Abstract
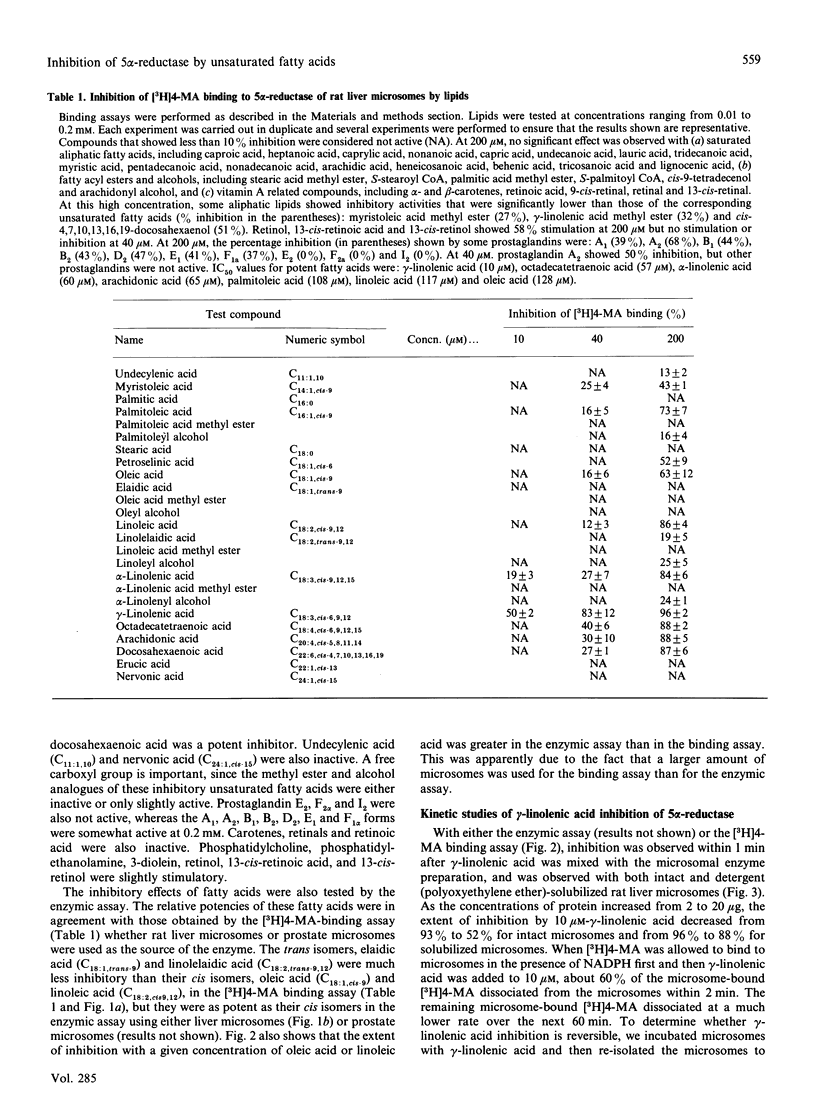
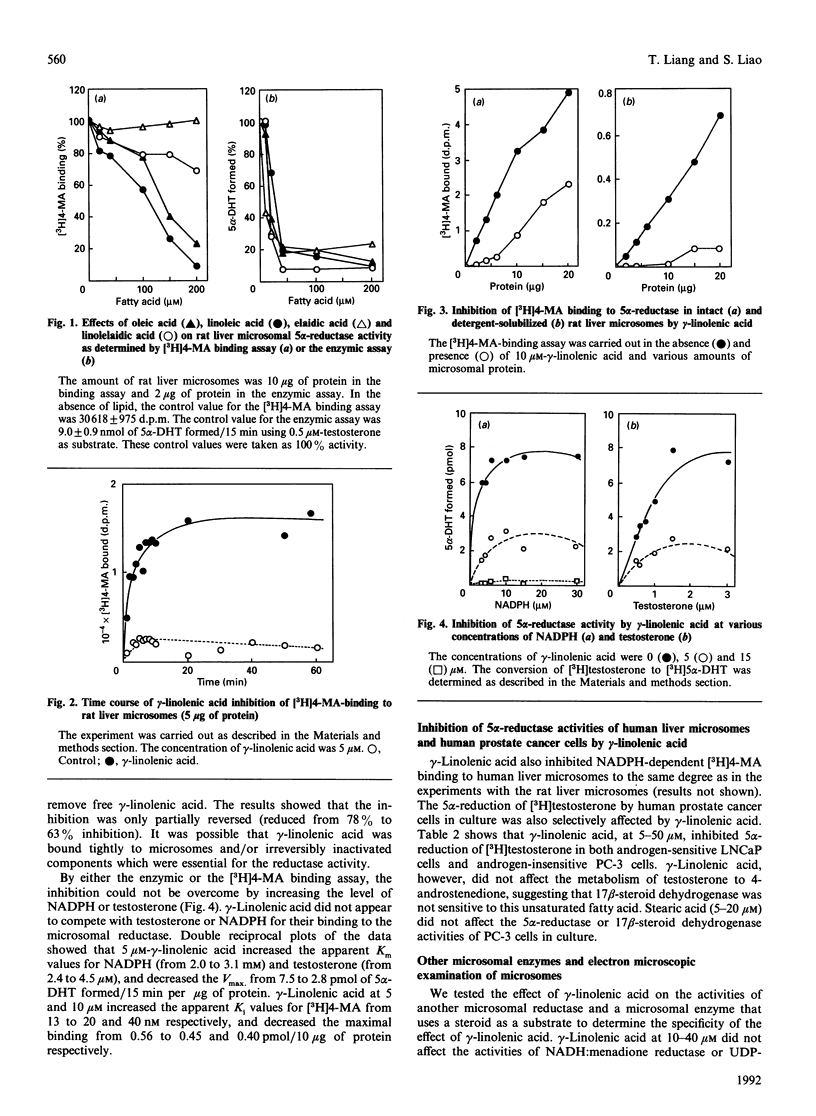
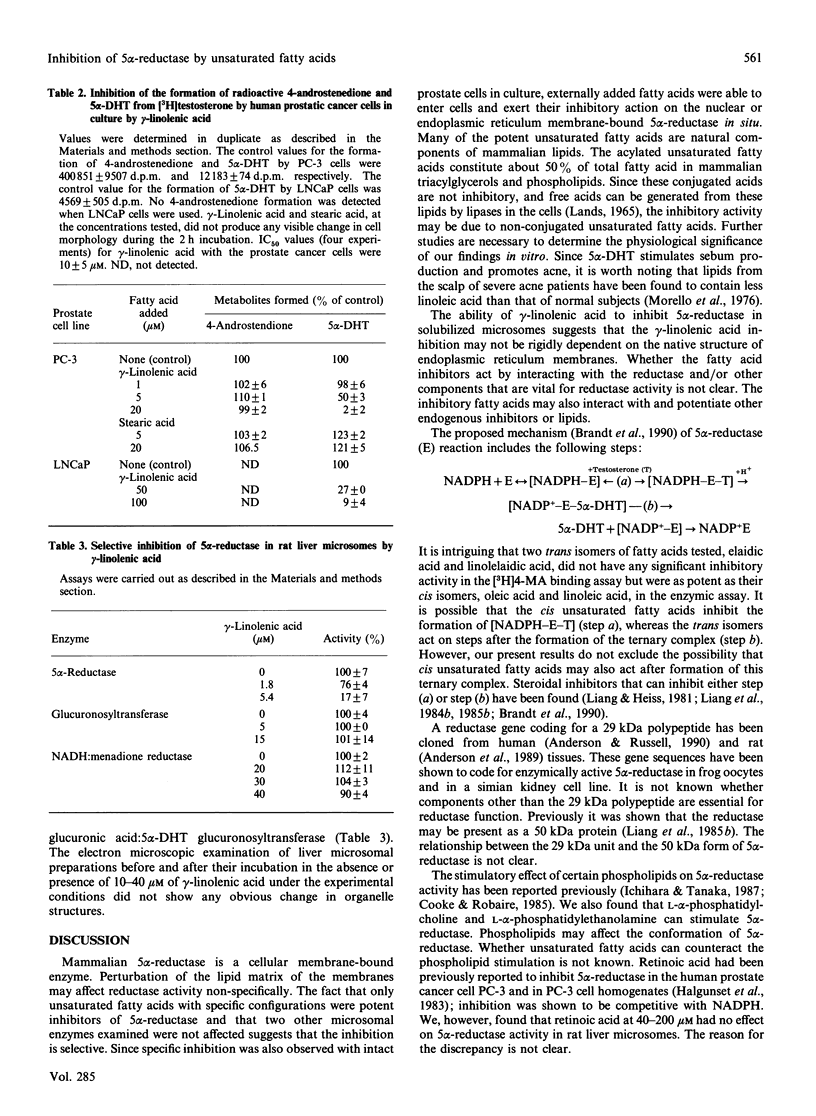
Human or rat microsomal 5 alpha-reductase activity, as measured by enzymic conversion of testosterone into 5 alpha-dihydrotestosterone or by binding of a competitive inhibitor, [3H]17 beta-NN-diethulcarbamoyl-4-methyl-4-aza-5 alpha-androstan-3-one ([3H]4-MA) to the reductase, is inhibited by low concentrations (less than 10 microM) of certain polyunsaturated fatty acids. The relative inhibitory potencies of unsaturated fatty acids are, in decreasing order: gamma-linolenic acid greater than cis-4,7,10,13,16,19-docosahexaenoic acid = cis-6,9,12,15-octatetraenoic acid = arachidonic acid = alpha-linolenic acid greater than linoleic acid greater than palmitoleic acid greater than oleic acid greater than myristoleic acid. Other unsaturated fatty acids such as undecylenic acid, erucic acid and nervonic acid, are inactive. The methyl esters and alcohol analogues of these compounds, glycerols, phospholipids, saturated fatty acids, retinoids and carotenes were inactive even at 0.2 mM. The results of the binding assay and the enzymic assay correlated well except for elaidic acid and linolelaidic acid, the trans isomers of oleic acid and linoleic acid respectively, which were much less active than their cis isomers in the binding assay but were as potent in the enzymic assay. gamma-Linolenic acid had no effect on the activities of two other rat liver microsomal enzymes: NADH:menadione reductase and glucuronosyl transferase. gamma-Linolenic acid, the most potent inhibitor tested, decreased the Vmax. and increased Km values of substrates, NADPH and testosterone, and promoted dissociation of [3H]4-MA from the microsomal reductase. gamma-Linolenic acid, but not the corresponding saturated fatty acid (stearic acid), inhibited the 5 alpha-reductase activity, but not the 17 beta-dehydrogenase activity, of human prostate cancer cells in culture. These results suggest that unsaturated fatty acids may play an important role in regulating androgen action in target cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Bishop R. W., Russell D. W. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989 Sep 25;264(27):16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Andersson S., Russell D. W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc Natl Acad Sci U S A. 1990 May;87(10):3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba A., Tatsuno T., Iwata H. Modulation by unsaturated fatty acids of norepinephrine- and adenosine-induced formation of cyclic AMP in brain slices. J Neurochem. 1984 Jan;42(1):192–197. doi: 10.1111/j.1471-4159.1984.tb09716.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandt M., Greway A. T., Holt D. A., Metcalf B. W., Levy M. A. Studies on the mechanism of steroid 5 alpha-reductase inhibition by 3-carboxy A-ring aryl steroids. J Steroid Biochem Mol Biol. 1990 Nov 30;37(4):575–579. doi: 10.1016/0960-0760(90)90403-8. [DOI] [PubMed] [Google Scholar]
- Brooks J. R., Berman C., Hichens M., Primka R. L., Reynolds G. F., Rasmusson G. H. Biological activities of a new steroidal inhibitor of delta 4-5 alpha-reductase. Proc Soc Exp Biol Med. 1982 Jan;169(1):67–73. [PubMed] [Google Scholar]
- Bégin M. E. Fatty acids, lipid peroxidation and diseases. Proc Nutr Soc. 1990 Jul;49(2):261–267. doi: 10.1079/pns19900029. [DOI] [PubMed] [Google Scholar]
- Cooke G. M., Robaire B. Modulation of epididymal delta 4-steroid 5 alpha-reductase activity in vitro by the phospholipid environment. J Biol Chem. 1985 Jun 25;260(12):7489–7495. [PubMed] [Google Scholar]
- Dell K. R., Severson D. L. Effect of cis-unsaturated fatty acids on aortic protein kinase C activity. Biochem J. 1989 Feb 15;258(1):171–175. doi: 10.1042/bj2580171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Kervina M. Subcellular fractionation of rat liver. Methods Enzymol. 1974;31:6–41. doi: 10.1016/0076-6879(74)31005-1. [DOI] [PubMed] [Google Scholar]
- George F. W., Johnson L., Wilson J. D. The effect of a 5 alpha-reductase inhibitor on androgen physiology in the immature male rat. Endocrinology. 1989 Nov;125(5):2434–2438. doi: 10.1210/endo-125-5-2434. [DOI] [PubMed] [Google Scholar]
- Gormley G. J., Stoner E., Rittmaster R. S., Gregg H., Thompson D. L., Lasseter K. C., Vlasses P. H., Stein E. A. Effects of finasteride (MK-906), a 5 alpha-reductase inhibitor, on circulating androgens in male volunteers. J Clin Endocrinol Metab. 1990 Apr;70(4):1136–1141. doi: 10.1210/jcem-70-4-1136. [DOI] [PubMed] [Google Scholar]
- Halgunset J., Sunde A., Lundmo P. I. Retinoic acid (RA): an inhibitor of 5 alpha-reductase in human prostatic cancer cells. J Steroid Biochem. 1987 Dec;28(6):731–736. doi: 10.1016/0022-4731(87)90405-5. [DOI] [PubMed] [Google Scholar]
- Herold P. M., Kinsella J. E. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. Am J Clin Nutr. 1986 Apr;43(4):566–598. doi: 10.1093/ajcn/43.4.566. [DOI] [PubMed] [Google Scholar]
- Ichihara K., Tanaka C. Specific stimulation of steroid 5 alpha-reductase solubilized from rat liver microsomes by endogenous phosphatidylserine. Biochem Biophys Res Commun. 1987 Dec 16;149(2):482–487. doi: 10.1016/0006-291x(87)90393-7. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Peterson R. E., Gautier T., Sturla E. Male pseudohermaphroditism secondary to 5 alpha-reductase deficiency--a model for the role of androgens in both the development of the male phenotype and the evolution of a male gender identity. J Steroid Biochem. 1979 Jul;11(1B):637–645. doi: 10.1016/0022-4731(79)90093-1. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Shackleton C., Orlic S., Stoner E. C19 and C21 5 beta/5 alpha metabolite ratios in subjects treated with the 5 alpha-reductase inhibitor finasteride: comparison of male pseudohermaphrodites with inherited 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1990 Mar;70(3):777–782. doi: 10.1210/jcem-70-3-777. [DOI] [PubMed] [Google Scholar]
- Karmali R. A., Marsh J., Fuchs C. Effect of omega-3 fatty acids on growth of a rat mammary tumor. J Natl Cancer Inst. 1984 Aug;73(2):457–461. doi: 10.1093/jnci/73.2.457. [DOI] [PubMed] [Google Scholar]
- Kato J. Arachidonic acid as a possible modulator of estrogen, progestin, androgen, and glucocorticoid receptors in the central and peripheral tissues. J Steroid Biochem. 1989;34(1-6):219–227. doi: 10.1016/0022-4731(89)90085-x. [DOI] [PubMed] [Google Scholar]
- LANDS W. E. LIPID METABOLISM. Annu Rev Biochem. 1965;34:313–346. doi: 10.1146/annurev.bi.34.070165.001525. [DOI] [PubMed] [Google Scholar]
- Liang T., Brady E. J., Cheung A., Saperstein R. Inhibition of luteinizing hormone (LH)-releasing hormone-induced secretion of LH in rat anterior pituitary cell culture by testosterone without conversion to 5 alpha-dihydrotestosterone. Endocrinology. 1984 Dec;115(6):2311–2317. doi: 10.1210/endo-115-6-2311. [DOI] [PubMed] [Google Scholar]
- Liang T., Cascieri M. A., Cheung A. H., Reynolds G. F., Rasmusson G. H. Species differences in prostatic steroid 5 alpha-reductases of rat, dog, and human. Endocrinology. 1985 Aug;117(2):571–579. doi: 10.1210/endo-117-2-571. [DOI] [PubMed] [Google Scholar]
- Liang T., Cheung A. H., Reynolds G. F., Rasmusson G. H. Photoaffinity labeling of steroid 5 alpha-reductase of rat liver and prostate microsomes. J Biol Chem. 1985 Apr 25;260(8):4890–4895. [PubMed] [Google Scholar]
- Liang T., Heiss C. E., Cheung A. H., Reynolds G. F., Rasmusson G. H. 4-Azasteroidal 5 alpha-reductase inhibitors without affinity for the androgen receptor. J Biol Chem. 1984 Jan 25;259(2):734–739. [PubMed] [Google Scholar]
- Liang T., Heiss C. E. Inhibition of 5 alpha-reductase, receptor binding, and nuclear uptake of androgens in the prostate by a 4-methyl-4-aza-steroid. J Biol Chem. 1981 Aug 10;256(15):7998–8005. [PubMed] [Google Scholar]
- Liang T., Heiss C. E., Ostrove S., Rasmusson G. H., Cheung A. Binding of a 4-methyl-4-aza-steroid to 5 alpha-reductase of rat liver and prostate microsomes. Endocrinology. 1983 Apr;112(4):1460–1468. doi: 10.1210/endo-112-4-1460. [DOI] [PubMed] [Google Scholar]
- Liang T., Hiipakka R. A., Stebbins J., Liao S. Anti-5 alpha-reductase autoantibodies in the serum of patients with prostatic cancer. J Clin Endocrinol Metab. 1990 Dec;71(6):1666–1668. doi: 10.1210/jcem-71-6-1666. [DOI] [PubMed] [Google Scholar]
- Liao S. S., Kokontis J., Sai T., Hiipakka R. A. Androgen receptors: structures, mutations, antibodies and cellular dynamics. J Steroid Biochem. 1989;34(1-6):41–51. doi: 10.1016/0022-4731(89)90064-2. [DOI] [PubMed] [Google Scholar]
- Morello A. M., Downing D. T., Strauss J. S. Octadecadienoic acids in the skin surface lipids of acne patients and normal subjects. J Invest Dermatol. 1976 May;66(5):319–323. doi: 10.1111/1523-1747.ep12482300. [DOI] [PubMed] [Google Scholar]
- Nalbone G., Grynberg A., Chevalier A., Leonardi J., Termine E., Lafont H. Phospholipase A activity of cultured rat ventricular myocyte is affected by the nature of cellular polyunsaturated fatty acids. Lipids. 1990 Jun;25(6):301–306. doi: 10.1007/BF02544337. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Phillipson B. E., Rothrock D. W., Connor W. E., Harris W. S., Illingworth D. R. Reduction of plasma lipids, lipoproteins, and apoproteins by dietary fish oils in patients with hypertriglyceridemia. N Engl J Med. 1985 May 9;312(19):1210–1216. doi: 10.1056/NEJM198505093121902. [DOI] [PubMed] [Google Scholar]
- Rittmaster R. S., Stoner E., Thompson D. L., Nance D., Lasseter K. C. Effect of MK-906, a specific 5 alpha-reductase inhibitor, on serum androgens and androgen conjugates in normal men. J Androl. 1989 Jul-Aug;10(4):259–262. doi: 10.1002/j.1939-4640.1989.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Szepesi B., Kamara A. K., Clarke S. D. Lack of specificity of polyunsaturated fats in the inhibition of rat liver glucose-6-phosphate dehydrogenase. J Nutr. 1989 Feb;119(2):161–165. doi: 10.1093/jn/119.2.161. [DOI] [PubMed] [Google Scholar]
- Tesoriere L., Bongiorno A., Livrea M. A., Bono A. Modulation by docosahexaenoic acid of the epinephrine-stimulated adenylate cyclase activity of the bovine retina. J Neurochem. 1988 Sep;51(3):704–709. doi: 10.1111/j.1471-4159.1988.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Vallette G., Christeff N., Bogard C., Benassayag C., Nunez E. Dynamic pattern of estradiol binding to uterine receptors of the rat. Inhibition and stimulation by unsaturated fatty acids. J Biol Chem. 1988 Mar 15;263(8):3639–3645. [PubMed] [Google Scholar]
- Vermeulen A., Giagulli V. A., De Schepper P., Buntinx A., Stoner E. Hormonal effects of an orally active 4-azasteroid inhibitor of 5 alpha-reductase in humans. Prostate. 1989;14(1):45–53. doi: 10.1002/pros.2990140106. [DOI] [PubMed] [Google Scholar]
- Ziboh V. A., Miller C. C. Essential fatty acids and polyunsaturated fatty acids: significance in cutaneous biology. Annu Rev Nutr. 1990;10:433–450. doi: 10.1146/annurev.nu.10.070190.002245. [DOI] [PubMed] [Google Scholar]
- Zuniga M. E., Lokesh B. R., Kinsella J. E. Disparate effects of dietary fatty acids on activity of 5'-nucleotidase of rat liver plasma membrane. J Nutr. 1989 Feb;119(2):152–160. doi: 10.1093/jn/119.2.152. [DOI] [PubMed] [Google Scholar]


