Abstract
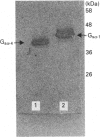
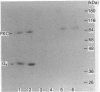
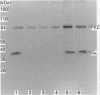
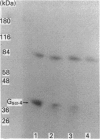
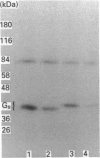
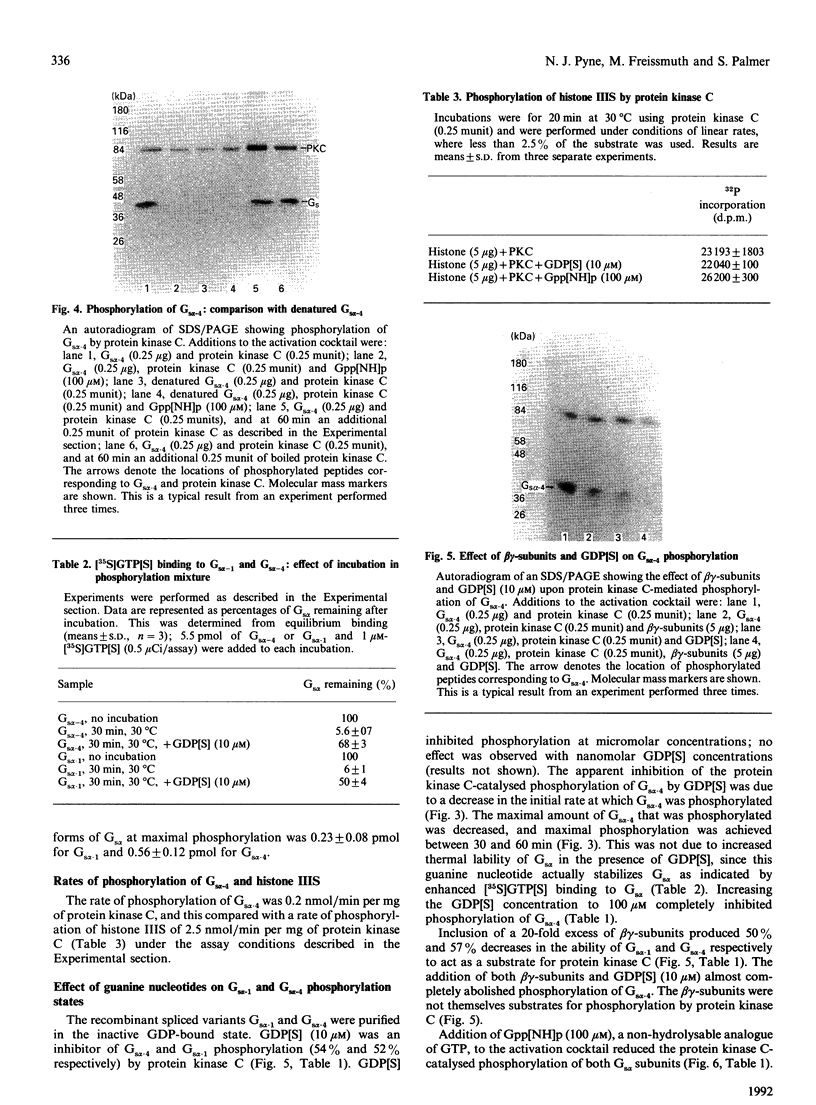
Recombinant forms of Gs alpha-1 and Gs alpha-4 were shown to act as substrates for a purified preparation of brain protein kinase C. Both forms of Gs alpha were thermally denatured during the incubation such that phosphorylation was virtually complete (greater than 90%) after 30 min. The quantity of phosphate incorporated into approximately equivalent starting amounts of the two forms of Gs alpha (4.8 pmol of Gs alpha-1 and 5.5 pmol of Gs alpha-4) at maximal phosphorylation were 0.23 +/- 0.08 pmol for Gs alpha-1 and 0.56 +/- 0.12 pmol for Gs alpha-4. Since both forms of Gs alpha were thermally denatured to the same extent after 30 min, the increased phosphorylation state of Gs alpha-4 provides evidence that Gs alpha-4 contains an additional phosphorylation site. Bray and co-workers [Bray, Carter, Simmons, Guo, Puckett, Kamhollz, Spiegel & Nirenberg (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 8893-8897] proposed that an additional phosphorylation site may exist at the splice junction in Gs alpha-4. The guanine-nucleotide-free form of Gs alpha appears to be the preferred substrate for phosphorylation. This interpretation is based upon the following observations. (i) Guanosine 5'-[beta-thio]diphosphate at micromolar concentrations inhibits the susceptibility of Gs alpha to phosphorylation; (ii) beta gamma-subunits, which inhibit GDP release from Gs alpha-GDP at millimolar Mg2+ concentrations, also inhibit the susceptibility of Gs alpha to phosphorylation; and (iii) guanosine 5'[beta gamma-imido]triphosphate inhibits the susceptibility of Gs alpha to act as a substrate for phosphorylation. These studies suggest that there is potential for cross-talk between receptors which trigger PtdIns(4,5)P2 hydrolysis and subsequently protein kinase C activation, and receptors which stimulate adenylate cyclase via Gs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L. Transduction of receptor signal into modulation of effector activity by G proteins: the first 20 years or so .... FASEB J. 1990 Nov;4(14):3178–3188. doi: 10.1096/fasebj.4.14.2172060. [DOI] [PubMed] [Google Scholar]
- Brandt D. R., Ross E. M. GTPase activity of the stimulatory GTP-binding regulatory protein of adenylate cyclase, Gs. Accumulation and turnover of enzyme-nucleotide intermediates. J Biol Chem. 1985 Jan 10;260(1):266–272. [PubMed] [Google Scholar]
- Braun S., Tolkovsky A. M., Levitzki A. Mechanism of control of the turkey erythrocyte beta-adrenoceptor dependent adenylate cyclase by guanyl nucleotides: a minimum model. J Cyclic Nucleotide Res. 1982;8(3):133–147. [PubMed] [Google Scholar]
- Bray P., Carter A., Simons C., Guo V., Puckett C., Kamholz J., Spiegel A., Nirenberg M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Griffiths S. L., Murphy G. J., Pyne N. J., Knowler J. T., Milligan G., Parker P. J., Mollner S., Houslay M. D. Diabetes-induced alterations in the expression, functioning and phosphorylation state of the inhibitory guanine nucleotide regulatory protein Gi-2 in hepatocytes. Biochem J. 1990 Oct 15;271(2):365–372. doi: 10.1042/bj2710365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushfield M., Murphy G. J., Lavan B. E., Parker P. J., Hruby V. J., Milligan G., Houslay M. D. Hormonal regulation of Gi2 alpha-subunit phosphorylation in intact hepatocytes. Biochem J. 1990 Jun 1;268(2):449–457. doi: 10.1042/bj2680449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione R. A., Staniszewski C., Benovic J. L., Lefkowitz R. J., Caron M. G., Gierschik P., Somers R., Spiegel A. M., Codina J., Birnbaumer L. Specificity of the functional interactions of the beta-adrenergic receptor and rhodopsin with guanine nucleotide regulatory proteins reconstituted in phospholipid vesicles. J Biol Chem. 1985 Feb 10;260(3):1493–1500. [PubMed] [Google Scholar]
- Cronin M. J., Summers S. T., Sortino M. A., Hewlett E. L. Protein kinase C enhances growth hormone releasing factor (1-40)-stimulated cyclic AMP levels in anterior pituitary. Actions of somatostatin and pertussis toxin. J Biol Chem. 1986 Oct 25;261(30):13932–13935. [PubMed] [Google Scholar]
- Daniel-Issakani S., Spiegel A. M., Strulovici B. Lipopolysaccharide response is linked to the GTP binding protein, Gi2, in the promonocytic cell line U937. J Biol Chem. 1989 Dec 5;264(34):20240–20247. [PubMed] [Google Scholar]
- Dobson P. R., Brown B. L., Michelangeli V. P., Short A. D., Moseley J. M., Russell R. G., Martin T. J. Interactive regulation of signalling pathways in bone cells: possible modulation of PGE2-stimulated adenylyl cyclase activity by protein kinase C. Biochim Biophys Acta. 1990 May 2;1052(2):323–326. doi: 10.1016/0167-4889(90)90228-6. [DOI] [PubMed] [Google Scholar]
- Freissmuth M., Gilman A. G. Mutations of GS alpha designed to alter the reactivity of the protein with bacterial toxins. Substitutions at ARG187 result in loss of GTPase activity. J Biol Chem. 1989 Dec 25;264(36):21907–21914. [PubMed] [Google Scholar]
- Freissmuth M., Selzer E., Marullo S., Schütz W., Strosberg A. D. Expression of two human beta-adrenergic receptors in Escherichia coli: functional interaction with two forms of the stimulatory G protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8548–8552. doi: 10.1073/pnas.88.19.8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graziano M. P., Freissmuth M., Gilman A. G. Expression of Gs alpha in Escherichia coli. Purification and properties of two forms of the protein. J Biol Chem. 1989 Jan 5;264(1):409–418. [PubMed] [Google Scholar]
- Houslay M. D. 'Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur J Biochem. 1991 Jan 1;195(1):9–27. doi: 10.1111/j.1432-1033.1991.tb15671.x. [DOI] [PubMed] [Google Scholar]
- Itoh H., Gilman A. G. Expression and analysis of Gs alpha mutants with decreased ability to activate adenylylcyclase. J Biol Chem. 1991 Aug 25;266(24):16226–16231. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Kitano T., Go M., Kikkawa U., Nishizuka Y. Assay and purification of protein kinase C. Methods Enzymol. 1986;124:349–352. doi: 10.1016/0076-6879(86)24026-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattera R., Graziano M. P., Yatani A., Zhou Z., Graf R., Codina J., Birnbaumer L., Gilman A. G., Brown A. M. Splice variants of the alpha subunit of the G protein Gs activate both adenylyl cyclase and calcium channels. Science. 1989 Feb 10;243(4892):804–807. doi: 10.1126/science.2536957. [DOI] [PubMed] [Google Scholar]
- Murphy G. J., Gawler D. J., Milligan G., Wakelam M. J., Pyne N. J., Houslay M. D. Glucagon desensitization of adenylate cyclase and stimulation of inositol phospholipid metabolism does not involve the inhibitory guanine nucleotide regulatory protein Gi, which is inactivated upon challenge of hepatocytes with glucagon. Biochem J. 1989 Apr 1;259(1):191–197. doi: 10.1042/bj2590191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Smigel M. D., Gilman A. G. Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem. 1986 Jul 25;261(21):9587–9590. [PubMed] [Google Scholar]