Highlights
-
•
The foreign population had a lower crude cancer mortality rate than the Colombian population.
-
•
However, the age-adjusted cancer mortality rate among the foreign population was higher compared to the Colombian population.
-
•
The proportional cancer mortality was lower among foreign population compared to the Colombian population.
Keywords: Colombian, Foreign, Immigrant, Cancer mortality, Latin America
Abstract
Purpose
We aimed to compare cancer mortality among foreign- and Colombian populations in Colombia during the period of 2006–2020.
Methods
This retrospective study utilized vital statistics from the Colombian National Department of Statistics (DANE). The dataset included variables such as age group, sex, country of permanent residency, insurance, education level, marital status, ethnicity, and cause of death. The population data to calculate rates was obtained from the Colombian census and the United Nations. Crude and adjusted rates as well as proportional mortality rates were calculated.
Results
A total of 561,932 cancer deaths occurred in Colombia from 2006 to 2020. The foreign population (country of permanent residency different to Colombia) had a lower crude cancer mortality rate (31.1 per 100,000 inhabitants) than the Colombian population (81.9 per 100,000 inhabitants). However, the age-adjusted cancer mortality rate among the foreign population was 253.6 per 100,000, compared to 86.1 per 100,000 among the Colombian population. The proportional cancer mortality was 10.4 % among foreign population compared to 17.4 % among Colombian population.
Conclusions
The proportional cancer mortality shows that the proportion of cancer-related deaths is greater among the Colombian population compared to the immigrant population. However, immigrants in Colombia have a higher age-adjusted cancer mortality rate than Colombians, indicating that immigrants have worse cancer outcomes than the Colombians even though the immigrant population is younger. This is likely due to the frequent barriers that immigrants encounter in accessing health care in Colombia. Future research needs to focus on access to care for the immigrant population by investigating cancer-related risk factors among immigrants and addressing their barriers to cancer prevention and treatment.
1. Background
Global cancer incidence and mortality are rising (Sung et al., 2021; Bray et al., 2018). In 2020, GLOBOCAN estimated 19.3 million new cancers and 10 million cancer deaths globally (Sung et al., 2021). Although cancer incidence rates were higher in countries that passed through epidemiological transitions compared to transitioning countries, cancer mortality rates for breast and cervical cancers were higher in transitioning countries (Sung et al., 2021). The global cancer burden is expected to increase for transitioning countries than transitioned countries (Sung et al., 2021; Lortet-Tieulent et al., 2020). The increase in cancer incidence is seen in Latin America with Colombia as one of the highest rate of cancer incidence increase (Integrated Cancer Control Initiative in Latin America 2021).
International migration is a growing challenge to health systems worldwide. Colombia is experiencing major migration influx from neighboring countries, such as Venezuela, with a younger immigrant population (Arango, 2021). As of 2020, Colombia had accepted approximately 1.8 million Venezuelan immigrants; however, that number is likely much higher due to undocumented migration (Bitar, 2022). The impact of migration on the health of native and migrant populations is not well understood for many diseases. Cancer mortality studies comparing native-born to foreign populations have been conducted in high-income countries such as Australia, Canada, Finland, Spain, Sweden, the Netherlands, and the United States (Abdoli et al., 2014; Oliva-Arocas et al., 2020; Hallowell et al., 2019; Kyrönlahti et al., 2020; Cheung et al., 2017; Stirbu et al., 2006; Feletto and Sitas, 2015). There have been fewer studies examining cancer mortality between native-born and foreign populations in low- and middle-income countries (LMICs). The only study in a LMIC looking at all cancers was conducted in Chile and reported that hospital cancer mortality rate was much higher in Chileans than immigrants (Oyarte et al., 2018).
Multiple factors influence morbidity and mortality among immigrants. Most studies conducted in high income countries have reported that immigrants have a lower cancer mortality than the native population (Abdoli et al., 2014; Oliva-Arocas et al., 2020; Hallowell et al., 2019; Kyrönlahti et al., 2020; Cheung et al., 2017; Stirbu et al., 2006). Even though there is no sufficient information about these factors in Colombia, it is expected that besides migration status (Murillo-Pedrozo et al., 2021), multiple barriers reported for accessing health care among Colombians such as fragmented health system, economic, geographic, and administrative barriers also negatively impact the access to health care for immigrants (Sardi et al., 2019; Vargas et al., 2010; Vargas et al., 2016; Cucunubá et al., 2017).
With cancer mortality on the rise in recent years and as the second cause of mortality from all causes, cancer control is a priority in Colombia (Integrated Cancer Control Initiative in Latin America 2021). Colombia's National 10-year Plan for Cancer Control focuses on reducing risk factors and improving cancer early detection (Integrated Cancer Control Initiative in Latin America 2021). However, cancer control for immigrants in Colombia has not been studied or addressed as data on immigrants is limited. Although there have been studies examining cancer incidence and mortality in Colombia, to our knowledge, there have not been any studies comparing cancer mortality between Colombian and foreign populations. The aim of this study was to compare cancer mortality rates between the Colombian population and the foreign population in Colombia from the period of 2006 to 2020.
2. Methods
2.1. Study design and data sources
Colombia's National Department of Statistics (DANE) collects mortality information from death certificates that are either certified by medical staff, authorized health personnel or medical examiners. DANE has annual vital statistics publicly available from 1979 to 2020. In this dataset, the following variables were included: country of habitual residence, age group, ethnicity, education, marital status, insurance type, and cancer type. Our database included other variables such as place of death, direct cause of death, and how the death was determined. However, these variables were not used in data analysis.
To examine mortality rates, proportional cancer mortality rates, and age-adjusted cancer mortality rates among Colombian and foreign populations, we analyzed mortality micro-data from 2006 to 2020 from DANE (Departamento Administrativo Nacional Estadistica). To calculate cancer mortality rates of Colombian, we used population estimates and projections from the national census. For the foreign population, we used data from the Population Division of the United Nations (UN) (United Nations Department of Economic and Social Affairs Population Division 2022). The UN calculated international migration based on foreign populations from census, estimates from the differences between overall population growth and natural increase, and the number of refugees from United Nations High Commissioner for Refugees (UNHCR) statistics (United Nations Department of Economic and Social Affairs Population Division 2022). Additionally, we used a demographic profile on Venezuelan immigrants in Colombia reported by DANE for the age distribution of immigrants (Arango, 2021). For this study, the terms foreign, immigrants, and international migrants are used interchangeably.
2.2. Data management and statistical analysis
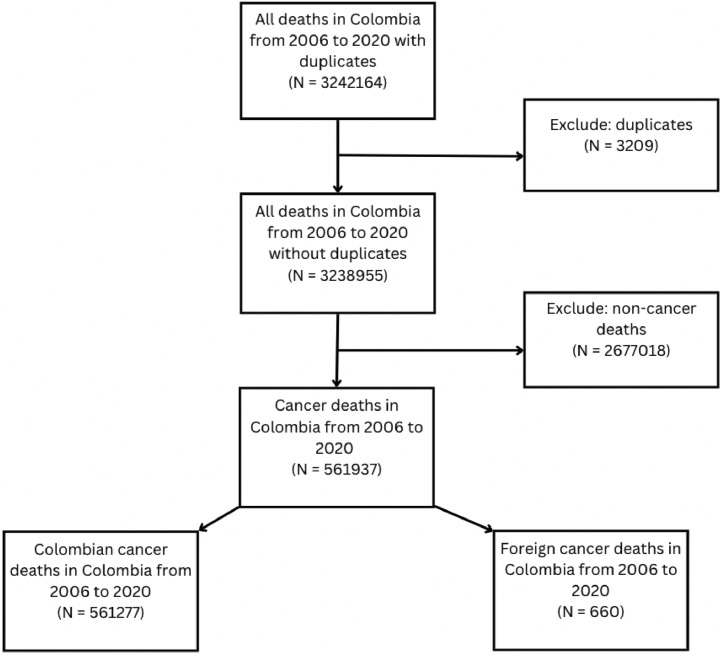
On the DANE website, we downloaded each mortality dataset by year, containing 35 variables. First, we transformed variables that had different categories across datasets to merge all years into one dataset. We eliminated duplicates that were completely the same in all variables. Then, we created a final dataset with only cancer-related deaths using the basic cause of death codes from ICD-10 (C00 to C97). Fig. 1 shows the number of cases at each stage of data management.
Fig. 1.
Data management from original dataset of mortality from 2006 to 2020 to the final dataset used for data analysis. This figure was created using Canva.
Marital status had 6 categories: “not married and living with partner for 2 or more years”, “not married and living with partner for less than 2 years”, “separated or divorced”, “widow”, “single”, and “married”. These categories were grouped into 3: “in a relationship”; “divorced, widowed, or separated”; and “single”. Those who were not married and living with their partner for 2 or more years were combined with those who were married. Those who were not married and living with partners for less than 2 years were combined with singles.
Age had 6 categories: “less than one year”, “1–4″, “5–14″, “15–44″, “45–64″, and “65 years and older”. These categories were grouped into 4 categories: less than one year-14, 15–44, 45–64, and 65+.
Education had 12 categories: preschool, primary completed, primary incomplete, secondary completed, secondary incomplete, undergraduate completed, undergraduate incomplete, specialization, Master's, doctorate, or no education. These categories were grouped into 4 categories: preschool or no education, primary, secondary, and undergraduate or more education.
Ethnicity originally had indigenous, Rom, Raizal from the Archipelago of San Andres and Providencia, Palenquero from San Basilio, Black or Afrocolombian, and none of the above. These categories were grouped into 2 categories: having an ethnicity and not having an ethnicity.
Insurance originally had 6 categories: contributive (private), subsidized (public), uninsured, out of pocket, other, and ignored (no answer). These categories were grouped into four categories: contributive, subsidized, uninsured or out of pocket, and other. Ignored became part of missing cases.
ICD-10 codes were categorized as follow: malignancies of the lip, oral cavity and pharynx (C00-C14), malignancies of digestive organs (C15-C26), malignancies of respiratory and intra-thoracic organs (C30-C39), malignancies of bones and articular cartilage (C40-C41), melanoma and other malignant skin tumors (C43-C44), malignancies of mesothelial tissues and soft tissues (C45-C49), malignancies of the breast (C50), malignancies of female genital organs (C51-C58), malignancies of male genital organs (C60-C63), malignancies of the urinary tract (C64-C68), malignancies of the eye, brain, and other parts of the central nervous system (C69-C72), malignancies of thyroid gland and other endocrine glands (C73-C75), ill-defined, secondary, and unspecified sites (C76-C80), maligncies of lymphatic tissue, hematopoietic organs, and related tissues (C81-C96), and malignant (primary) tumors of independent multiple sites (C97).
We also created the following categories to explore if infections played a role in cancer mortality among immigrants: oral (C10), liver (C22), stomach (C16), anal (C21), head and neck (C76.0), and cervical (C53) cancers.
Some cases (N = 1880) had missing “country of habitual residence” but reported data in the variable “department of habitual residence (this is a political division of the Colombian territory similar to the concept of states in other countries), these cases were given Colombia as the country of origin; thus, creating a new variable. Based on the new variable of country of origin, a dichotomous variable was created to compare Colombian to foreign. Those cases with a country code of 170 were coded as Colombian, while the rest were coded as foreign.
A chi squared test was conducted using Statistical Package for the Social Sciences (SPSS) to determine if there was a significance difference between each variable when comparing Colombian and foreign populations.
The crude cancer mortality rates of Colombian and foreign individuals were calculated as follows:
The proportional mortality rates of Colombian and foreign individuals were calculated as follows:
We calculated age-adjusted rates using direct standardization method using Epidat (Consellería de Sanidade 2016). The age groups used for the Colombian and standard populations were: 0 to 14 years, 15 to 44 years, 45 to 64 years, and 65 and older. The population by age groups for Colombian were obtained from estimates and projections from national census data from DANE, and using WHO data for the world standard population (Ahmad et al., 2001). Because of lack of data on the age distribution of immigrant population in Colombia, we used a demographic profile done on Venezuelan immigrants in Colombia from DANE, which only included percentages of each age group for the years 2014 to 2019 (Arango, 2021). Therefore, we used 2014′s age distribution for 2006 to 2013 analysis and 2019′s age distribution to calculate 2020. The age groups for immigrants in the report differed from that of the Colombian and standard population. The groups were as followed: 0 to 20 years, 21 to 45 years, 46 to 65 years, and 65 and older. The first two age groups differed by a few years but given the lack of data, we assumed those were the closest approximations we could obtain with the available data on immigrants.
3. Results
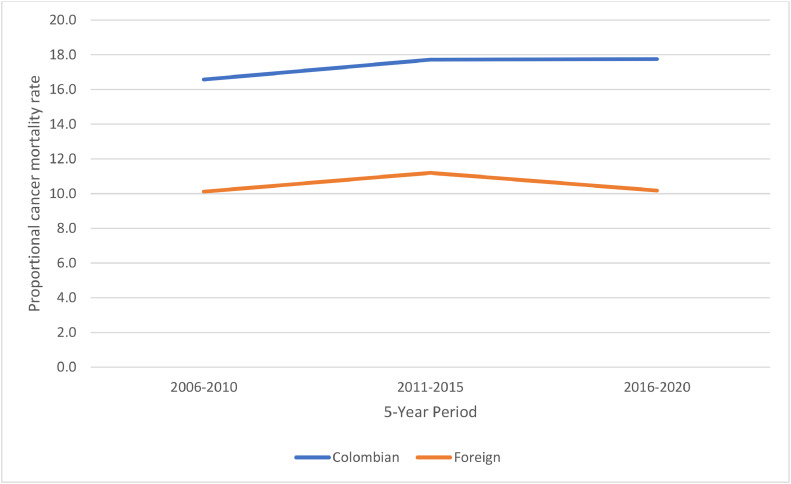
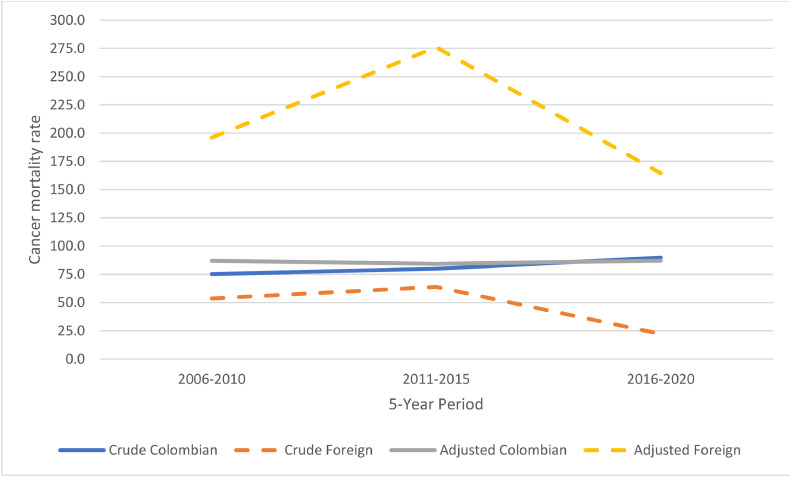
From 2006 to 2020, a total of 561,932 deaths from cancers occurred in Colombia, of these 660 occurred in foreign population. Out of 660 foreign deaths, the main countries that immigrants came from were as follows: Venezuela (40.8 %), Aruba (10.8 %), United States (9.7 %), the Netherlands (5.3 %), and Ecuador (3.2 %). Crude and proportional cancer mortality rates increased steadily among the Colombian population, while sharp fluctuations were observed among the immigrant population (Fig. 2, Fig. 3). Overall, the foreign population had a lower crude cancer mortality rate (31.1 per 100,000 persons) than the Colombian population (81.9 per 100,000 persons). Similarly, the proportional cancer mortality rate was lower among foreign population (10.4 % vs. 17.4 %) (Fig. 2). However, age-adjusted cancer mortality rate showed a much higher rate among the immigrant population (253.6 per 100,000) when compared to the Colombian population (86.1 per 100,000) (Fig. 3).
Fig. 2.
Proportional cancer mortality rate by 5-year periods and country of origin. This figure was created using Excel.
Fig. 3.
Crude and age-adjusted cancer mortality rate among Colombian and foreign by 5-year periods. This figure was created using Excel.
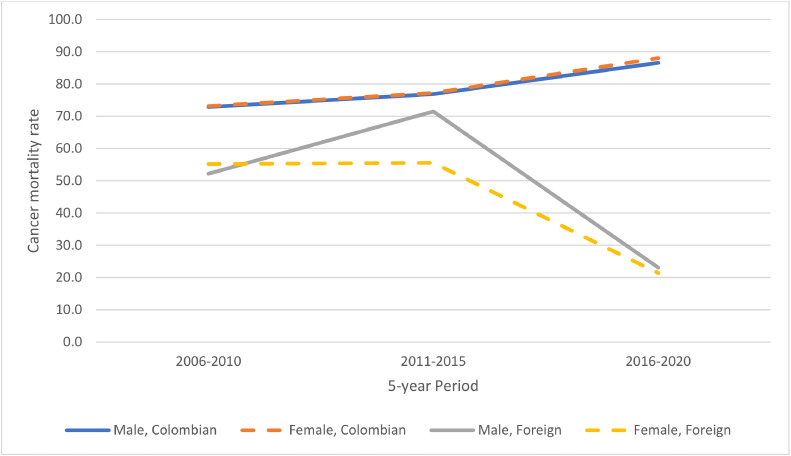
Regarding sex, there was a significant higher number of cancer deaths among foreign men than foreign women (p < 0.031). We also analyzed crude mortality rate by sex (Fig. 4). In terms of age, there was more cancer deaths among older adults in the Colombian than the foreign population (p < 0.001). In regard to ethnicity, there was a greater percentage of foreign individuals (10.9 %) compared to Colombian individuals (5.5 %) that had one of the following ethnicities: indigenous, Rom, Raizal from the Archipelago of San Andres and Providencia, Palenquero from San Basilio, Black or Afrocolombian. A greater percentage of foreign individuals had either none or only primary education compared to Colombian individuals (p < 0.001) (Table 1). More foreign individuals also had undergraduate or higher education than Colombian individuals (p < 0.001) (Table 1).
Fig. 4.
Crude cancer mortality among Colombian and foreign by 5-year periods and sex. This figure was created using Excel.
Table 1.
Socio-demographic characteristics of 561,932 cancer mortality cases in Colombia by habitual residence.
| Variable | Total population (N = 561,932) | Colombian (N = 561,277) | Foreign (N = 660) | p* |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Age groups (in years) | N=561,672 | N=561,015 | N=657 | <0.001 |
| Less than 1 to 14 | 7680 (1.4) | 7657 (1.4) | 23 (3.5) | |
| From 15 to 44 | 51,867 (9.2) | 51,723 (9.2) | 144 (21.9) | |
| From 45 to 64 | 170,479 (30.4) | 170,229 (30.3) | 250 (38.1) | |
| From 65 and older | 331,646 (59.0) | 331,406 (59.1) | 240 (36.5) | |
| Marital status | N=456,117 | N=455,615 | N=502 | <0.001 |
| Single | 92,412 (20.3) | 92,271 (20.3) | 141 (28.1) | |
| In a relationship | 238,298 (52.2) | 238,018 (52.2) | 280 (55.8) | |
| Separated, widowed, or divorced | 125,407 (27.5) | 125,326 (27.5) | 81 (16.1) | |
| Education | N=442,419 | N=442,019 | N=400 | <0.001 |
| Preschool or none | 146,998 (33.2) | 146,852 (33.2) | 146 (36.5) | |
| Primary | 239,697 (54.2) | 239,568 (54.2) | 129 (32.3) | |
| Secondary | 46,226 (10.4) | 46,124 (10.4) | 102 (25.5) | |
| Undergraduate or higher | 9498 (2.1) | 9475 (2.1) | 23 (5.8) | |
| Ethnicity | N=478,452 | N=477,866 | N=586 | <0.001 |
| Yes | 26,121 (5.5) | 26,057 (5.5) | 64 (10.9) | |
| No | 452,331 (94.5) | 451,809 (94.5) | 522 (89.1) | |
| Insurance type | N=557,806 | N=557,159 | N=647 | <0.001 |
| Contributive | 265,948 (47.7) | 265,796 (47.7) | 152 (23.5) | |
| Subsidized | 244,802 (43.9) | 244,715 (43.9) | 87 (13.4) | |
| Uninsured or out of pocket | 29,517 (5.3) | 29,481 (5.3) | 36 (5.6) | |
| Other | 17,539 (3.1) | 17,167 (3.1) | 372 (57.5) | |
| Sex | N=561,932 | N=561,277 | N=660 | <0.031 |
| Male | 276,193 (49.2) | 275,841 (49.1) | 352 (53.3) | |
| Female | 285,744 (50.8) | 285,436 (50.9) | 308 (46.7) |
Note: *The p-values provided describe the level of significance for the comparisons between Colombian and foreign individuals. Having an ethnicity includes the following ethnicities: indigenous, Rom, Raizal from the Archipelago of San Andres and Providencia, Palenquero from San Basilio, and Black or Afrocolombian.
Cancer types between Colombian and foreign individuals was significantly different (Table 2). For foreign individuals, the following types were the most common cause of death: first, malignancies of the digestive organs (26.7 %); second, malignancies of lymphatic tissue, hematopoietic organs and related tissues (18.6 %); third, malignancies of the female genital organs (12.4 %); and fourth, malignancies of the respiratory and intrathoracic organs (11.4 %) (Table 2). For Colombians, the following cancers were the most common cause of death: first, malignancies of digestive organs (35.4 %); second, malignancies of respiratory and intrathoracic organs (12.9 %); third, malignancies of lymphatic tissue, hematopoietic organs and related tissues (9.6 %); and fourth, malignancies of female genital organs (8.5 %) (Table 2).
Table 2.
Cancer mortality cases in Colombia by cancer type and habitual residence.
| Variable | Total population (N = 561,932) | Colombian (N = 561,277) | Foreign (N = 660) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Cancer type | |||
| Malignant tumors of the digestive organs (C15-C26) | 198,947 (35.4) | 198771a (35.4) | 176b (26.7) |
| Malignant tumors [neoplasms] of lymphatic tissue, hematopoietic organs, and related tissues (C81-C96) | 54,104 (9.6) | 53981a (9.6) | 123b (18.6) |
| Malignant tumors of the female genital organs (C51-C58) | 48,047 (8.6) | 47965a (8.5) | 82b (12.4) |
| Malignant tumors of the respiratory and intrathoracic organs (C30-C39) | 72,729 (12.9) | 72654a (12.9) | 75a (11.4) |
| Malignant tumor of the breast (C50) | 41,182 (7.3) | 41138a (7.3) | 44a (6.7) |
| Malignant tumors [neoplasms] of ill-defined, secondary, and unspecified sites (C76-C80) | 30,051 (5.3) | 30013a (5.3) | 38a (5.8) |
| Malignant tumors of the eye, brain, and other parts of the central nervous system (C69-C72) | 17,282 (3.1) | 17253a (3.1) | 29b (4.4) |
| Malignant tumors of the urinary tract (C64-C68) | 15,001 (2.7) | 14979a (2.7) | 22a (3.3) |
| Malignant tumors of the male genital organs (C60-C63) | 44,420 (7.9) | 44404a (7.9) | 16b (2.4) |
| Malignant tumors of the lip, oral cavity and pharynx (C00-C14) | 9187 (1.6) | 9173a (1.6) | 14a (2.1) |
| Melanoma and other malignant skin tumors (C43-C44), Malignant tumors of mesothelial tissues and soft tissues | 11,061 (2.0) | 11047a (2.0) | 14a (2.1) |
| (C45-C49) | 8133 (1.4) | 8123a (1.4) | 10a (1.5) |
| Malignant tumors of the thyroid gland and other endocrine glands (C73-C75) | 5769 (1.0) | 5759a (1.0) | 10a (1.5) |
| Malignant tumors of the bones and articular cartilage (C40-C41) | 4573 (0.8) | 4567a (0.8) | 6a (0.9) |
| Malignant (primary) tumors [neoplasms] of independent multiple sites (C97) | 1451 (0.3) | 1450a (0.3) | 1a (0.2) |
Note: Chi-square was used for the comparison between Colombian and foreign individuals had a p < 0.001. Having an ‘a’ and ‘b’ subscript denotes proportions that differ significantly by column at an alpha level of 0.05 according to a proportion z test using SPSS.
We investigated oral (C10), liver (C22), stomach (C16), anal (C21), head and neck (C76.0), and cervical (C53) cancers to examine if the foreign population had a higher number of cancer deaths due to infections such as HPV, helicobacter pillory, and HIV. The foreign population did not have a significantly higher cancer deaths in these cancers when compared to the Colombian population.
Foreign individuals mainly resided in the following departments at the time of death: Bogota (28.5 %), North Santander, bordered with Venezuela (23.8 %), Valley of Cauca (14.5 %), and Antioquia (10.5 %). Colombian individuals mainly resided in the following departments at the time of death: Bogota (20.8 %), Antioquia (16.7 %), Valley of Cauca (12.3 %), and Atlantic (5.3 %).
4. Discussion
This study reveals several interesting observations. First, immigrants in Colombia had a higher cancer mortality rate than Colombian individuals, after adjusting for age. Second, the proportion of cancer deaths was higher in men than women in most cancer types, in both Colombian and foreign populaitons. Third, foreign population did not have significantly higher cancer mortality caused by infectious agents.
Regarding the first observation of cancer mortality, lower crude cancer mortality rate among immigrants is likely due to a younger immigrant population. Venezuelans are the largest group of immigrants in Colombia with a large influx of Venezuelans starting in 2014, with a younger age than Colombians (Arango, 2021). Similarly, results from high-income countries showed lower crude cancer mortality in immigrant population than in the native population (Abdoli et al., 2014; Oliva-Arocas et al., 2020; Hallowell et al., 2019; Cheung et al., 2017). However, in this study, the age-adjusted cancer mortality rate was higher among the immigrant population. Even though the immigrant population is younger, the age distribution of the main types of cancer is similar to that of the Colombians. For example, there was an increase in death from tumors of male genital organs with aging for both Colombian and foreign populations.
In terms of the second observation of cancer deaths by sex, men had a higher mortality for most cancers than women, in both the immigrant and the Colombian populations. GLOBOCAN also shows that men have a higher cancer incidence and mortality than women worldwide (Ferlay et al., 2020). According to DANE, there was a larger proportion of men to women among the immigrant population and vice versa among the Colombian population (Arango, 2021). Future studies should analyze sex-adjusted cancer mortality among both populations. This could not be performed in this study due to limited data on the distribution of immigrants by sex.
Regarding the third observation of cancers caused by infections, although the majority of the immigrants in Colombia are from Venezuela, with a collapsed health system (Alarcón et al., 2022), the immigrant population does not have a significantly higher proportion of cancer deaths when compared to the Colombian population for the following: oral (C10), liver (C22), stomach (C16), anal (C21), head and neck (C76.0), and cervical (C53). With high rates of HIV and little to no HIV treatment in Venezuela (Arenas-Suarez et al., 2021), we expected a significant difference in cancer deaths linked to infections among Colombian and foreign individuals, but this analysis did not show any differences.
Considering the department/province of residence at time of death, the highest proportion of deaths occurred in Bogota for both Colombian and foreign individuals. However, the next largest proportion for foreign individuals occurred in North Santander. The North Santander department borders Venezuela. This shared border can help explain that difference of distribution across departments among Colombian and foreign individuals. The four departments at time of death with the highest percentages for Colombians have the four biggest cities in Colombia: Bogota, Medellin, Cali, and Barranquilla.
Previous research has shown that immigrant in foreign countries have a lower crude cancer mortality than native populations (Abdoli et al., 2014; Oliva-Arocas et al., 2020; Hallowell et al., 2019; Cheung et al., 2017). Some theories on migration selectivity offer possible explanations. Migration selectivity tends to rely on the health status of the migrant (Wallace and Kulu, 2014). Because young people are often in better health than older people, young people tend to migrate more than their counterparts (Wallace and Kulu, 2014). The healthy immigrant effect is that immigrants are healthier than those who remain in their country of origin (Wallace and Kulu, 2014). In contrast, the salmon bias is that migrants return to their country of origin when they fall ill (Wallace and Kulu, 2014). These two theories on migration selectivity can help explain why immigrants in Colombia have a lower crude cancer mortality rate, despite the increased number of barriers that immigrants face. However, the healthy immigrant effect does not explain why immigrants have a higher age-adjusted cancer mortality rate.
Most studies on migration and cancer examined migrants from LMICs migrating to high-income countries (Abdoli et al., 2014; Hallowell et al., 2019; Kyrönlahti et al., 2020; Cheung et al., 2017; Stirbu et al., 2006; Wallace and Kulu, 2014). Our study examined migrants from low-, middle-, and high-income countries migrating to a middle-income country. Further research should focus on migration from a higher income country to a lower income country to examine differences in health benefits for the immigrant population. It is likely that similar results would be found in other Latin American countries that have similar migration patterns.
Strengths of this study include being the first in Colombia and one of the first studies in Latin America to examine the relationship between cancer mortality and immigration. Because the present study used information from death certificates collected by DANE, it included data on demographics like country of origin, ethnicity, education level, insurance type, and marital status. These variables are important in determining factors influencing cancer mortality. We also examined mortality over a long period and adjusted for age. We analyzed cancer mortality rather than incidence because it is more reliable data as there is no national cancer registry.
Study limitations include quality of migrant information in Colombia because of the high amount of non-documented migration. Certain demographic information, such as length of residency and citizenship status for foreign individuals was not available. Other studies in different countries have shown that length of residency and citizenship play a role in cancer mortality rates among immigrants (Stirbu et al., 2006). Because of the lack of data regarding immigrants in Colombia, we were unable to obtain the exact number of immigrants per age group and year and had to use estimates when adjusting for age.
In summary, immigrants in Colombia had a higher cancer mortality rate than the Colombian population, when adjusting for age. Although immigrants had a lower crude cancer mortality rate, this may be in part, due to a younger population and migration selectivity such as the healthy immigrant effect. Future studies on screening and barriers to cancer prevention and treatment of immigrants in Colombia can be crucial for clarifying the current access to care among immigrants. More complete data are crucial to migrant health such as information on length of residency and documentation status. Other countries facing an influx of immigrants will need to determine the difference in cancer mortality among native and immigrant populations to further improve upon migrant health.
Funding sources
Diana Hernandez and Almira Lewis were supported by the Cancer Epidemiology and Education in Special Populations (CEESP) Program through funding from the National Cancer Institute grant R25CA112383 (PI: Amr Soliman, MD, PhD). Diana Hernandez was also partially supported by financial assistance from the Institute of Latin American Studies at Columbia University.
Compliance with ethical standards
Ethics approval
This study used anonymous mortality data from 2006 to 2020 from a publicly available databases provided by the Colombian government. Neither Columbia University Mailman School of Public Health nor University of Antioquia considered the study falling under human subjects’ research or requested ethical approval.
CRediT authorship contribution statement
Diana M. Hernandez: Writing – original draft, Visualization, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Amr S. Soliman: Writing – review & editing, Resources, Funding acquisition, Conceptualization. Almira G.C. Lewis: Writing – review & editing. Isabel C. Garcés-Palacio: Writing – review & editing, Supervision, Resources, Methodology, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The data that support the findings of this study are publicly available from the National Department of Statistics (DANE) by year:https://microdatos.dane.gov.co/index.php/catalog/SAL-Microdatos. Combined mortality data from 2006 to 2020 are however available from the authors upon reasonable request.
References
- Abdoli G., Bottai M., Moradi T. Cancer mortality by country of birth, sex, and socioeconomic position in Sweden, 1961-2009. PLoS ONE. 2014;9(3):e93174. doi: 10.1371/journal.pone.0093174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad O.B., Boschi-Pinto C., Lopez A.D., Murray C.J., Lozano R., Inoue M. Vol. 9. World Health Organization; Geneva: 2001. pp. 1–4. (Age Standardization of Rates: A new WHO Standard). [Google Scholar]
- Alarcón R.D., Lozano-Vargas A., Velásquez E., Gaviria S., Ordoñez- Mancheno J., Lucio M., et al. Venezuelan Migration in Latin America: history and sociodemographic aspects. Rev. Neuropsiquiatr. 2022;85(2):107–116. [Google Scholar]
- Arango J.D.O. Un Panorama Usando La Gran Encuesta Integrada de Hogares National Department of Statistics (DANE) 2021. Perfil demográfico, laboral y educativo de la migración venezolana, 2014-2021.https://www.dane.gov.co/files/investigaciones/notas-estadisticas/dic-2021-nota-estadistica-perfil-demografico-laboral-poblacion-venezolana-en-colombia-2014-2021-presentacion.pdf Dec 17 [cited 2023 Jul 14]. Available from: [Google Scholar]
- Arenas-Suarez N.E., Cuervo L.I., Avila E.F., Duitama-Leal A., Pineda-Peña A.C. The impact of immigration on tuberculosis and HIV burden between Colombia and Venezuela and across frontier regions. Cad Saúde Pública. 2021;37(5) doi: 10.1590/0102-311X00078820. [DOI] [PubMed] [Google Scholar]
- Bitar S. UNDP Latin America and the Caribbean; 2022. Migration in Colombia and Public Policy Responses.https://www.undp.org/latin-america/publications/migration-colombia-and-public-policy-responses [cited 2023 Jul 24]. Available from: [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Cheung M.C., Earle C.C., Fischer H.D., Camacho X., Liu N., Saskin R., et al. Impact of immigration status on cancer outcomes in Ontario, Canada. J. Oncol. Pract. 2017;13(7):e602–e612. doi: 10.1200/JOP.2016.019497. [DOI] [PubMed] [Google Scholar]
- Consellería de Sanidade . Epidat: programa para análisis epidemiológico de datos; 2016. Xunta De Galicia, España, Organización Panamericana De La Salud (OPS-OMS), Universidad CES, Colombia.https://www.sergas.es/Saude-publica/EPIDAT?idioma=es [cited 2023 Jul 19]. Available from: [Google Scholar]
- Cucunubá Z.M., Manne-Goehler J.M., Díaz D., Nouvellet P., Bernal O., Marchiol A., et al. How universal is coverage and access to diagnosis and treatment for Chagas disease in Colombia? A health systems analysis. Soc. Sci. Med. 2017;175:187–198. doi: 10.1016/j.socscimed.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Departamento Administrativo Nacional Estadistica. Catálogo Central de Datos . [cited 2023 Jul 19]. Available from: https://microdatos.dane.gov.co/index.php/catalog/central/about.
- Feletto E., Sitas F. Quantifying disparities in cancer incidence and mortality of Australian residents of New South Wales (NSW) by place of birth: an ecological study. BMC Public Health. 2015;15:823. doi: 10.1186/s12889-015-2141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., et al. 2020. Global Cancer Observatory: Cancer Today.http://gco.iarc.fr/today/home [cited 2023 Jul 24]. Available from: [Google Scholar]
- Hallowell B.D., Endeshaw M., McKenna M.T., Senkomago V., Razzaghi H., Saraiya M. Cancer mortality rates among US and foreign-born individuals: United States 2005-2014. Prev. Med. 2019;126 doi: 10.1016/j.ypmed.2019.105755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Cancer Control Initiative in Latin America . Integrated Cancer Control Initiative in Latin America; 2021. Addressing the Rising Burden of Cancer in Colombia: Challenges & Opportunities: An Analysis of Colombia's Health System and Cancer Control Policies; pp. 1–125.https://www.uicc.org/resources/addressing-rising-burden-cancer-colombia-challenges-opportunities Apr [cited 2023 Jun 29]Available from. [Google Scholar]
- Kyrönlahti A., Madanat-Harjuoja L., Pitkäniemi J., Rantanen M., Malila N., Taskinen M. Childhood cancer mortality and survival in immigrants: a population-based registry study in Finland. Int. J. Cancer. 2020;146(10):2746–2755. doi: 10.1002/ijc.32625. [DOI] [PubMed] [Google Scholar]
- Lortet-Tieulent J., Georges D., Bray F., Vaccarella S. Profiling global cancer incidence and mortality by socioeconomic development. Int. J. Cancer. 2020;147(11):3029–3036. doi: 10.1002/ijc.33114. [DOI] [PubMed] [Google Scholar]
- Murillo-Pedrozo A.M., Martínez-Herrera E., Ronda-Pérez E., Agudelo-Suárez A.A. A qualitative study of the health perceptions in the Venezuelan immigrant population in Medellín (Colombia) and its conditioning factors. Int. J. Environ. Res. Public Health. 2021;18(8):3897. doi: 10.3390/ijerph18083897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva-Arocas A., Pereyra-Zamora P., Copete J.M., Nolasco A. Cancer mortality trends in Spain (2000-2016): differences between immigrant and native populations. Int. J. Environ. Res. Public Health. 2020;17(14):5127. doi: 10.3390/ijerph17145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyarte M., Delgado I., Pedrero V., Agar L., Cabieses B. Hospitalizations for cancer in international migrants versus local population in Chile. Rev. Saude Publica. 2018;52:36. doi: 10.11606/S1518-8787.2018052000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi A., Orozco-Urdaneta M., Velez-Mejia C., Perez-Bustos A.H., Munoz-Zuluaga C., El-Sharkawy F., et al. Overcoming barriers in the implementation of programs for breast and cervical cancers in Cali, Colombia: a pilot model. J. Glob. Oncol. 2019;(5):1–9. doi: 10.1200/JGO.19.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirbu I., Kunst A.E., Vlems F.A., Visser O., Bos V., Deville W., et al. Cancer mortality rates among first and second generation migrants in the Netherlands: convergence toward the rates of the native Dutch population. Int. J. Cancer. 2006;119(11):2665–2672. doi: 10.1002/ijc.22200. [DOI] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- United Nations Department of Economic and Social Affairs Population Division . 2022. World Population Prospects.https://population.un.org/wpp/Download/Standard/MostUsed/ [cited 2023 Jul 19]. Available from: [Google Scholar]
- Vargas I., Mogollón-Pérez A.S., De Paepe P., Ferreira Da Silva M.R., Unger J.P., Vázquez M.L. Barriers to healthcare coordination in market-based and decentralized public health systems: a qualitative study in healthcare networks of Colombia and Brazil. Health Policy Plan. 2016;31(6):736–748. doi: 10.1093/heapol/czv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I., Vázquez M.L., Mogollón-Pérez A.S., Unger J.P. Barriers of access to care in a managed competition model: lessons from Colombia. BMC Health Serv. Res. 2010;10(1):297. doi: 10.1186/1472-6963-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M., Kulu H. Migration and Health in England and Scotland: a study of migrant selectivity and Salmon bias: migration and health in England and Scotland. Popul. Space Place. 2014;20(8):694–708. [Google Scholar]