SUMMARY.
We investigated the feasibility of testing feathers as a complementary approach to detect low pathogenic influenza A viruses (IAVs) in wild duck populations. Feathers on the ground were collected at four duck capture sites during 2010 and 2011, in Minnesota, U. S. A. IAVs were isolated from both feathers and cloacal swabs sampled from ducks at the time of capture. Although virus isolation rates from feather and cloacal swabs were inconsistent between collections, the overall rate of isolation was greatest from the feather samples. Viruses isolated from feathers also reflected the subtype diversity observed in cloacal swab isolates but resulted in many more isolates that contained more than one virus. Our study suggests that testing feathers may represent an alternative noninvasive approach to recover viruses and estimate subtype abundance and diversity.
Keywords: avian influenza, mallard, wild birds, virus isolation, subtype diversity, Minnesota
RESUMEN.
Nota de Investigación—Aislamiento del virus de la influenza aviar de patos silvestres y de plumas en Minnesota (2010–2011).
Se investigó la viabilidad de analizar las plumas como un enfoque complementario para detectar al virus de influenza aviar tipo A de baja patogenicidad (IAV) en poblaciones de patos silvestres. Se recolectaron plumas del suelo de cuatro sitios de captura de patos durante los años 2010 y 2011, en Minnesota, en los Estados Unidos. Los virus de influenza aviar de baja patogenicidad fueron aislados de las plumas y de los hisopos cloacales recolectados de los patos en el momento de la captura. Aunque los porcentajes de aislamiento del virus de las plumas y de los hisopos cloacales fueron inconsistentes entre las recolecciones, la tasa global de aislamiento era mayor en las muestras de plumas. Los virus aislados a partir de las plumas también reflejaron la diversidad de subtipos observados en los aislados de hisopos cloacales pero resultaron en muchos más aislamientos que contenían más de un virus. Nuestro estudio sugiere que el análisis de las plumas puede representar un enfoque alternativo no invasivo para recuperar los virus y estimar la abundancia de subtipos y su diversidad.
In a recent study, Delogu et al. (1) hypothesized that preening behavior could facilitate influenza A virus (IAV) accumulation on duck feathers. Attachment of IAV to feathers from contaminated water was facilitated by preening oil produced by the uropygial gland (1). Based on feather swabs performed on wild ducks and on feathers experimentally impregnated with preening oil, it was demonstrated that IAV could be concentrated from water with this mechanism (1). These findings suggested that feather preening may facilitate virus infection in wild ducks and provide a means for viral movement to noninfected birds. From a surveillance standpoint, these results also suggest that sampling detached feathers could be an alternative method for detecting viruses in wild duck populations.
We investigated the feasibility of feather sampling as an alternative approach to detect viruses in wild ducks. Sampling methods for IAV isolation in wild birds have primarily relied on cloacal and oropharyngeal swabs, and fecal samples (e.g., 6,16). Environmental samples (e.g., water, mud) also have successfully led to the isolation of viruses and detection of viral RNA; however, low detection rates have been reported (7,13). Although direct sampling of birds and sampling of fresh feces are reliable approaches to IAV surveillance, these methods are not always possible under all field conditions. The objectives of this work were to determine whether there were differences between sampling methods with regard to: 1) the probability of isolating IAV between feathers and cloacal swabs collected at the same time and place, and 2) the diversity of subtypes detected.
MATERIALS AND METHODS
Samples were collected in September 2010 and 2011 at the Thief Lake Wildlife Management Area (WMA; Marshall County, 48u29912.850N, 95u57902.170W), Roseau River WMA (Roseau County, 48u58939.770N, 96u00932.080W), and two locations (Mud River and Tamarac Pools) at Agassiz National Wildlife Refuge (NWR; Marshall County, 48u18902.900N, 95u58949.680W). Sampled bird species were mainly dabbling ducks, such as mallard (Anas platy-rhynchos), blue-winged teal (Anas discors), and American green-winged teal (Anas carolinensis), but also wood duck (Aix sponsa); details that relate to the duck species diversity at the study site have been previously described (17). Ducks were captured using rocket-nets, and cloacal swabs were collected as described by Hanson et al. (4). Whole feathers were collected randomly from the ground, with a distance between collected feathers of at least 50 cm, without regard to feather type, size, or species, immediately after ducks were captured (Fig. 1). Gloves were changed between individual samples, and single feathers were placed in individual sterile polypropylene tubes (i.e., one feather per tube; Corning Inc., Corning, NY) containing 2 ml of transport media (4). Feathers were not grounded and were submerged in the transport media. All samples were stored at −80 C until viral isolation.
Fig. 1.

Feathers remaining on the ground after ducks were captured with rocket-nets.
Samples were processed as described by Wilcox et al. (17). Briefly, tubes containing cloacal swabs or individual feathers were vortexed and centrifuged at 1500 × g for 15 min. The supernatant was inoculated into three 9–11-day-old specific-pathogen-free (SPF) embryonating chicken eggs (0.33 ml/egg) via the allantoic route (14). Eggs were incubated at 37 C for 120 hr, and the amnio allantoic fluid was tested by hemagglutination assay (5). RNA was extracted from the amnio allantoic fluid using the QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA) as per the manufacturer’s instructions, and tested by avian influenza virus (IAV) matrix real-time reverse-transcriptase polymerase chain reaction (rtRT-PCR) as described previously (3). Identification of 13 hemagglutinin (HA) and nine neuraminidase (NA) subtypes was performed by screening each IAV-positive sample against every possible HA and NA subtype using standard PCR: H2, H4, H6, H8, H9, H11, H12, H13 (8); H1, H3, H9, H10, and N1 (15); N2, N3, N4, N5, N6, N7, and N8 (9); N9 (2); and H5 and H7 (SmartCycler, Roche, Valencia, CA) (11,12). PCR products were run on 2% agarose gels and visualized with ethidium bromide.
Statistical analyses were done in program R 2.13.1 (10). A Pearson correlation coefficient was calculated to measure the association between the proportion of IAV-positive samples from feathers and cloacal swabs. A chi-square test was used to test for differences in virus detection rates from the two types of sampling methods. A Mann-Whitney nonparametric test was used to compare the proportion of detected HA and NA subtypes originating from cloacal swabs and feather samples.
RESULTS AND DISCUSSION
Of the 156 feather samples and 497 cloacal swabs collected, 61 (39%) and 138 (28%), respectively, were IAV positive (i.e., successful virus isolation and positive Matrix rtRT-PCR). Sample size and virus detection rates for the different locations and dates are presented in Table 1. The prevalence of infected ducks observed in 2010 (13%) was comparable to values reported during previous years in Minnesota (11% during 2007; 24% during 2008) (17). In 2011, a higher prevalence was measured; 38% of sampled birds tested positive for IAV.
Table 1.
Influenza A virus detection rate in cloacal swabs and feathers from wild ducks in northwestern Minnesota, U. S. A.
| Date | Location | Sample type | Sample size | Detection rate (%) |
|---|---|---|---|---|
|
| ||||
| 2010 | ||||
| Sep. 8 | Mud River | Cloacal swabs | 72 | 19 |
| Feathers | 10 | 100 | ||
| Sep. 9 | Tamarac Pool | Cloacal swabs | 56 | 11 |
| Feathers | 22 | 27 | ||
| Sep. 14 | Mud River | Cloacal swabs | 72 | 8 |
| Feathers | 31 | 61 | ||
| 2011 | ||||
| Sep. 7 | Tamarac Pool | Cloacal swabs | 101 | 20 |
| Feathers | 24 | 0 | ||
| Thief Lake | Cloacal swabs | 135 | 61 | |
| Feathers | 24 | 100 | ||
| Sep. 15 | Mud River | Cloacal swabs | 45 | 13 |
| Feathers | 24 | 0 | ||
| Sep. 22 | Roseau River | Cloacal swabs | 16 | 25 |
| Feathers | 21 | 10 | ||
Isolation success from feathers was not correlated to the prevalence of infected birds as measured from cloacal samples (r = 0.49, df = 5, P = 0.26). This suggests that while results from feather samples may represent an alternative way to detect circulating viruses in locations where bird trapping or fecal sampling is difficult, this approach does not provide accurate estimates of IAV prevalence at the population level. Overall, we also found that virus detection rate in feather samples was significantly higher than in cloacal swabs (χ2 = 6.67, df = 1, P < 0.01), but low detection rates were observed from feather samples (0–10%), as compared to cloacal samples (13–25%), at three of seven capture sites (Tamarac Pool, Mud River, and Roseau River, in 2011; Table 1). The source of this variation is unknown but may relate to environmental variables such as air temperature, humidity, ultraviolet (UV) radiation, or feather degeneration. The variables potentially affecting successful recovery of virus from feathers need to be better understood before this sampling and testing approach can be adapted for field use; experimental work may provide important insights on the effects of environmental and climatic variables.
In both feather samples and cloacal swabs, H4 and N6 were the most common subtypes (Fig. 2), with no apparent variation between sampling sites and years. These HA and NA subtypes previously have been reported as the most common subtypes detected in Minnesota ducks, along with H3 and N8 (4,17), suggesting that sampling feathers likely provides an adequate representation of the proportion of these subtypes in studied populations. Additionally, the number of detected subtypes in feather samples was significantly greater than in cloacal swabs, both when considering the HA (W = 4970, P < 0.001; mean number of detected subtype per sample: feathers = 1.93, swabs = 1.22) and the NA (W = 5334, P < 0.001; mean number of detected subtype per sample: feathers = 2.56, swabs = 1.41) subtypes. This finding supports the assertion that sampling for feathers can provide a suitable method by which to characterize the diversity of circulating subtypes in wild duck populations, especially when the prevalence of infected ducks is low and requires a high number of cloacal swab samples to accurately estimate IAV diversity.
Fig. 2.
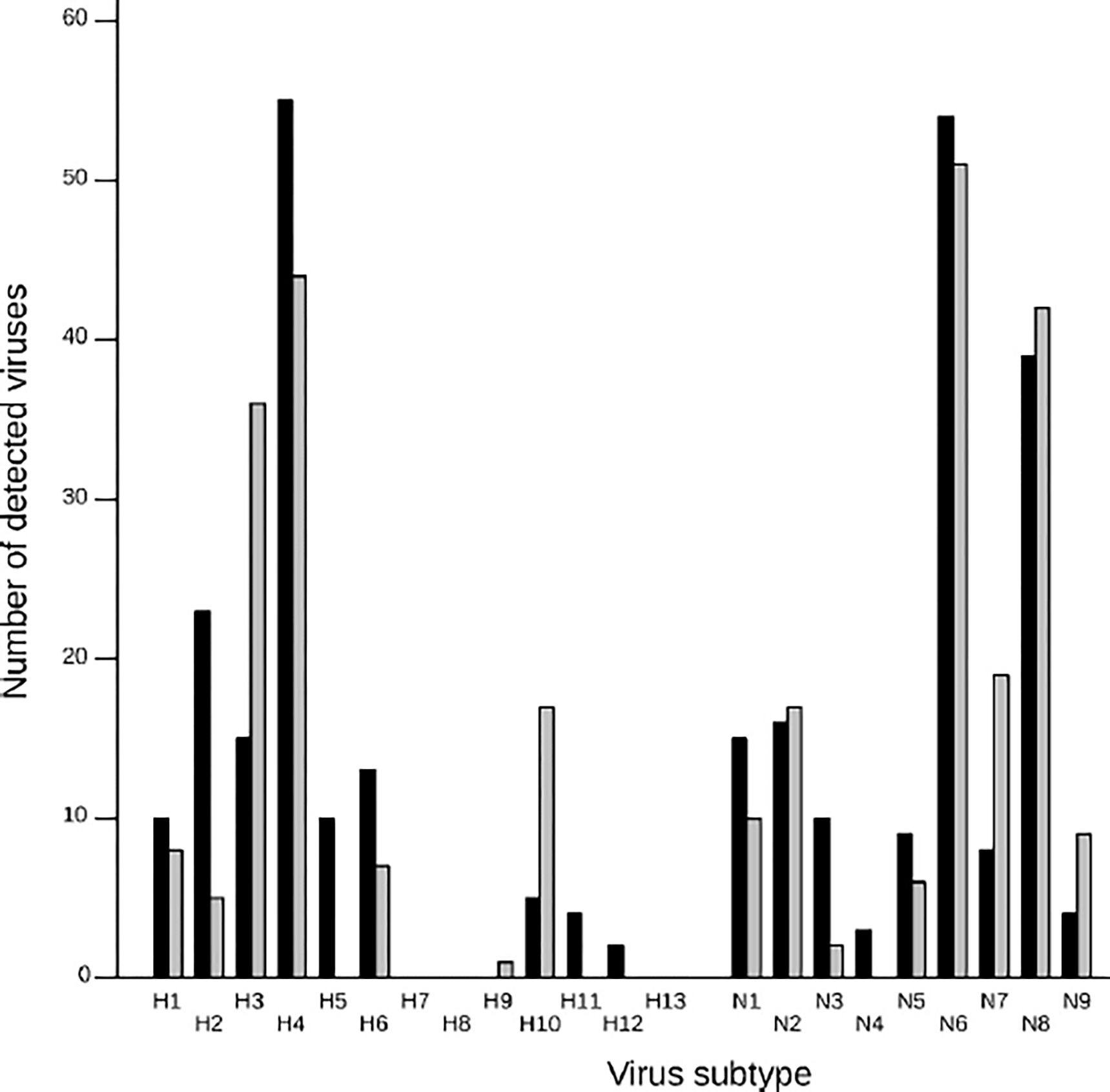
Number of hemagglutinin and neuraminidase subtypes detected from cloacal swabs (black bars) and feather samples (gray bars), collected from wild ducks in northwestern Minnesota, U. S. A.
Although samples from feathers reflected the overall subtype diversity of that measured from cloacal swab isolates, virus subtypes such as H5, H11, and H12 were never detected in feathers (Fig. 2). The opposite pattern also was observed with the isolation of H10 IAV from feathers at Thief Lake in 2011 (12/24 isolated viruses), but only from a few cloacal swabs collected during the same capture event (4/82 isolated viruses). These differences may reflect variation related to the load and duration of viral shedding, as well as the ability of viruses to persist in aquatic habitats. Such phenomena could affect the probability of IAV attachment to duck feathers.
The high IAV isolation rate observed in Minnesota, as well as that reported by Delogu et al. in Italy (1), underlines the supposition that virus concentration in feathers may be a common and underestimated mechanism for IAV transmission in duck populations or as a source of infection for other species. The role of virus accumulation on duck feathers in the epidemiology of IAV needs to be clarified. Future research directions will have to consider behavioral (e.g., self- and allo-preening behaviors), physiologic (e.g., molecular interaction between virus and preening oil), and ecologic aspects (e.g., role of environmental factors in the maintenance of virus in feathers) to determine the importance of this mechanism in IAV transmission in wild ducks and waterbirds, as compared to waterborne transmission.
ACKNOWLEDGMENTS
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN266200700007C. The funding agencies did not have any involvement in the study design, implementation, or publishing of this study and the research presented herein represents the opinions of the authors, but not necessarily the opinions of the funding agencies.
Abbreviations:
- IAV
influenza A virus
- NWR
National Wildlife Refuge
- SPF
specific pathogen free
- WMA
Wildlife Management Area
REFERENCES
- 1.Delogu M, De Marco MA, Di Trani L, Raffini E, Cotti C, Puzelli S, Ostanello F, Webster RG, Cassone A, and Donatelli I. Can preening contribute to influenza A virus infection in wild waterbirds? PLoS ONE 5:e11315. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fereidouni SR, Starick E, Grund C, Globig A, Mettenleiter TC, Beer M, and Harder T. Rapid molecular subtyping by reverse transcription polymerase chain reaction of the neuraminidase gene of avian influenza A viruses. Vet. Microbiol. 135:253–260. 2009. [DOI] [PubMed] [Google Scholar]
- 3.Fouchier RAM, Bestebroer TM, Herfst S, Van Der Kemp L, Rimmelzwaan GF, and Osterhaus AD. Detection of influenza viruses from different species by PCR amplification of conserved sequences in matrix gene. J. Clin. Microbiol. 38:4096–4101. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanson BA, Stallknecht DE, Swayne DE, Lewis LA, and Senne DA. Avian influenza viruses in Minnesota ducks during 1998–2000. Avian Dis. 47:867–871. 2003. [DOI] [PubMed] [Google Scholar]
- 5.Hirst G The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus. Science 94:22–23. 1941. [DOI] [PubMed] [Google Scholar]
- 6.Krauss S, Walker D, Pryor SP, Niles L, Li CH, Hinshaw VS, and Webster RG. Influenza A viruses of migrating wild aquatic birds in North America. Vector-Borne Zoonot. Dis. 4:177–189. 2004. [DOI] [PubMed] [Google Scholar]
- 7.Lang AS, Kelly A, and Runstadler JA. Prevalence and diversity of avian influenza viruses in environmental reservoirs. J. Gen. Virol. 89:509–519. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Chang PC, Shien JH, Cheng MC, and Shieh SK. Identification and subtyping of avian influenza viruses by reverse transcription PCR. J. Virol. Methods 97:13–22. 2001. [DOI] [PubMed] [Google Scholar]
- 9.Qiu BF, Liu WJ, Peng DX, Hu SL, Tang YH, and Liu XF. A reverse transcription-PCR for subtyping of the neuraminidase of avian influenza viruses. J. Virol. Methods 155:193–198. 2009. [DOI] [PubMed] [Google Scholar]
- 10.R Development Core Team. R: a language and environment for statistical computing [Internet; modified 8 July 2011]. R Foundation for Statistical Computing, Vienna, Austria, Available from: http://www.R-project.org [Google Scholar]
- 11.Spackman E, Ip HS, Suarez DL, Slemons RD, and Stallknecht DE. Analytical validation of a real-time reverse transcription polymerase chain reaction test for pan-American lineage H7 subtype avian influenza viruses. J. Vet. Diagn. Invest. 20:612–616. 2008. [DOI] [PubMed] [Google Scholar]
- 12.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, and Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J. Clin. Microbiol. 40:3256–3260. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stallknecht DE, Goekjian VH, Wilcox BR, Poulson RL, and Brown JD. Avian influenza virus in aquatic habitats: what do we need to learn? Avian Dis. 54:461–465. 2010. [DOI] [PubMed] [Google Scholar]
- 14.Stallknecht DE, Shane SM, Zwank PJ, Senne DA, and Kearney MT. Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Avian Dis. 34:398–405. 1990. [PubMed] [Google Scholar]
- 15.Tsukamoto K, Ashizawa T, Nakanishi K, Kaji N, Suzuki K, Shishido M, Okamatsu M, and Mase M. Use of reverse transcriptase PCR to subtype N1 to N9 neuraminidase genes of avian influenza viruses. J. Clin. Microbiol. 47:2301–2303. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallensten A, Munster VJ, Latorre-Margalef N, Brytting M, Elmberg J, Fouchier RAM, Fransson T, Haemig PD, Karlsson M, Lundkvist A, Osterhaus ADME, Stervander M, Waldenström J, and Björn O. Surveillance of influenza A virus in migratory waterfowl in Northern Europe. Emerging Infect. Dis. 13:404–411. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox BR, Knutsen GA, Berdeen J, Goekjian V, Poulson R, Goyal S, Sreevatsan S, Cardona C, Berghaus RD, Swayne DE, Yabsley MJ, and Stallknecht DE. Influenza-A viruses in ducks in northwestern Minnesota: fine scale spatial and temporal variation in prevalence and subtype diversity. PLoS ONE 6:e24010. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


