Abstract
Objective
Epicardial adipose tissue (EAT) is implicated in the pathogenesis and progression of coronary artery disease (CAD). Limited data exists on the interplay between EAT and atherosclerosis in young individuals. Our study aims to explore the relationship between EAT and CAD in a young cohort.
Methods
All young (18–45 years) patients without prior CAD, referred for coronary computed tomography angiography (CCTA) from 2016 to 2022 were included. EAT volume and coronary artery calcium (CAC) were calculated from dedicated non-contrast scans. Coronary plaque presence, extent, and volume were quantified from CCTA. Multivariable logistic regression models for the presence of CAD, defined as any coronary atherosclerosis, were performed.
Results
Overall, 712 patients (39±4.8 years, 54 % female) with 45 % Hispanic, and 21 % non-Hispanic Black were included. Patients with CAD had higher EAT volume than those without (80.80 mL ± 36.00 vs 55.16 mL ± 27.92; P < 0.001). In those with CAC=0, higher EAT was associated with the presence of CAD compared to lower EAT volume (P < 0.001). An EAT volume >76 mL was associated with higher CAC (P < 0.001), segment involvement score (P < 0.001), and quantitative total, non-calcified, and low-attenuation plaque volumes (P < 0.002). At multivariable analysis, EAT volume (per 10 mL, OR: 1.21; 95 %CI: 1.12–1.30; P < 0.0001) was independently associated with the presence of CAD.
Conclusion
In a diverse cohort of young adults without history of CAD and undergoing a clinically indicated CCTA, EAT volume was independently associated with the presence of CAD. Our findings highlight EAT potential as a novel marker for CAD risk-assessment and a potential therapeutic target in young patients.
Keywords: Epicardial adipose tissue, CCTA, Coronary plaque, Young adults
1. Introduction
Despite a recent decline in overall cardiovascular disease (CVD) mortality in the US, young adults exhibit an opposite trend with increasing mortality [1]. Although risk estimators are widely recommended, they prove less effective in individual cases, particularly for young adults, as they overly emphasize age over other standard modifiable cardiovascular disease risk factors (SMuRFs), resulting in an estimated 10-year ASCVD under the threshold for preventive therapies [2]. Unsurprisingly, most young adults experiencing a myocardial infarction would not have qualified for statin therapy according to the current guidelines before the onset of their cardiac event [3]. Coronary artery calcium (CAC) and computed tomography coronary angiography (CCTA) may allow for early detection of coronary atherosclerosis in young individuals [[4], [5], [6], [7]]. We have reported previously that the presence of coronary plaque in young symptomatic adults (18–45 years) is not negligible [8], and others have shown that finding minimal CAC in this subgroup, is associated with an exponential increase in cardiovascular events [9]. However, little is known about imaging markers of atherosclerosis risk in young individuals.
Epicardial adipose tissue (EAT), is a unique fat deposit with paracrine effects on the heart and an emerging novel cardiovascular disease (CVD) risk marker [10,11]. EAT contributes to coronary atherosclerosis through various mechanisms, including inflammation, immune response, oxidative stress, and lipid accumulation [10]. Higher EAT volume has been associated with plaque burden, high-risk plaque features including low-attenuation non-calcified plaque, significant coronary stenosis, myocardial ischemia, and MACE in middle-aged symptomatic patients [[12], [13], [14]]. Moreover, it has been suggested that EAT may play a role in the early stages of atherosclerosis in asymptomatic individuals [15]. However, the EAT characteristics and interplay with CAD in young adults remain unknown. We explored the relationship between EAT volume and attenuation with CAD in a diverse cohort of young patients referred for CCTA.
2. Methods
2.1. Study population
All symptomatic young adults (age 18–45 years) referred for a clinically indicated CCTA for suspicion of CAD at Montefiore Healthcare System, from June 2016 to December 2022, were identified from our CCTA registry. Patients with known CAD (defined as prior myocardial infarction or coronary revascularization) or presenting through the emergency department with suspected acute coronary syndrome, and those without a dedicated gated non-contrast CAC scan at the time of CCTA, were excluded. Demographic data and cardiovascular risk factors such as smoking status, body mass index (BMI), hyperlipidemia, hypertension, diabetes mellitus, family history of CAD, and lipid levels, were through retrospective chart review. Hypertension, dyslipidemia, diabetes, family history of CAD, and smoking status were defined as either diagnosed by a physician or receiving medical treatment for these conditions. Race/ethnicity was self-identified at the time of registration. Symptoms and pretest probability were classified and estimated according to the latest 2021 AHA/ACC Chest Pain guidelines [16]. Our Institutional Review Board (Office of Human Research Affairs at Albert Einstein College of Medicine) approved the study. Data is available upon reasonable request.
2.2. Cardiac computed tomography
All scans were performed according to the Society of Cardiovascular CT guidelines [17]. The CCTA acquisition was performed using a 64-slice Philips IQon scanner or a 64-slice GE Lightspeed VCT scanner using 80 to 100 mL of Isovue-370 contrast. Acquisition parameters (kVp and mA) were adjusted according to the patient's BMI. As per institutional guidelines, a prospective ECG-triggered scan was used whenever possible. The detailed CCTA acquisition parameters are reported in Supplemental Table 1.
2.3. Analysis of atherosclerotic plaque
Experienced board-certified level III cardiac imagers interpreted all CAC and CCTA images. CAD was defined as the visual evidence of plaque by CCTA and/or CAC (score) ≥1; while the absence of plaque by CCTA and CAC=0 were considered as normal coronaries. The presence of obstructive CAD was defined as stenosis ≥50 % in any coronary vessel.
Calcium score was calculated by the Agatston method. It was categorized into: CAC=0, 1–10, 11–100, and CAC>100 [18]. Standard semiautomated software reconstructed CCTA images were graded by CAD reporting and data system (CAD-RADS) as recommended by guidelines [19]. Segment involvement score (SIS) was retrospectively calculated as previously described by Min et al. [20]. Quantitative plaque analysis was performed (Fig. 1) in coronary segments ≥2 mm in diameter, using semi-automated software (Autoplaque version 2.5, Cedars-Sinai Medical Center, Los Angeles, CA, USA). Level III imaging readers (DL and PP), blinded to patient characteristics, conducted the measurements. For patients with normal coronary arteries, the plaque volumes and burden were set to 0 as previously published [21]. Total plaque, non-calcified plaque (NCP), LAP, and calcified plaque (CP) volumes (mm3) and burden (%) were quantified in each patient with visual evidence of coronary plaque, through an adaptive threshold method. The plaque burden or percent atheroma volume was estimated by dividing the plaque volume by the vessel volume of diseased segments and multiplying by 100 [22].
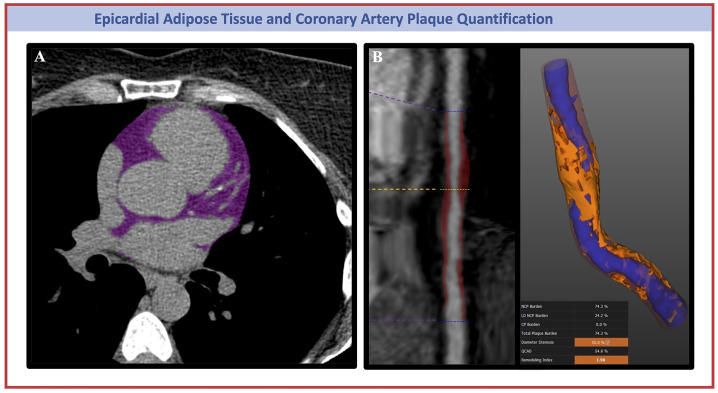
Fig. 1.
Representative Coronary Computed Tomography (CCT) Images of Epicardial Adipose Tissue (A) and Coronary Artery Plaque (B) Quantification. Panel B shows on the left a straight MPR reconstruction of the mid-RCA artery; on the right a plaque characterization showing lumen (in blue) with a 55 % diameter stenosis, and high burden of both noncalcified plaque (in red) and low-attenuation plaque (in orange). MPR, multiplanar reconstruction; RCA, right coronary artery.
2.4. Epicardial adipose tissue quantification
EAT volume and attenuation were quantified from gated non-contrast CAC scans using a previously validated semi-automated dedicated software (QFAT; v2.0; Cedars-Sinai Medical Center, Los Angeles, CA, USA) [23,24] (Fig. 1). Using this method, the pericardium was automatically segmented from the non-contrast CT datasets. The superior limit of the heart was defined as the pulmonary artery bifurcation, and the inferior limit was defined as the posterior descending artery. EAT volume (mL) and mean attenuation (Hounsfield Units, HU) were automatically calculated from 3-dimensional fat voxels between the HU limits of −190 to −30 HU enclosed by the visceral pericardium. The results were reviewed and manually optimized by one experienced CT analyst (AF). QFAT uses convolutional deep-learning for fully automated quantification of EAT volume.
2.5. Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) when normally distributed, and as median and interquartile ranges [IQR] when not normally distributed. Categorical data are presented as counts with percentages. For two-group comparisons of continuous variables, the two-sample T-test, and for the categorical variable the Pearson Chi-square test were used. The relationship between EAT and the risk of CAD was further explored with logistic regression models by entering the EAT volume measurement as a restricted cubic spline with 3 knots. Univariable and multivariable odds ratios (OR) with 95 % confidence intervals (CI) were calculated using logistic regression analysis to assess the association between CAD and EAT. The multivariable logistic regression model included the following variables (among those significantly associated with CAD in our cohort and historical studies): EAT volume, age, sex, BMI, hypertension, family history of CAD, diabetes, hyperlipidemia, statin therapy, and smoking status (active and former). A ROC curve was performed to assess the discrimination of CAD using EAT volume. The optimal cut-off for the EAT volume was identified by the value corresponding to the highest Youden index. Subgroup analyses were performed to test the interaction between EAT and variables of interest on CAD diagnosis. Pearson correlation analysis and linear regression analyses have been performed to investigate the relationship among LAP, EAT, and CAC. Multivariable linear regression analysis was conducted to identify factors associated with EAT volume. The analysis included the following variables that were clinically relevant and significantly different stratifying the population by EAT volume cut-off: age, sex, BMI, hypertension, diabetes, smoking status (active and former), hyperlipidemia, family history of CAD, statin therapy. All statistical tests were 2-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using R, version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline population characteristics
A total of 712 patients were included in this study. The median age was 39±4.8 years, 45.6 % were male and more frequently Hispanic (44.7 %). Baseline characteristics, stratified by the presence of CAD, are summarized in Table 1. A total of 127 patients (17.8 %) had CAD, among which most (89 %) had non-obstructive CAD and 11 % (n = 14) had obstructive disease.
Table 1.
Baseline population characteristics, according to the presence of CAD.
| Overall N = 712 |
CAD N = 127 |
No CAD N = 585 |
P Value |
|
|---|---|---|---|---|
| Age, y | 39 ± 4.83 | 40.5 ± 4.4 | 38.7 ± 4.9 | <0.001 |
| Male | 325 (45.6) | 83 (65.4) | 242 (41.4) | <0.001 |
| Race/ethnicity* | ||||
| Hispanic | 318 (44.7) | 50 (39.4) | 268 (45.8) | 0.221 |
| Non-Hispanic Asian | 28 (3.9) | 8 (6.3) | 20 (3.4) | 0.207 |
| Non-Hispanic Black | 153 (21.5) | 28 (22.0) | 125 (21.4) | 0.960 |
| Non-Hispanic White | 33 (4.6) | 13 (10.2) | 20 (3.4) | 0.002 |
| Cardiovascular risk factors | ||||
| BMI, kg/m2 | 31.57 ± 8.22 | 33.31 ± 7.89 | 31.16 ± 8.25 | 0.009 |
| Diabetes Mellitus | 81 (11.6) | 21 (16.7) | 60 (10.5) | 0.070 |
| Hypertension | 229 (32.8) | 56 (44.4) | 173 (30.2) | 0.003 |
| Hyperlipidemia | 186 (26.6) | 53 (42.1) | 133 (23.2) | <0.001 |
| Family History of CAD | 92 (13.8) | 33 (27.5) | 59 (10.8) | <0.001 |
| Smoking History | ||||
| Current | 108 (15.5) | 22 (18) | 86 (15.0) | 0.479 |
| Former | 110 (15.8) | 27 (22.1) | 83 (14.5) | 0.049 |
| Never | 478 (68.7) | 73 (59.8) | 405 (70.6) | 0.027 |
| Primary Presenting Symptom | ||||
| Cardiac Chest Pain | 303 (42.6) | 85 (66.9) | 218 (37.3) | <0.001 |
| Non Cardiac Chest Pain | 228 (32.0) | 18 (14.2) | 210 (35.9) | <0.001 |
| Dyspnea | 106 (14.9) | 31 (24.4) | 75 (12.8) | 0.001 |
| Others | 215 (30.2) | 27 (21.3) | 188 (32.1) | 0.021 |
| Pre-test Probability | <0.001 | |||
| Low | 625 (87.8) | 97 (76.4) | 528 (90.3) | |
| Intermediate | 87 (12.2) | 30 (23.6) | 57 (9.7) | |
| Baseline laboratory values | ||||
| Total Cholesterol, mg/dL | 182.59 ± 42.84 | 186.09 ± 44.32 | 181.51 ± 42.39 | 0.361 |
| HDL-C, mg/dL | 46.23 ± 11.83 | 43.03 ± 11.47 | 47.03 ± 11.79 | 0.003 |
| LDL-C, mg/dL | 111.50 ± 36.70 | 116.44 ± 38.92 | 110.25 ± 36.07 | 0.142 |
| Non-HDL-C, mg/dL | 134.15 ± 46.31 | 143.06 ± 42.902 | 130.12 ± 47.32 | 0.023 |
| Triglycerides, mg/dL | 131.78 ± 108.73 | 144.29 ± 95.00 | 128.61 ± 111.84 | 0.207 |
| HbA1c | 6.08 ± 1.46 | 6.08 ± 1.46 | NA | NA |
| Creatinine, mg/dL | 0.89 ± 0.55 | 1.01 ± 1.07 | 0.86 ± 0.31 | 0.008 |
| Baseline Statin | ||||
| Statin | 126 (17.8) | 27 (21.6) | 99 (17.0) | 0.269 |
| Epicardial Adipose Tissue on CCT | ||||
| Volume, mL | 59.73 ± 31.08 | 80.80 ± 36.00 | 55.16 ± 27.92 | <0.001 |
| Mean density, HU | −75.80 ± 5.61 | −76.31 ± 6.30 | −75.70 ± 5.45 | 0.266 |
| Quartile | <0.001 | |||
| I | 189 (26.5) | 13 (10.2) | 176 (30.1) | |
| II | 172 (24.2) | 22 (17.3) | 150 (25.6) | |
| III | 174 (24.4) | 22 (17.3) | 152 (26.0) | |
| IV | 177 (24.9) | 70 (55.1) | 107 (18.3) |
Values are presented as n (%); mean ± SD; median [IQR].
BMI, body mass index; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; HbA1c, hemoglobin A1C; HDL-C, high-density lipoprotein cholesterol; HU, Hounsfield unit; LDL-C, low-density lipoprotein cholesterol.
Race/Ethnicity (n = 532).
Baseline demographic and clinical characteristics according to sex and race/ethnicity are shown in Supplemental Table 2 and 3.
3.2. Coronary computed tomography angiography, CAC, and plaque
A total of 622 patients had CAC=0 (87.4 %), 33 patients CAC 1–10 (4.6 %), 42 patients CAC 11–100 (5.9 %), and 15 patients CAC>100 (2.1 %). Among patients with CAC=0, the mean age was 38.8 years, 42 % were male, 46 % Hispanic; 5.9 % were found to have coronary artery plaque. In the subgroup of subjects with CAC=0 and plaque, the average plaque volume was 318.4 ± 201.3mm [3], and the LAP volume was 43.8 ± 29.65mm [3]. The detailed characterization of plaque morphology and burden is reported in Supplemental Table 4.
In those with CAD, the total plaque volume was 414.8 ± 337 mm3, while the total plaque burden was 39.9 ± 11.2 %. The presence of any coronary artery plaque was higher in men compared with women (25.5% vs 11.4 %, P < 0.001). However, there were no differences in plaque characteristics and burden by sex (Supplemental Table 2). There was no difference in the frequency of obstructive CAD between men and women (13.3 % in men vs 6.8 % in women, P = 0.421).
3.3. Epicardial adipose tissue and presence of CAD
Overall, the mean EAT volume was 59.73±31.08 mL, while the mean EAT attenuation was −75.80±5.61 HU. Men showed higher EAT volumes than women (P < 0.001) (Fig. 2A). The EAT volume and EAT mean attenuation varied by race/ethnicity (Supplemental Table 3). The EAT volume was significantly higher in men compared to women (66.15±32.53 mL vs 54.33±28.77 mL; EAT volume indexed for BMI: 2.12±1.05 vs 1.80±0.9, P < 0.001), no significant differences were observed in EAT attenuation by sex (P = 0.352).
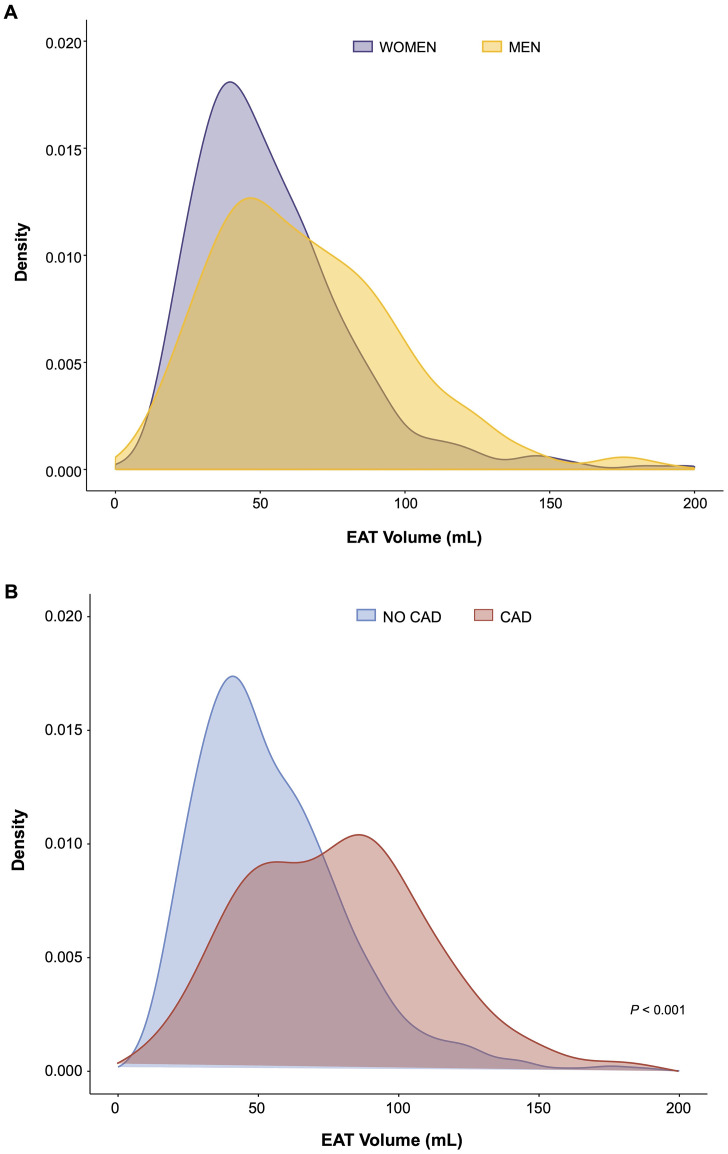
Fig. 2.
Association Between Epicardial Adipose Tissue and Sex (B), or Coronary Artery Disease (B). The curves show density plots: on the X axis represents the EAT volume (mL), on the Y axis the density, expressed as a probability density function. CAD, coronary artery disease; EAT, epicardial adipose tissue.
EAT volume was significantly higher in patients with CAD (80.80±36.00 mL) compared with those without CAD (55.16±27.92 mL, P < 0.001) (Fig. 2B). No significant differences were observed in EAT attenuation in the CAD and non-CAD groups (P = 0.266) (Table 1). When comparing obstructive and non-obstructive CAD, no differences were recorded in terms of EAT volume (70.29±16.94 mL vs 82.1 ± 37.54 mL, P = 0.248) or attenuation (−76.64±3.15 HU vs −76.27±6.59 HU, P = 0.834). At multivariable logistic regression analysis, EAT volume (per 10 mL, OR: 1.21; 95 % CI: 1.12–1.30; P < 0.0001), male sex (OR: 2.28; 95 % CI: 1.43–3.62; P < 0.001), age (per 1 year, OR: 1.08; 95 % CI: 1.02–1.14; P = 0.007), and family history of CAD (OR: 3.22; 95 % CI: 1.85–5.61; P < 0.0001) were independently associated with CAD, after adjustment for BMI, diabetes, hyperlipidemia, statin therapy, hypertension, and smoking (Central Illustration).
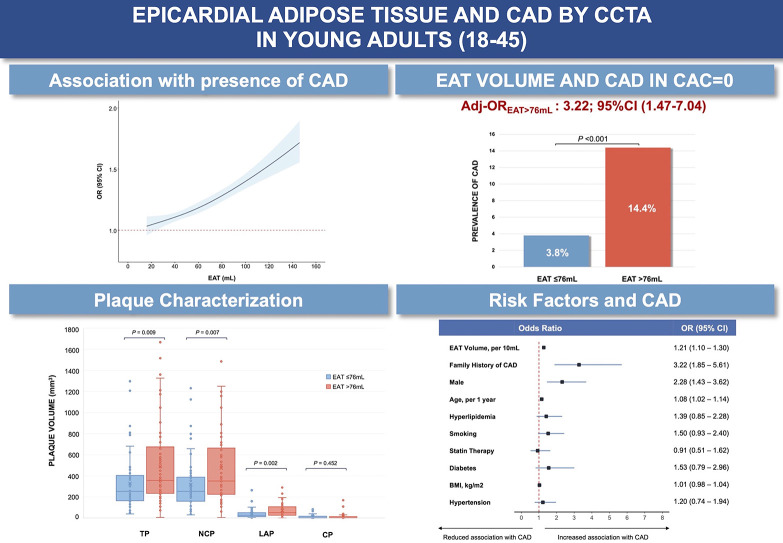
Central Illustration.
Epicardial Adipose Tissue and CAD by CCTA in Young Adults (18–45). (Top Left) Association between epicardial adipose tissue and coronary artery disease. (Top Right) Relationship between epicardial adipose tissue and coronary artery disease in CAC=0 population. (Bottom left) Coronary artery plaque characterization by epicardial adipose tissue volume. (Bottom right) Multivariable logistic regression analysis on coronary artery disease. BMI, body mass index; CAD, coronary artery disease; EAT, epicardial adipose tissue; LAP, low-attenuation plaque; CP, calcified plaque; NCP, non-calcified plaque; TP, total plaque.
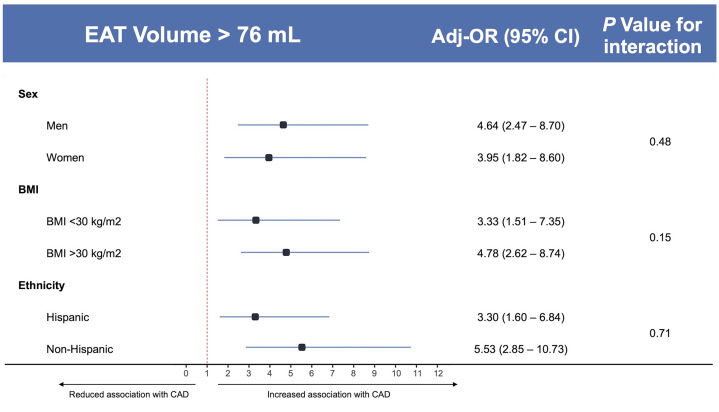
A restricted cubic spline regression analysis showed a linear relationship between EAT measured as a continuous variable and the presence of CAD (nonlinearity P = 0.39) with a stronger association per increasing volume of EAT (Central Illustration). In those with CAC=0, higher EAT volume was associated with a higher prevalence of CAD, compared to low EAT volume (P < 0.001) (Central Illustration). In addition, ROC analysis for the prediction of CAD using EAT showed acceptable discrimination (AUC=0.73, 95 % CI 0.68–0.78 (Supplemental Fig. 1, panel A). The optimal cut-off for CAD was identified, using Youden index, at 76 mL of EAT (Youden=0.3727) corresponding to the lower end of the 4th quartile, with an accuracy of 76.8 %, a sensitivity of 55.9 %, and a specificity of 81.4 %, (Supplemental Fig. 1, panel B). At multivariable linear regression analysis, BMI (b = 0.94; P < 0.001), male sex (b = 0.36; P < 0.001); age (b = 0.9: P < 0.001), and hypertension (b = 6.94; P < 0.01), were identified as independently associated with EAT volume, after adjustment for diabetes, hyperlipidemia, statin therapy, smoking, and family history of CAD (Supplemental Table 5). As shown in Table 2, when dividing the cohort by this cut-off, the EAT >76 mL group had a substantially higher presence of CAD (39.5% vs 10.7 %, P < 0.001). In subgroup analyses, EAT volume was confirmed to be significantly associated with CAD with no interaction with sex (adj-Pinteraction=0.48), ethnicity (adj-Pinteraction=0.71), or BMI (adj-Pinteraction = 0.15), after adjustment for sex, age, BMI, hypertension, hyperlipidemia, and family history of CAD (Fig. 3). At a multivariable logistic regression analysis in those with CAC=0, EAT volume >76 mL was independently associated with the presence of coronary artery disease (OR: 3.22; 95 %CI: 1.47–7.04), (Central Illustration). Analysis of demographics, laboratory values, and CT findings per the EAT cut-off value (i.e., 76 mL) are reported in Table 2 and Supplemental Table 6.
Table 2.
Quantitative and qualitative coronary atherosclerosis disease analysis on coronary computed tomography angiography, stratified by EAT.
| EAT >76 mL (N = 177) |
EAT ≤76 mL (N = 535) |
P value | |
|---|---|---|---|
| CAD | 70 (39.5) | 57 (10.7) | <0.001 |
| Obstructive CAD | 7 (10) | 7 (12.3) | 0.902 |
| Total Plaque Volume, mm3 | 490.50 [374.41] | 327.91 [265.46] | 0.009 |
| Total NCP Volume, mm3 | 475.79 [354.9] | 316.97 [254.92] | 0.007 |
| Total LAP Volume, mm3 | 72.78 [60.70] | 41.06 [44.51] | 0.002 |
| Total CP Volume, mm3 | 14.70 [33.06] | 10.94 [17.09] | 0.452 |
| Total Plaque Burden,% | 40.08 ± 11.99 | 39.65 ± 10.45 | 0.836 |
| Total NCP Burden,% | 38.98 ± 11.83 | 38.01 ± 10.49 | 0.641 |
| Total LAP Burden,% | 6.33 ± 4.02 | 5.01 ± 3.35 | 0.061 |
| Total CP Burden,% | 1.10 ± 1.69 | 1.64 ± 3.18 | 0.248 |
| CAC (AU) | |||
| 0 | 125 (70.6) | 497 (92.9) | <0.001 |
| 1–10 | 21 (11.9) | 12 (2.2) | <0.001 |
| 11–100 | 24 (13.6) | 18 (3.4) | <0.001 |
| >100 | 7 (4.0) | 8 (1.5) | 0.095 |
| SIS | <0.001 | ||
| ≤ 2 | 38 (21.5) | 46 (8.6) | |
| 3 - 4 | 23 (13) | 28 (3.9) | |
| 5 - 7 | 7 (4) | 11 (1.5) | |
| ≥ 8 | 2 (1.1) | 3 (0.4) |
Values are presented as n (%); mean ± SD; median [IQR]. AU, angaston units; CAC, coronary artery calcium score; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; CP, calcified plaque; EAT, epicardial adipose tissue; NCP, non-calcified plaque; LAP, low-attenuation plaque; SIS, segment involvement score.
Fig. 3.
Subgroup Analyses of Coronary Artery Disease per EAT Volume. Odds Ratios and P values for interaction are adjusted for: sex, age, BMI, hypertension, hyperlipidemia, and family history of CAD. BMI, body mass index; CAD, coronary artery disease; EAT, epicardial adipose tissue.
3.4. Epicardial adipose tissue, plaque extent, and quantitative plaque analysis
The linear regression analyses revealed a positive association between EAT and CAC. Moreover, EAT and CAC were positively correlated with LAP (P < 0.001, R2=0.10; P < 0.0001, R2=0.26, respectively) (Supplemental Fig. 2). Analyzing the relationship between EAT volume and CAC; higher EAT volume was associated with higher LAP volume in those with CAC >0 (P = 0.008) (Supplemental Fig. 3).
When using the previously determined EAT volume threshold of 76 mL, a higher EAT volume had a higher CAC score and SIS compared with those with EAT volume ≤76 mL (P < 0.001, Table 2).
When stratified by the optimal cut-off of EAT volume (i.e., 76 mL), total plaque volume (490.50±374.41 vs 327.91±265.46 mm3, P = 0.009), NCP volume (475.79±354.99 mm3 vs 316.97±254.92 mm3, P = 0.007), and LAP volume (72.78±60.70 mm3 vs 41.06±44.51 mm3, P = 0.002) were significantly higher in the group with higher EAT, (Central Illustration). No differences were found in the prevalence of obstructive CAD (10% vs 12 %, P = 0.902), the overall CP volume (14.70±33.06 mm3 vs 10.94±17.09 mm3, P = 0.452), and plaque burden between both groups (1.10±1.69% vs 1.64±3.18%, P = 0.248) (Table 2).
4. Discussion
This study represents the first effort, as far as we are aware, to investigate the interplay between EAT and CAD among symptomatic young adults referred for a CCTA. The main findings are the following: 1) EAT volume was independently associated with the presence of CAD; 2) An EAT optimal cut-off volume of >76 mL was strongly associated with CAD, independently of SMuRFs; 3) EAT volume >76 mL was also related with greater extent of CAD (by CAC or SIS) and higher volume of total, non-calcified and low-attenuation plaques, indicating a potential higher lifetime risk for ASCVD events; and 4) EAT volume >76 mL was independently associated with CAD in those with CAC=0.
The relation of EAT with the presence and severity of CAD has been well-established in both asymptomatic and symptomatic older adults [14], however data from asymptomatic individuals is more extensive. In the Framingham Heart Study (N = 1155, age 63 years, 55 % female), a significant association was found between EAT and CAC, which remained significant after adjustment for SMuRFs [25]. A similar association was noted in other community-based prospective cohorts like MESA (Multi-Ethnic Study of Atherosclerosis, N = 6814, age 45–84) [26], Heinz Nixdorf Recall Study (N = 4093, age 59.4 years, 53 % female) [27], and Rotterdam [28] (N = 2298, age 69.4 ± 6.6 years, 53 % female) studies. Beyond the presence and extent of CAD, EAT was also found to be a predictor of myocardial infarction in EISNER [29,30] (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research, (N = 2068, 56±9 years, 41 % female) and Heinz Nixdorf Recall [27]. In 3948 symptomatic individuals (age 60.6 ± 8.3 years; 51 % female) from the PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial, EAT volume increased and attenuation decreased proportionally to the CAC score [12]. Furthermore, the extent of plaque by CCTA was higher (SIS ≥4) in those with higher EAT volume and lower attenuation. Similarly, in the mostly White SCOT-HEART (Scottish Computed Tomography of the Heart) cohort (N = 1558, age 58 [47–69], 43 % female), a deep-learning quantified EAT volume was found to be predictor of obstructive (≥50 %) CAD (OR 1.13; 95 %CI: 1.04–1.30; P = 0.01) [31]. The association of EAT and the presence and extent of plaque by CCTA was also demonstrated among patients with diabetes and abnormal fasting glucose presenting with chest pain [32]. In addition, a meta-analysis by Nerlekar et al. demonstrated an association of EAT volume with high-risk plaque features as measured by CCTA or intracoronary imaging with a pooled OR 1.26 (95 %CI: 1.11–1.43; P < 0.001) [33].
Our study expands on the interplay between EAT volume and CAD in a significantly younger and diverse cohort (mean age 39±4.83 years, 54 % female, 44 % Hispanic). Moreover, we found that EAT volume was associated with CAD extent, evaluated by CAC and SIS, and with plaque volume by software-derived quantitative analysis. Our findings are in line with data from the Heinz Nixdorf study that found that EAT was associated with the progression of atherosclerosis in particular in young individuals, suggesting that EAT may promote early atherosclerosis development [27]. Since atherosclerosis takes decades to progress from early lesions to calcified plaques [34], it is not surprising that younger patients most commonly have a CAC score of 0 [6]. In our study, even in the absence of coronary calcification (i.e., CAC = 0), higher EAT volume was associated with the presence of non-calcified plaque. This underlines the potential role of EAT volume as a predictor of early CAD in young individuals. Moreover, the measurement of EAT volume could add value to a CAC=0 (power of zero) in young individuals.
Qualitative plaque burden, especially LAP, has shown to be the strongest predictor of cardiac events in the SCOT-HEART trial, even independent of CAC score [21]. The association of EAT with NCP and LAP is this young population represents a novel finding. The fact that the amount of CP by advanced plaque characterization did not differ between the subgroups with high vs low EAT could be related to an early stage of CAD but also to a potentially different atherosclerosis phenotype in these young high EAT patients.
In this young cohort, we found a relatively lower EAT volume predicting cut-off (>76 mL) in comparison with previous works. Nafakhi et al., studied an intermediate pretest probability CCTA cohort and found that an EAT >100 mL was significantly associated with plaque and obstructive CAD but not with CAC [35]. Similarly, Schlett et al., reported an EAT volume of 145 cm3, as the most accurate predictor for high-risk plaque and a volume <74 cm3 excluded the presence of coronary high-risk lesions [36]. It is possible that, as our population is significantly younger, with a low prevalence of obstructive CAD, our findings related to an earlier stage of atherosclerosis.
Regarding EAT attenuation, we did not find any association with CAD, however, as it has been shown, this relation is still controversial [37].
As EAT can be measured in non-contrast CT scans, it could potentially serve as an imaging biomarker even prior to the development of calcified plaque detected by CAC or as a maker of the increased risk for presence of non-calcified plaque in young patients below the threshold for statin initiation therapy. Imaging in young adults with measurement of EAT volume could potentially represent an unique opportunity for early initiation of intensive preventive therapy, aiming for plaque regression with potentially higher impact on ASCVD prevention [[38], [39], [40]]. Data from the PESA study by Mendieta et al. suggests that preventive interventions targeting smoking cessation, blood pressure, and LDL-C in particular in early stages of atherosclerosis in young adults can lead to significant plaque regression [41]. The chances of plaque regression were inversely related to fibrinogen levels, a marker of inflammation. Epicardial adipose tissue under certain conditions, can become a site of deranged adipogenesis, leading to production of proinflammatory adipokines [10,42] and a proinflammatory state. Similarly, it has been recently suggested that EAT volume and composition may change as a response to known cardiometabolic drugs [43]. In a BELLES (Beyond Endorse Lipid Lowering with Electrom Beam Tomography Scanning) sub-study, Alexopoulos et al. found that atorvastatin 80 mg led to a significant decrease in epicardial adipose tissue (EAT), independently of LDL-C reduction [44]. Moreover, Raggi et al. demonstrated statin-induced changes in EAT attenuation with statin therapy [45]. Furthermore, treatment of patients with type 2 diabetes mellitus with sodium-glucose cotransporter2 inhibitors [46] and glucagon-like peptide-1 receptor agonists [47,48] have shown changes in EAT volume, and it would be expected that such changes may also be seen in patients with obesity without diabetes who are treated with these agents [49]. Treatment of EAT as a novel risk factor may therefore represent a potential target for therapy of those at risk or with early stages of atherosclerosis and a potential surrogate for clinical trials in young adults where clinical endpoints are low. Further studies are needed on the identification of an integral high-risk imaging phenotype in young adults to guide therapeutic intervention, and whether treatment of EAT (or its associated risk factors) may prevent plaque progression or, ultimately more importantly, the development of CAD.
5. Limitations
Our study has several limitations. First, although our population is diverse and our healthcare system involves multiple centers from outpatient clinics to a quaternary medical center, our results may not be extrapolated to other populations. Second, there is likely to be a referral bias as our study included only symptomatic patients referred for CCTA. Third, the retrospective nature of the study implies the possibility of unmeasured confounders playing a role in the relation of EAT and CAD. Fourth, although data continues to accumulate in the realm of advanced plaque characterization with CCTA [50], more evidence is still needed in regards to standardization across different platforms or scan parameters and the impact in future events. Moreover, the optimal cutoff identified in our study will require external validation in further studies. Finally, this study was not sufficiently powered to provide information about outcomes that would have required a substantially bigger population and longer follow-up.
6. Conclusions
EAT volume was associated with the presence, extent, and quantitative burden of CAD, in young symptomatic adults from a diverse real-world cohort. The interplay between EAT and coronary atherosclerosis, even in patients with no calcific atherosclerotic disease, may represent a unique opportunity for preventive medical therapies.
Authors declaration
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
CRediT authorship contribution statement
Annalisa Filtz: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Daniel Lorenzatti: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation, Data curation, Conceptualization. Andrea Scotti: Writing – review & editing, Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Pamela Piña: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Carol Fernandez-Hazim: Investigation, Data curation. Dou Huang: Investigation, Data curation. Paul Ippolito: Investigation, Data curation. John P Skendelas: Investigation, Data curation. Toshiki Kuno: Investigation, Data curation. Carlos J Rodriguez: Writing – review & editing, Supervision, Investigation, Conceptualization. Aldo L Schenone: Writing – review & editing, Visualization, Methodology, Conceptualization. Azeem Latib: Writing – review & editing, Visualization, Supervision, Conceptualization. Carl J Lavie: Writing – review & editing, Visualization, Supervision, Conceptualization. Leslee J. Shaw: Writing – review & editing, Visualization, Supervision, Conceptualization. Ron Blankstein: Writing – review & editing, Visualization, Supervision, Conceptualization. Michael D Shapiro: Writing – review & editing, Visualization, Supervision, Conceptualization. Mario J Garcia: Writing – review & editing, Visualization, Supervision, Conceptualization. Daniel S Berman: Writing – review & editing, Visualization, Supervision, Conceptualization. Damini Dey: Writing – review & editing, Visualization, Supervision, Software, Resources, Investigation, Conceptualization. Salim S Virani: Writing – review & editing, Visualization, Supervision, Investigation, Conceptualization. Leandro Slipczuk: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
Annalisa Filtz, Daniel Lorenzatti, and Leandro Slipczuk are supported by institutional grants from Amgen and Philips. Damini Dey received grant support from the National Heart, Lung, and Blood Institute, software royalties from Cedars-Sinai Medical Center, and hold a patent (US8885905B2in USA and WO patent WO2011069120A1, Method and System for Plaque Characterization). Dr Lavie is a Consultant and Promotional Speaker for Amgen, a consultant to Novartis, and on a DSMB for NovoNordisk. Michael Shapiro reports grants (to institution) from PCORI, DCRI, Amgen, Boehringer Ingelheim, 89 Bio, Esperion, Genentech, Novartis, Ionis, Merck, New Amsterdam; Scientific Advisory Boards with Amgen, Agepha, Ionis, Novartis, Precision BioScience, Novo Nordisk, New Amsterdam; and as a Consultant with Ionis, Novartis, Regeneron, Aidoc, Shanghai Pharma Biotherapeutics, Kaneka. Others have nothing to disclose.
Funding
No funding was received for this project.
Footnotes
Short tweet (150 characters): Epicardial adipose tissue was associated with the presence, extent, and burden of CAD in a diverse young symptomatic patient cohort.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2024.100711.
Appendix. Supplementary materials
References
- 1.Rallidis L.S., Xenogiannis I., Brilakis E.S., Bhatt D.L. Causes, angiographic characteristics, and management of premature myocardial infarction. J Am Coll Cardiol. 2022;79:2431–2449. doi: 10.1016/j.jacc.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Stone N.J., Smith S.C., Orringer C.E., et al. Managing atherosclerotic cardiovascular risk in young adults: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:819–836. doi: 10.1016/j.jacc.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Singh A., Collins B.L., Gupta A., et al. Cardiovascular risk and statin eligibility of young adults after an MI: partners YOUNG-MI registry. J Am Coll Cardiol. 2018;71:292–302. doi: 10.1016/j.jacc.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichikawa K., Susarla S., Budoff M.J. The use of coronary artery calcium scoring in young adults. J Cardiovasc Comput Tomogr. 2023;17:242–247. doi: 10.1016/j.jcct.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sama C., Abdelhaleem A., Velu D., et al. Non-calcified plaque in asymptomatic patients with zero coronary artery calcium score: a systematic review and meta-analysis. J Cardiovasc Comput Tomogr. 2023;S1934-5925(23) doi: 10.1016/j.jcct.2023.10.002. 00438–0. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen M.B., Gaur S., Frimmer A., et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7:36. doi: 10.1001/jamacardio.2021.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agha A.M., Pacor J., Grandhi G.R., et al. The prognostic value of CAC zero among individuals presenting with chest pain: a meta-analysis. JACC Cardiovasc Imaging. 2022;15:1745–1757. doi: 10.1016/j.jcmg.2022.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzatti D., Piña P., Huang D., et al. Interaction between risk factors, coronary calcium, and CCTA plaque characteristics in patients age 18-45. Eur Heart J Cardiovasc Imaging. 2024 doi: 10.1093/ehjci/jeae094. In press. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen M.B., Dzaye O., Bødtker H., et al. Interplay of risk factors and coronary artery calcium for CHD risk in young patients. JACC: Cardiovascul Imaging. 2021;14:2387–2396. doi: 10.1016/j.jcmg.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19:593–606. doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scotti A., Slipczuk L., Latib A. Aortic stenosis and heart failure with preserved ejection fraction: a shared phenotypic puzzle. Eur J Heart Fail. 2023;25:1959–1961. doi: 10.1002/ejhf.3050. [DOI] [PubMed] [Google Scholar]
- 12.Foldyna B., Zeleznik R., Eslami P., et al. Epicardial adipose tissue in patients with stable chest pain: insights from the PROMISE trial. JACC Cardiovasc Imaging. 2020;13:2273–2275. doi: 10.1016/j.jcmg.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Kuno T., Arce J., Fattouh M., et al. Cardiometabolic predictors of high-risk CCTA phenotype in a diverse patient population. Am J Prev Cardiol. 2023;15 doi: 10.1016/j.ajpc.2023.100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancio J., Azevedo D., Saraiva F., et al. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2018;19:490–497. doi: 10.1093/ehjci/jex314. [DOI] [PubMed] [Google Scholar]
- 15.Mancio J., Pinheiro M., Ferreira W., et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int J Cardiol. 2017;249:419–425. doi: 10.1016/j.ijcard.2017.09.178. [DOI] [PubMed] [Google Scholar]
- 16.Gulati M., Levy P.D., Mukherjee D., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain. J Cardiovasc Comput Tomogr. 2022;16:54–122. doi: 10.1016/j.jcct.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Abbara S., Blanke P., Maroules C.D., et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2016;10:435–449. doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Ansari S., Pourafkari L., Kinninger A., Manubolu V., Budoff M.J. Risk stratifying individuals with zero, minimal, and mild coronary artery calcium for cardiovascular disease by determining coronary plaque burden. J Cardiovasc Comput Tomogr. 2023 doi: 10.1016/j.jcct.2023.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Leipsic J., Abbara S., Achenbach S., et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8:342–358. doi: 10.1016/j.jcct.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Min J.K., Shaw L.J., Devereux R.B., et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 21.Williams M.C., Kwiecinski J., Doris M., et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART) Circulation. 2020;141:1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rosendael A.R., Lin F.Y., Ma X., et al. Percent atheroma volume: optimal variable to report whole-heart atherosclerotic plaque burden with coronary CTA, the PARADIGM study. J Cardiovasc Comput Tomogr. 2020;14:400–406. doi: 10.1016/j.jcct.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Nakazato R., Shmilovich H., Tamarappoo B.K., et al. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr. 2011;5:172–179. doi: 10.1016/j.jcct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey D., Wong N.D., Tamarappoo B., et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosito G.A., Massaro J.M., Hoffmann U., et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 26.McClain J., Hsu F., Brown E., et al. Pericardial adipose tissue and coronary artery calcification in the Multi-ethnic Study of Atherosclerosis (MESA) Obes (Silver Spring) 2013;21:1056–1063. doi: 10.1002/oby.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahabadi A.A., Lehmann N., Kälsch H., et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging. 2014;7:909–916. doi: 10.1016/j.jcmg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Bos D., Shahzad R., van Walsum T., et al. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur Heart J Cardiovasc Imaging. 2015;16:1264–1269. doi: 10.1093/ehjci/jev086. [DOI] [PubMed] [Google Scholar]
- 29.Cheng V.Y., Dey D., Tamarappoo B., et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg E., McElhinney P.A., Commandeur F., et al. Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.119.009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West H.W., Siddique M., Williams M.C., et al. Deep-learning for epicardial adipose tissue assessment with computed tomography: implications for cardiovascular risk prediction. JACC Cardiovasc Imaging. 2023;16:800–816. doi: 10.1016/j.jcmg.2022.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Versteylen M.O., Takx R.A.P., Joosen I.A.P.G., et al. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. Eur Heart J Cardiovasc Imaging. 2012;13:517–523. doi: 10.1093/ehjci/jes024. [DOI] [PubMed] [Google Scholar]
- 33.Nerlekar N., Brown A.J., Muthalaly R.G., et al. Association of epicardial adipose tissue and high-risk plaque characteristics: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori H., Torii S., Kutyna M., Sakamoto A., Finn A.V., Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11:127–142. doi: 10.1016/j.jcmg.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Nafakhi H., Al-Mosawi A., Al-Nafakh H., Tawfeeq N. Association of pericardial fat volume with coronary atherosclerotic disease assessed by CT angiography. Br J Radiol. 2014;87 doi: 10.1259/bjr.20130713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlett C.L., Ferencik M., Kriegel M.F., et al. Association of pericardial fat and coronary high-risk lesions as determined by cardiac CT. Atherosclerosis. 2012;222:129–134. doi: 10.1016/j.atherosclerosis.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monti C.B., Capra D., Zanardo M., et al. CT-derived epicardial adipose tissue density: systematic review and meta-analysis. Eur J Radiol. 2021;143 doi: 10.1016/j.ejrad.2021.109902. [DOI] [PubMed] [Google Scholar]
- 38.Pencina M.J., Pencina K.M., Lloyd-Jones D., Catapano A.L., Thanassoulis G., Sniderman A.D. The expected 30-year benefits of early versus delayed primary prevention of cardiovascular disease by lipid lowering. Circulation. 2020;142:827–837. doi: 10.1161/CIRCULATIONAHA.120.045851. [DOI] [PubMed] [Google Scholar]
- 39.Domanski M.J., Tian X., Wu C.O., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro M.D., Bhatt D.L. “Cholesterol-years” for ASCVD risk prediction and treatment. J Am Coll Cardiol. 2020;76:1517–1520. doi: 10.1016/j.jacc.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Mendieta G., Pocock S., Mass V., et al. Determinants of progression and regression of subclinical atherosclerosis over 6 years. J Am Coll Cardiol. 2023;82:2069–2083. doi: 10.1016/j.jacc.2023.09.814. [DOI] [PubMed] [Google Scholar]
- 42.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 43.Myasoedova V.A., Parisi V., Moschetta D., et al. Efficacy of cardiometabolic drugs in reduction of epicardial adipose tissue: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22:23. doi: 10.1186/s12933-023-01738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexopoulos N., Melek B.H., Arepalli C.D., et al. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning) J Am Coll Cardiol. 2013;61:1956–1961. doi: 10.1016/j.jacc.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 45.Raggi P., Gadiyaram V., Zhang C., Chen Z., Lopaschuk G., Stillman A.E. Statins reduce epicardial adipose tissue attenuation independent of lipid lowering: a potential pleiotropic effect. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato T., Aizawa Y., Yuasa S., et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutour A., Abdesselam I., Ancel P., et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabet Obes Metab. 2016;18:882–891. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 48.Iacobellis G., Villasante Fricke A.C. Effects of semaglutide versus dulaglutide on epicardial fat thickness in subjects with type 2 diabetes and obesity. J Endocr Soc. 2020;4:bvz042. doi: 10.1210/jendso/bvz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 50.Williams M.C., Earls J.P., Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2022;16:124–137. doi: 10.1016/j.jcct.2021.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.