Abstract
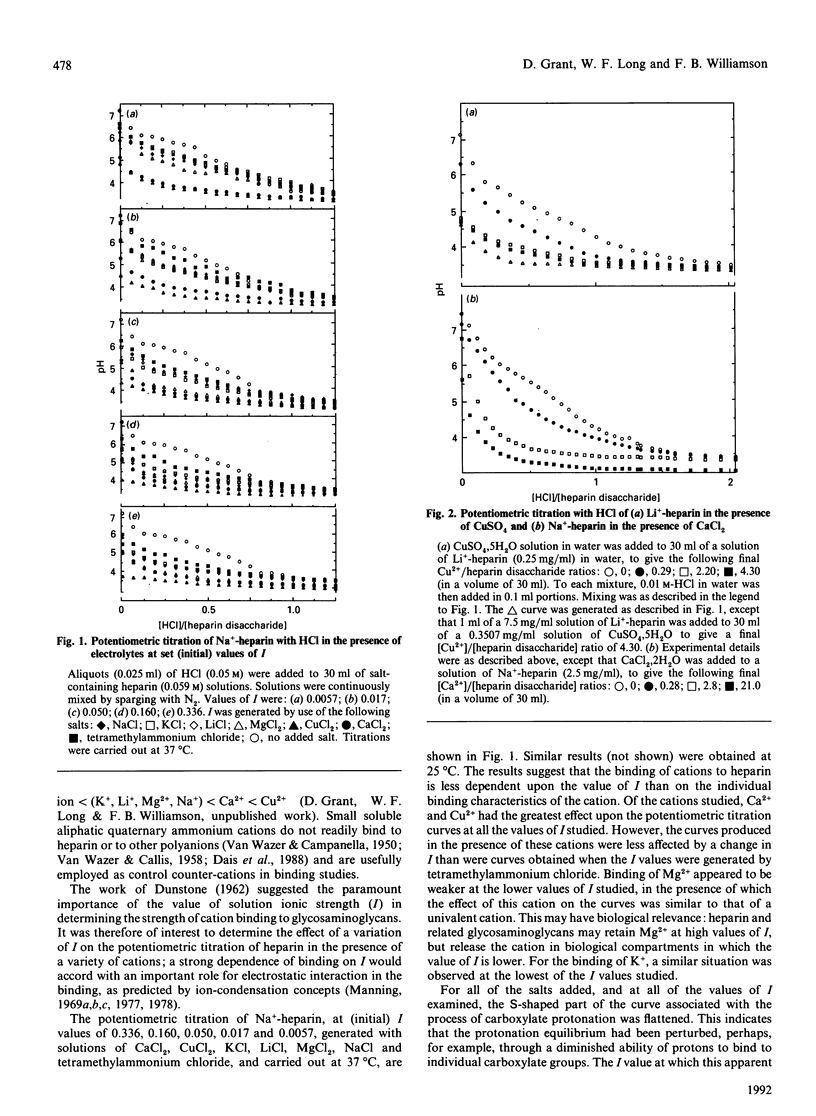
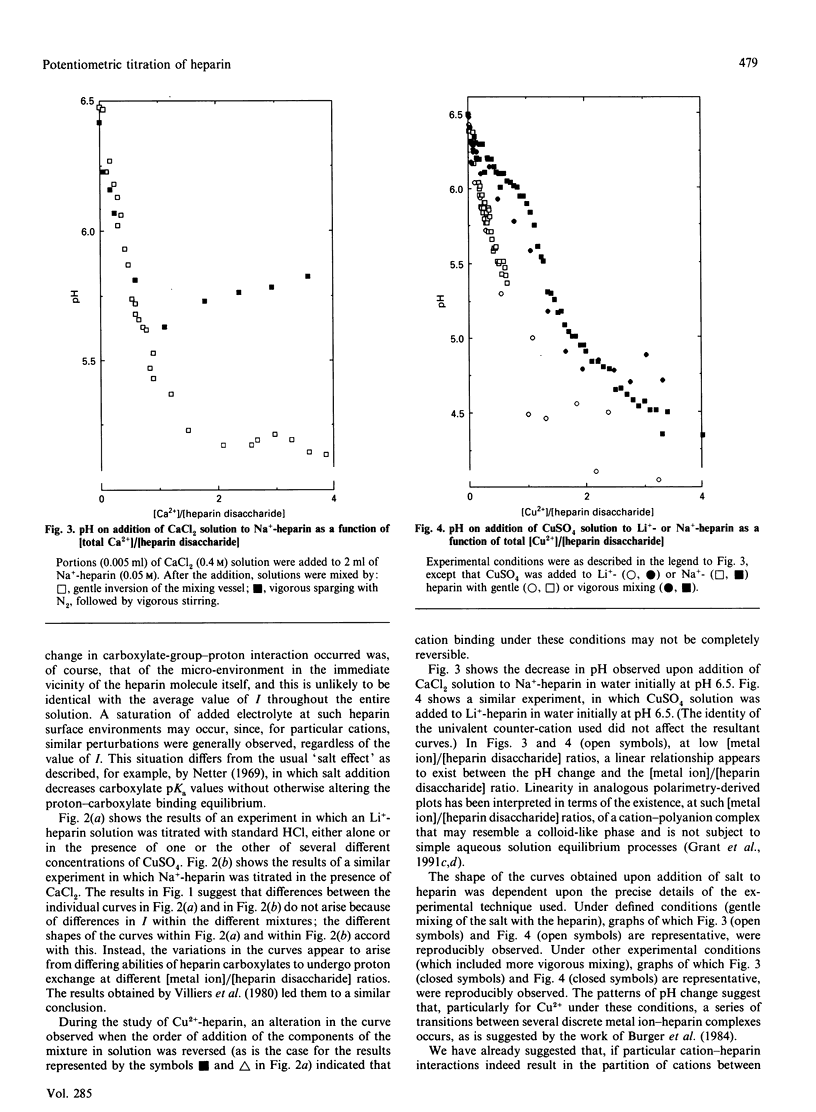
Potentiometric titrations, at ionic strengths (I) ranging from 0.0057 to 0.336, suggest that Ca2+, Cu2+, Li+ and Na+ bind to heparin in a manner that depends on cation identity. These interactions were less affected by the value of I than those of heparin with Mg2+, which binds weakly below I0.050 and with K+, which binds weakly at I0.0057. Of the interactions studied, that of heparin with Cu2+ was the least readily reversible.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessandri G., Raju K., Gullino P. M. Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res. 1983 Apr;43(4):1790–1797. [PubMed] [Google Scholar]
- Bernal J. D. The structure of water and its biological implications. Symp Soc Exp Biol. 1965;19:17–32. [PubMed] [Google Scholar]
- Dunstone J. R. Ion-exchange reactions between acid mucopolysaccharides and various cations. Biochem J. 1962 Nov;85(2):336–351. doi: 10.1042/bj0850336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Grant D., Long W. F., Moffat C. F., Williamson F. B. A study of Ca(2+)-heparin complex-formation by polarimetry. Biochem J. 1992 Mar 1;282(Pt 2):601–604. doi: 10.1042/bj2820601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D., Long W. F., Moffat C. F., Williamson F. B. Infrared spectroscopy of heparins suggests that the region 750-950 cm-1 is sensitive to changes in iduronate residue ring conformation. Biochem J. 1991 Apr 1;275(Pt 1):193–197. doi: 10.1042/bj2750193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D., Long W. F., Williamson F. B. Infrared spectroscopy of heparin-cation complexes. Biochem J. 1987 May 15;244(1):143–149. doi: 10.1042/bj2440143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D., Long W. F., Williamson F. B. The dependence on counter-cation of the degree of hydration of heparin. Biochem Soc Trans. 1990 Dec;18(6):1283–1284. doi: 10.1042/bst0181283. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Raju K. S., Alessandri G., Ziche M., Gullino P. M. Ceruloplasmin, copper ions, and angiogenesis. J Natl Cancer Inst. 1982 Nov;69(5):1183–1188. [PubMed] [Google Scholar]
- Rej R. N., Holme K. R., Perlin A. S. Marked stereoselectivity in the binding of copper ions by heparin. Contrasts with the binding of gadolinium and calcium ions. Carbohydr Res. 1990 Oct 25;207(2):143–152. doi: 10.1016/0008-6215(90)84044-u. [DOI] [PubMed] [Google Scholar]
- Shing Y. Heparin-copper biaffinity chromatography of fibroblast growth factors. J Biol Chem. 1988 Jun 25;263(18):9059–9062. [PubMed] [Google Scholar]
- Woodhead N. E., Long W. F., Williamson F. B. Binding of zinc ions to heparin. Analysis by equilibrium dialysis suggests the occurrence of two, entropy-driven, processes. Biochem J. 1986 Jul 1;237(1):281–284. doi: 10.1042/bj2370281. [DOI] [PMC free article] [PubMed] [Google Scholar]


