Abstract
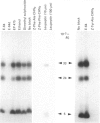
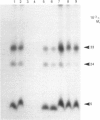
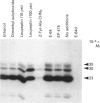
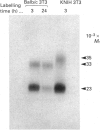
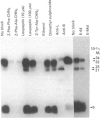
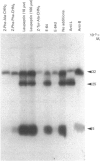
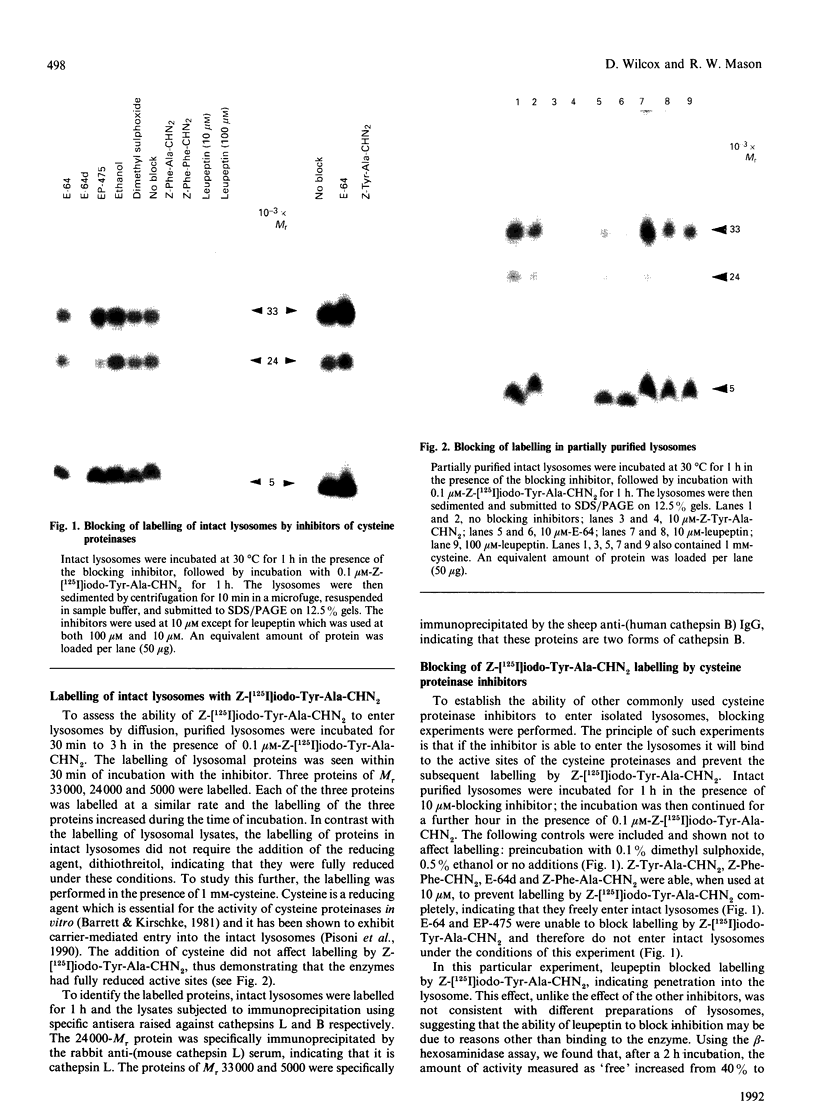
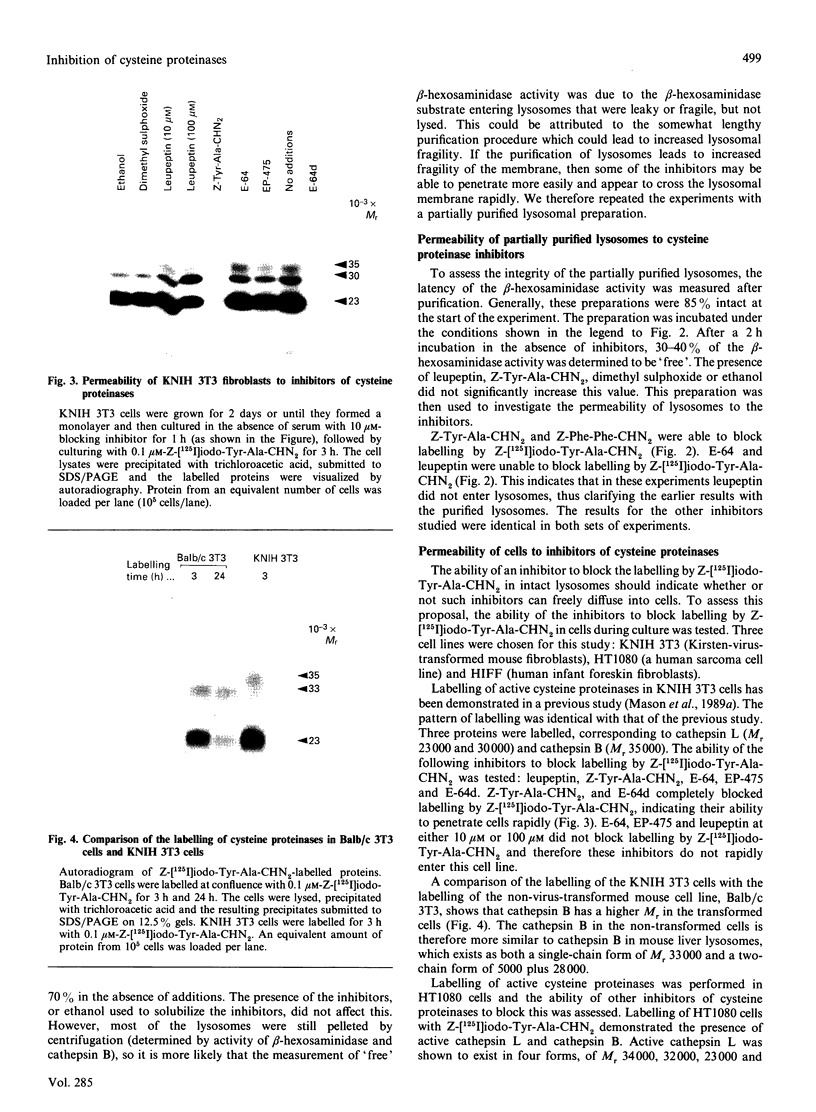
Inhibitors of cysteine proteinases have been used extensively to dissect the roles of these proteinases in cells. Surprisingly though, little work has been performed to demonstrate unequivocally that the inhibitors reach and inactivate their target proteinases in cell culture or in vivo. In the present study, the permeability of lysosomes and whole cells has been studied. Benzyloxycarbonyl (Z)-[125I]iodo-Tyr-Ala-diazomethane (CHN2), an inhibitor of cathepsins L and B, has been shown to label active forms of these enzymes in lysosomes and whole cells. The ability of other cysteine proteinase inhibitors to block this labelling has been used to indicate the permeation of these compounds. All the inhibitors were able to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2 in lysosomal extracts. In intact lysosomes or cells, however, only N-[N-(L-3-trans-ethoxycarbonyloxirane-2-carbonyl)-L-leucyl]-3- methylbutylamine ('E-64d') Z-Tyr-Ala-CHN2, Z-Phe-Ala-CHN2 and Z-Phe-Phe-CHN2 were able to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2. N-[N-(L-3-trans-Carboxyoxirane-2-carbonyl)-L-leucyl]amino-4-gua nidinobutane (E-64) and leupeptin were unable to block labelling by Z-[125I]iodo-Tyr-Ala-CHN2 in lysosomes or in cells. The ability to block labelling in lysosomes is an indication of the ability of the inhibitor to diffuse across membranes. Thus E-64 and leupeptin do not readily permeate membranes and therefore their uptake into cells probably only occurs via pinocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baricos W. H., Zhou Y., Mason R. W., Barrett A. J. Human kidney cathepsins B and L. Characterization and potential role in degradation of glomerular basement membrane. Biochem J. 1988 May 15;252(1):301–304. doi: 10.1042/bj2520301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bird S. J., Forster S., Lloyd J. B. Translocation of sugars into rat liver lysosomes. Evidence against a common carrier for D-glucose and D-ribose. Biochem J. 1987 Aug 1;245(3):929–931. doi: 10.1042/bj2450929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Macrophage protein turnover. Evidence for lysosomal participation in basal proteolysis. Biochem J. 1979 May 15;180(2):339–345. doi: 10.1042/bj1800339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Grinde B. The thiol proteinase inhibitors, Z-Phe-PheCHN2 and Z-Phe-AlaCHN2, inhibit lysosomal protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1983 May 4;757(1):15–20. doi: 10.1016/0304-4165(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Shaw E. Rapid interaction of cathepsin L by Z-Phe-PheCHN12 and Z-Phe-AlaCHN2. Biochem Biophys Res Commun. 1981 Jul 30;101(2):454–458. doi: 10.1016/0006-291x(81)91281-x. [DOI] [PubMed] [Google Scholar]
- Mason R. W., Bartholomew L. T., Hardwick B. S. The use of benzyloxycarbonyl[125I]iodotyrosylalanyldiazomethane as a probe for active cysteine proteinases in human tissues. Biochem J. 1989 Nov 1;263(3):945–949. doi: 10.1042/bj2630945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W. Species variants of cathepsin L and their immunological identification. Biochem J. 1986 Nov 15;240(1):285–288. doi: 10.1042/bj2400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. W., Wilcox D., Wikstrom P., Shaw E. N. The identification of active forms of cysteine proteinases in Kirsten-virus-transformed mouse fibroblasts by use of a specific radiolabelled inhibitor. Biochem J. 1989 Jan 1;257(1):125–129. doi: 10.1042/bj2570125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E. B., Becker E., Detwiler T. C. Inhibition of calpain in intact platelets by the thiol protease inhibitor E-64d. Biochem Biophys Res Commun. 1989 Jan 31;158(2):432–435. doi: 10.1016/s0006-291x(89)80065-8. [DOI] [PubMed] [Google Scholar]
- Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem Sci. 1991 Apr;16(4):150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- Murata M., Miyashita S., Yokoo C., Tamai M., Hanada K., Hatayama K., Towatari T., Nikawa T., Katunuma N. Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro. FEBS Lett. 1991 Mar 25;280(2):307–310. doi: 10.1016/0014-5793(91)80318-w. [DOI] [PubMed] [Google Scholar]
- Noda T., Isogai K., Katunuma N., Tarumoto Y., Ohzeki M. Effects of cathepsin B, H, and D in pectoral muscle of dystrophic chickens (line 413) of in vivo administration of E-64-c (N-[N-(L-3-transcarboxyoxirane-2-carbonyl)-L-leucyl]-3-methyl-butylamine). J Biochem. 1981 Sep;90(3):893–896. doi: 10.1093/oxfordjournals.jbchem.a133548. [DOI] [PubMed] [Google Scholar]
- Nonaka I., Ishiura S., Takagi A., Sugita H. Therapeutic trial with protease inhibitor (leupeptin) in chicken muscular dystrophy. A histologic and histochemical study. Acta Neuropathol. 1982;58(4):279–285. doi: 10.1007/BF00688610. [DOI] [PubMed] [Google Scholar]
- Parkes C., Kembhavi A. A., Barrett A. J. Calpain inhibition by peptide epoxides. Biochem J. 1985 Sep 1;230(2):509–516. doi: 10.1042/bj2300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni R. L., Acker T. L., Lisowski K. M., Lemons R. M., Thoene J. G. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J Cell Biol. 1990 Feb;110(2):327–335. doi: 10.1083/jcb.110.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Nelson-Rees W. A., Toth E. M., Arnstein P., Gardner M. B. Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer. 1974 Apr;33(4):1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W. C., HOGEBOOM G. H. Intracellular distribution of enzymes. IX. Certain purine-metabolizing enzymes. J Biol Chem. 1952 Mar;195(1):161–166. [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kasai Y., Senshu M., Iwashita S., Imahori K. Thiol protease-specific inhibitor E-64 arrests human epidermoid carcinoma A431 cells at mitotic metaphase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):146–150. doi: 10.1073/pnas.85.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O., Bohley P., Münchmeyer R., Bergner H., Hückel C. Studies on the in vivo-action of leupeptin on the nitrogen retention in rats. Acta Biol Med Ger. 1977;36(11-12):1923–1927. [PubMed] [Google Scholar]
- Tamai M., Matsumoto K., Omura S., Koyama I., Ozawa Y., Hanada K. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986 Aug;9(8):672–677. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- Tamai M., Omura S., Kimura M., Hanada K., Sugita H. Prolongation of life span of dystrophic hamster by cysteine proteinase inhibitor, loxistation (EST). J Pharmacobiodyn. 1987 Nov;10(11):678–681. doi: 10.1248/bpb1978.10.678. [DOI] [PubMed] [Google Scholar]
- Yamada H., Hayashi H., Natori Y. A simple procedure for the isolation of highly purified lysosomes from normal rat liver. J Biochem. 1984 Apr;95(4):1155–1160. doi: 10.1093/oxfordjournals.jbchem.a134704. [DOI] [PubMed] [Google Scholar]