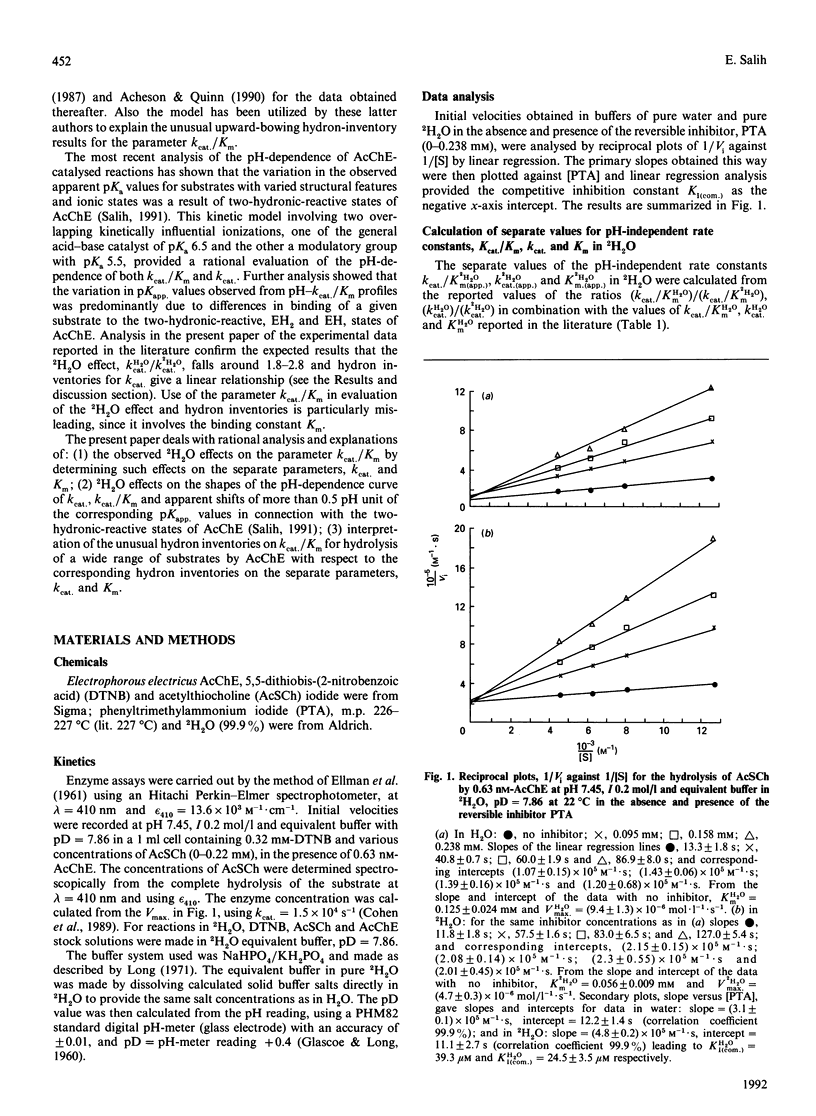
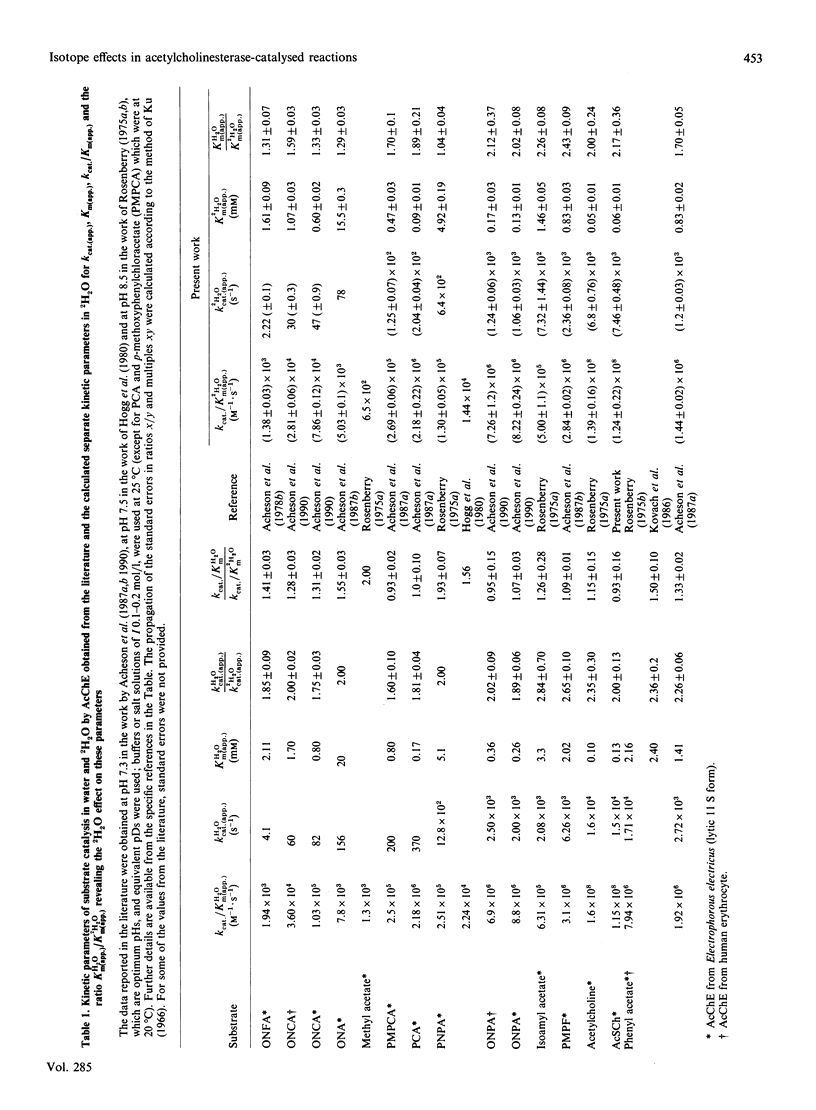
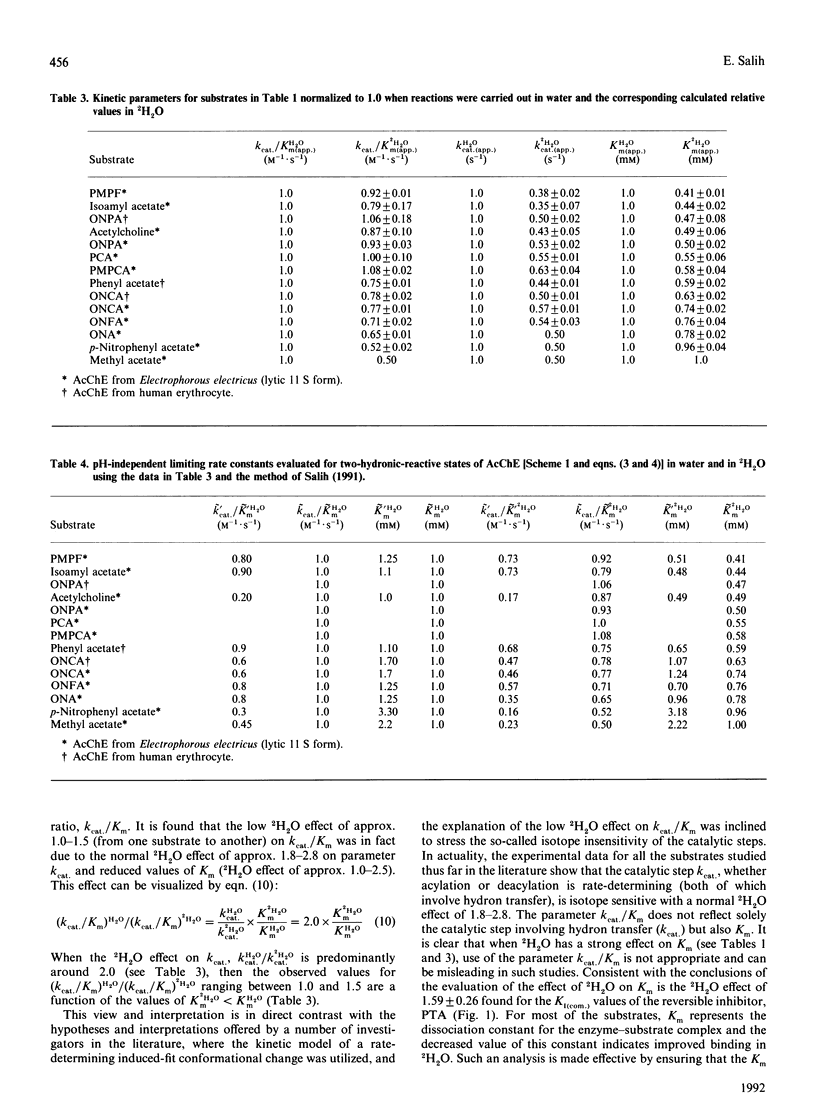
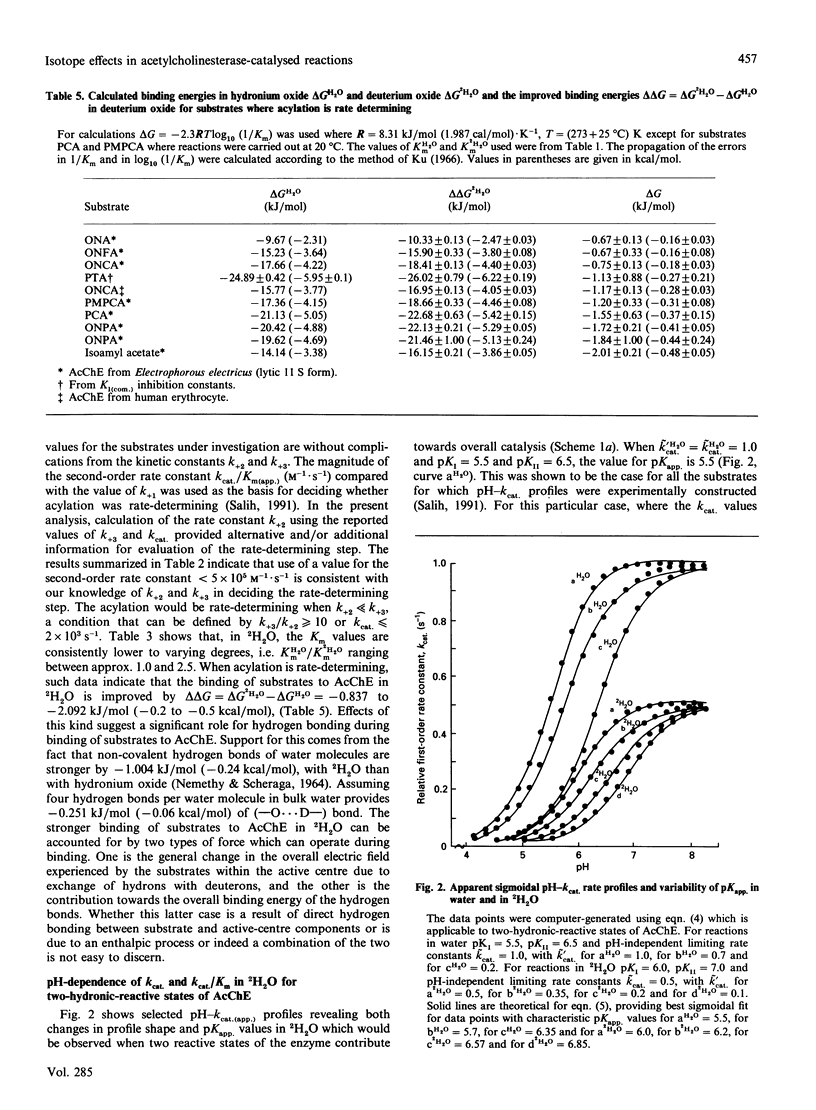
Abstract
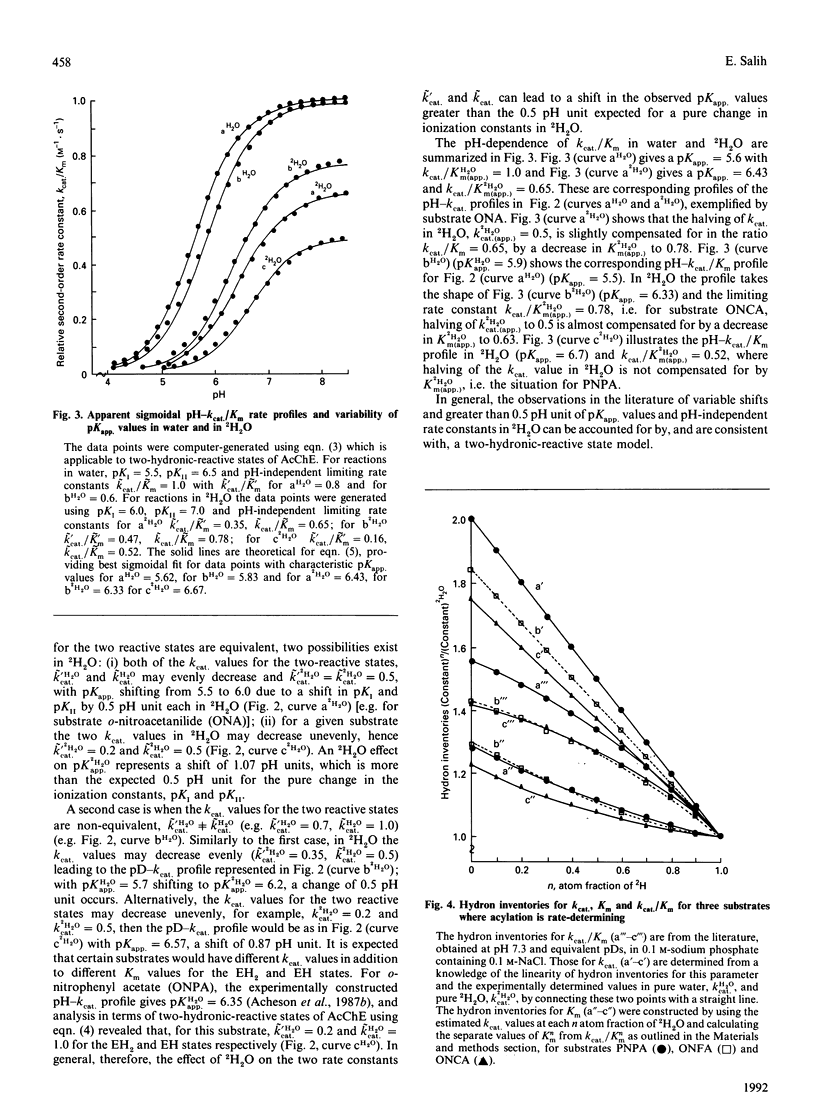
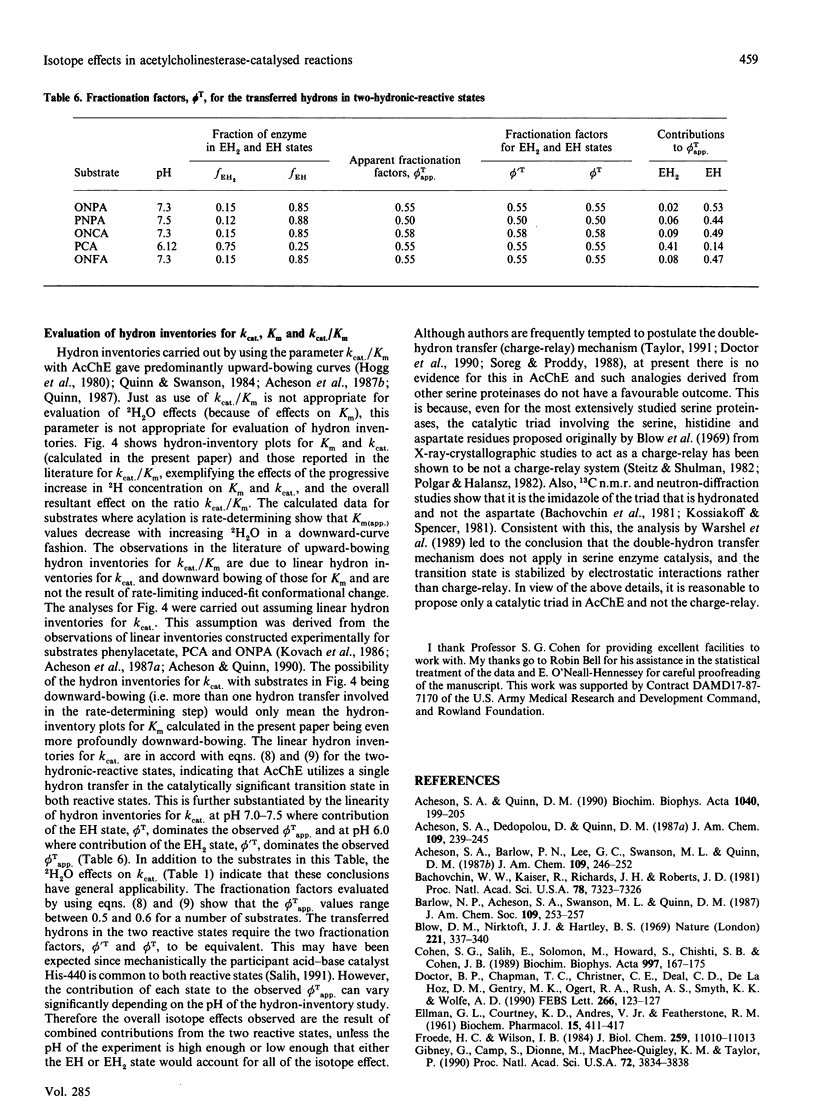
Low 2H2O effects (1.0-1.5) for the parameter k(cat.)/Km in the hydrolysis of various substrates by acetylcholinesterase (AcChE) is due to normal 2H2O effects (1.8-2.8) for the parameter k(cat.) and 2H2O effects of 1.0-2.5 for the parameter Km. The analyses and interpretations of 2H2O effects in the literature utilizing the parameter k(cat.)/Km, which led to the proposal of 'isotope insensitivity' of the catalytic steps and the hypothesis of a rate-limiting substrate-induced-fit conformational change, are incorrect. Since k(cat.) is the only parameter that can represent the hydron-transfer step solely, the 2H2O effect can most appropriately be evaluated by using this parameter. Calculations and comparison of acylation (k+2) and deacylation (k+3) rate constants show that acylation is rate-determining for most substrates and the improved binding -0.84 to -2.09 kJ/mol (-0.2 to -0.5 kcal/mol) in 2H2O obscures the normal 2H2O effect on k(cat.) when the ratio k(cat.)/Km is utilized. Consistent with this, measurements of the inhibition constant (KI(com.)) for a reversible inhibitor, phenyltrimethylammonium, lead to KI(com.)H2O = 39 +/- 3 microM and KI(com.)2H2O = 24.5 +/- 3.5 microM, an 2H2O effect of 1.59 +/- 0.26. pH-dependence of k(cat.) in 2H2O is subject to variability of the pK(app.) values, as evaluated in terms of the two-hydronic-reactive states (EH and EH2) of AcChE, and is due to an uneven decrease in 2H2O of the kinetic parameters k'cat. for the EH2 state relative to k(cat.) for the EH state, thus leading to variable shifts in pK(app.) values of between 0.5 and 1.2 pH units for this parameter. The observed pH-independent limiting rate constants for k(cat.)/Km(app.) are made to vary between 0.5 and 1.0 in 2H2O by effects on kinetic parameters for the EH2 state, k'cat./K'm varying between 0.2 and 0.7 relative to the EH state, with k(cat.)/Km varying between 0.4 and 1.0. The effects observed on k(cat.)/Km(app.) are ultimately the result of variable effects of 2H2O on k'cat. and K'm for the EH2 state relative to k(cat.) and Km for the EH state of AcChE. These effects are responsible for the variable shifts and more than 0.5 pH unit of the pK(app.) values in 2H2O for pH-k(cat.)/Km profiles. The upward-bowing hydron inventories for k(cat.)/Km are the result of linear hydron inventories for k(cat.) and downward-bowing on Km and are not due to the rate-limiting substrate-induced fit process as claimed in the literature.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson S. A., Quinn D. M. Anatomy of acetylcholinesterase catalysis: reaction dynamics analogy for human erythrocyte and electric eel enzymes. Biochim Biophys Acta. 1990 Sep 3;1040(2):199–205. doi: 10.1016/0167-4838(90)90076-r. [DOI] [PubMed] [Google Scholar]
- Bachovchin W. W., Kaiser R., Richards J. H., Roberts J. D. Catalytic mechanism of serine proteases: reexamination of the pH dependence of the histidyl 1J13C2-H coupling constant in the catalytic triad of alpha-lytic protease. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7323–7326. doi: 10.1073/pnas.78.12.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. G., Salih E., Solomon M., Howard S., Chishti S. B., Cohen J. B. Reactions of 1-bromo-2-[14C]pinacolone with acetylcholinesterase from Torpedo nobiliana. Effects of 5-trimethylammonio-2-pentanone and diisopropyl fluorophosphate. Biochim Biophys Acta. 1989 Aug 31;997(3):167–175. doi: 10.1016/0167-4838(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Doctor B. P., Chapman T. C., Christner C. E., Deal C. D., De La Hoz D. M., Gentry M. K., Ogert R. A., Rush R. S., Smyth K. K., Wolfe A. D. Complete amino acid sequence of fetal bovine serum acetylcholinesterase and its comparison in various regions with other cholinesterases. FEBS Lett. 1990 Jun 18;266(1-2):123–127. doi: 10.1016/0014-5793(90)81522-p. [DOI] [PubMed] [Google Scholar]
- Froede H. C., Wilson I. B. Direct determination of acetyl-enzyme intermediate in the acetylcholinesterase-catalyzed hydrolysis of acetylcholine and acetylthiocholine. J Biol Chem. 1984 Sep 10;259(17):11010–11013. [PubMed] [Google Scholar]
- Kossiakoff A. A., Spencer S. A. Direct determination of the protonation states of aspartic acid-102 and histidine-57 in the tetrahedral intermediate of the serine proteases: neutron structure of trypsin. Biochemistry. 1981 Oct 27;20(22):6462–6474. doi: 10.1021/bi00525a027. [DOI] [PubMed] [Google Scholar]
- Polgár L., Halász P. Current problems in mechanistic studies of serine and cysteine proteinases. Biochem J. 1982 Oct 1;207(1):1–10. doi: 10.1042/bj2070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L. Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Catalysis by acetylcholinesterase: evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3834–3838. doi: 10.1073/pnas.72.10.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih E. Two-hydronic-reactive states of acetylcholinesterase, mechanistically relevant acid-base catalyst of pKa 6.5 and a modulatory group of pKa 5.5. Biochim Biophys Acta. 1991 Jan 23;1073(1):183–194. doi: 10.1016/0304-4165(91)90200-z. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W., Vale W. W. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schowen K. B., Schowen R. L. Solvent isotope effects of enzyme systems. Methods Enzymol. 1982;87:551–606. [PubMed] [Google Scholar]
- Soreq H., Prody C. A. Sequence similarities between human acetylcholinesterase and related proteins: putative implications for therapy of anticholinesterase intoxication. Prog Clin Biol Res. 1989;289:347–359. [PubMed] [Google Scholar]
- Steitz T. A., Shulman R. G. Crystallographic and NMR studies of the serine proteases. Annu Rev Biophys Bioeng. 1982;11:419–444. doi: 10.1146/annurev.bb.11.060182.002223. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Taylor P. The cholinesterases. J Biol Chem. 1991 Mar 5;266(7):4025–4028. [PubMed] [Google Scholar]
- Venkatasubban K. S., Schowen R. L. The proton inventory technique. CRC Crit Rev Biochem. 1984;17(1):1–44. doi: 10.3109/10409238409110268. [DOI] [PubMed] [Google Scholar]
- Warshel A., Naray-Szabo G., Sussman F., Hwang J. K. How do serine proteases really work? Biochemistry. 1989 May 2;28(9):3629–3637. doi: 10.1021/bi00435a001. [DOI] [PubMed] [Google Scholar]


