI. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev 2011; 35:977–91. [DOI] [PubMed] [Google Scholar]
- 112. Cizman M, Plankar Srovin T. Antibiotic consumption and resistance of gram-negative pathogens (collateral damage). GMS Infect Dis 2018; 6:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Barbosa C, Breck A, King G, et al. Impact analysis of expanding narrow-spectrum antibiotic use for children with ear, sinus and throat infections. J Comp Eff Res 2022; 11:89–98. [DOI] [PubMed] [Google Scholar]
- 114. Deshpande A, Richter SS, Haessler S, et al. De-escalation of empiric antibiotics following negative cultures in hospitalized patients with pneumonia: rates and outcomes. n Infect Dis 2021; 72:1314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Britt NS, Khader K, He T, et al. Examining the clinical impact of rapid multiplex polymerase chain reaction-based diagnostic testing for bloodstream infections in a national cohort of the Veterans Health Administration. Pharmacotherapy 2023; 43:24–34. [DOI] [PubMed] [Google Scholar]
- 116. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.