Abstract
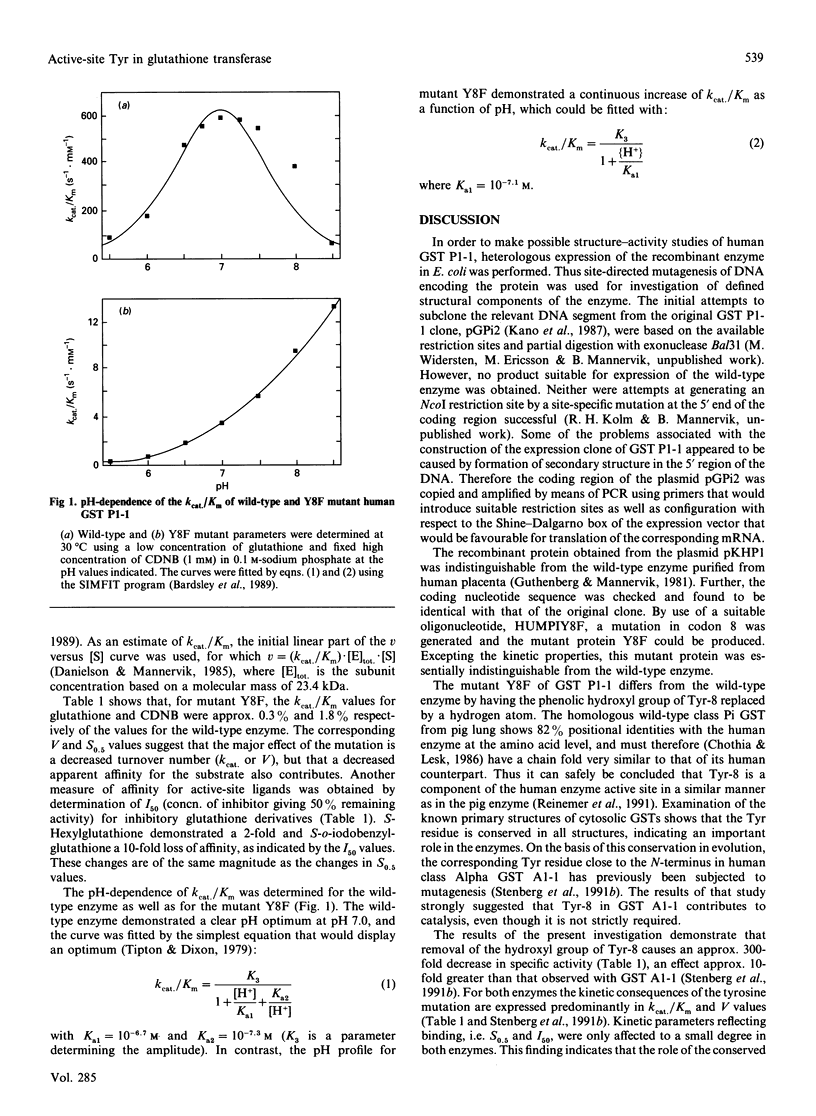
The coding region of cDNA corresponding to human class Pi glutathione transferase P1-1 was amplified by the PCR, subcloned into an expression vector, pKHP1, expressed in Escherichia coli, and characterized. The physicochemical and catalytic properties of recombinant glutathione transferase P1-1 were indistinguishable from those of the enzyme previously isolated from human placenta. The active-site residue Tyr-8 of the wild-type enzyme was converted into Phe by means of oligonucleotide-directed mutagenesis. The mutant enzyme Y8F displayed a 300-fold decrease in specific activity, ascribable mainly to a lowered k(cat.) (or V) value. Kinetic parameters reflecting binding affinity, S0.5 (substrate concn. giving 1/2V) and I50 (concn. of inhibitor giving 50% remaining activity), were only moderately elevated in the mutant enzyme. These results indicate that Tyr-8 contributes primarily to catalysis as such, rather than to binding of the substrates. The dependence of k(cat.)/Km on pH shows an optimum at pH 7.0, defined by acidic and basic ionic dissociation constants with pKa1 = 6.7 and pKa2 = 7.3 respectively. The mutant enzyme Y8F does not display the basic limb of the k(cat.)/Km versus pH profile, but shows a monotonic increase of k(cat.)/Km with an apparent pKa1 of 7.1. The results indicate that the phenolic hydroxyl group of Tyr-8 in un-ionized form, but not the phenolate of Tyr-8, contributes to catalysis by glutathione transferase P1-1.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björnestedt R., Widersten M., Board P. G., Mannervik B. Design of two chimaeric human-rat class alpha glutathione transferases for probing the contribution of C-terminal segments of protein structure to the catalytic properties. Biochem J. 1992 Mar 1;282(Pt 2):505–510. doi: 10.1042/bj2820505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. The use of pH studies to determine chemical mechanisms of enzyme-catalyzed reactions. Methods Enzymol. 1982;87:390–405. doi: 10.1016/s0076-6879(82)87024-9. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Mannervik B. Kinetic independence of the subunits of cytosolic glutathione transferase from the rat. Biochem J. 1985 Oct 15;231(2):263–267. doi: 10.1042/bj2310263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graminski G. F., Kubo Y., Armstrong R. N. Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry. 1989 Apr 18;28(8):3562–3568. doi: 10.1021/bi00434a062. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Mannervik B. Glutathione S-transferase (transferase pi) from human placenta is identical or closely related to glutathione S-transferase (transferase rho) from erythrocytes. Biochim Biophys Acta. 1981 Oct 13;661(2):255–260. doi: 10.1016/0005-2744(81)90012-7. [DOI] [PubMed] [Google Scholar]
- Kano T., Sakai M., Muramatsu M. Structure and expression of a human class pi glutathione S-transferase messenger RNA. Cancer Res. 1987 Nov 1;47(21):5626–5630. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Awasthi Y. C., Board P. G., Hayes J. D., Di Ilio C., Ketterer B., Listowsky I., Morgenstern R., Muramatsu M., Pearson W. R. Nomenclature for human glutathione transferases. Biochem J. 1992 Feb 15;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reinemer P., Dirr H. W., Ladenstein R., Schäffer J., Gallay O., Huber R. The three-dimensional structure of class pi glutathione S-transferase in complex with glutathione sulfonate at 2.3 A resolution. EMBO J. 1991 Aug;10(8):1997–2005. doi: 10.1002/j.1460-2075.1991.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G., Board P. G., Carlberg I., Mannervik B. Effects of directed mutagenesis on conserved arginine residues in a human Class Alpha glutathione transferase. Biochem J. 1991 Mar 1;274(Pt 2):549–555. doi: 10.1042/bj2740549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G., Board P. G., Mannervik B. Mutation of an evolutionarily conserved tyrosine residue in the active site of a human class Alpha glutathione transferase. FEBS Lett. 1991 Nov 18;293(1-2):153–155. doi: 10.1016/0014-5793(91)81174-7. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F., Dixon H. B. Effects of pH on enzymes. Methods Enzymol. 1979;63:183–234. doi: 10.1016/0076-6879(79)63011-2. [DOI] [PubMed] [Google Scholar]
- Widersten M., Pearson W. R., Engström A., Mannervik B. Heterologous expression of the allelic variant mu-class glutathione transferases mu and psi. Biochem J. 1991 Jun 1;276(Pt 2):519–524. doi: 10.1042/bj2760519. [DOI] [PMC free article] [PubMed] [Google Scholar]


