Abstract
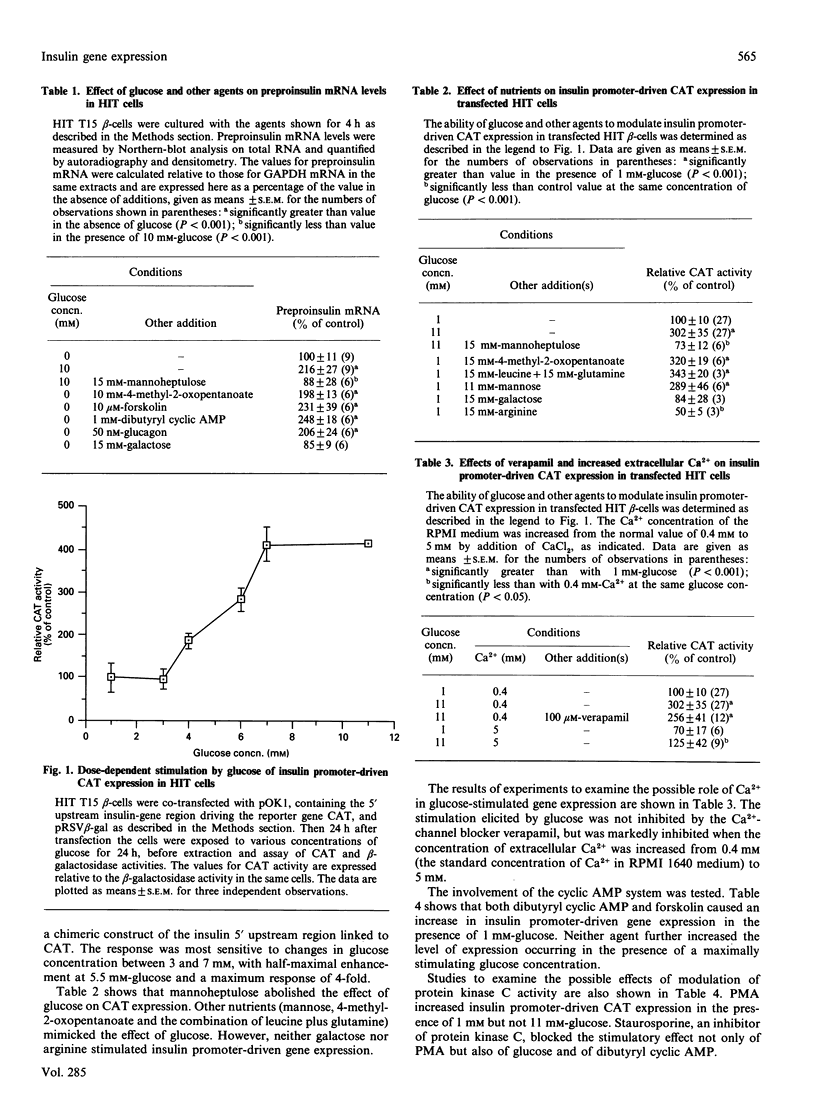
Northern-blot analysis was used to demonstrate that an increase in extracellular glucose concentration increased the content of preproinsulin mRNA 2.3-fold in the beta-cell line HIT T15. A probe for the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase was used as a control. Mannoheptulose blocked this effect of glucose. A stimulatory effect on preproinsulin mRNA levels was also observed in response to mannose and to 4-methyl-2-oxopentanoate. However, galactose and arginine were ineffective. Glucagon, forskolin and dibutyryl cyclic AMP also elicited an increase in HIT-cell preproinsulin mRNA. The ability of the 5' upstream region of the preproinsulin gene to mediate the effect of glucose and other metabolites on transcription was studied by using a bacterial reporter gene technique. HIT cells were transfected with a plasmid, pOK1, containing the upstream region of the rat insulin-1 gene (-345 to +1) linked to chloramphenicol acetyltransferase (CAT). Co-transfection with a plasmid pRSV beta-gal containing beta-galactosidase driven by the Rous sarcoma virus promoter was used as a control for the efficiency of transfection; expression of CAT activity in transfected HIT cells was normalized by reference to expression of beta-galactosidase. Glucose caused a dose-dependent increase in expression of CAT activity, with a half-maximal effect at 5.5 mM and a maximum response of 4-fold. Mannoheptulose blocked this effect of glucose. Other metabolites (mannose, 4-methyl-2-oxopentanoate and leucine plus glutamine) were also able to increase insulin promoter-driven CAT expression, but galactose and arginine were ineffective. The stimulatory effect of glucose on CAT expression was not blocked by verapamil and was inhibited by increasing extracellular Ca2+ from 0.4 to 5 mM. Both dibutyryl cyclic AMP and forskolin caused an increase in insulin promoter-driven gene expression in the presence of 1 mM-glucose, but neither agent further increased the level of expression occurring in the presence of a maximally stimulating glucose concentration. The phorbol ester phorbol 12-myristate 13-acetate (PMA) also increased insulin promoter-driven CAT expression in the presence of 1 mM-, but not 11 mM-glucose. Staurosporine blocked the stimulatory effect not only of PMA but also of glucose and of dibutyryl cyclic AMP. We conclude that the 5' upstream region of the insulin gene contains sequences responsible for mediating the stimulatory effect of glucose on insulin-gene transcription.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. Stimulaiton of insulin biosynthesis in isolated mouse islets by L-leucine, 2-aminonorbornane-2-carboxylic acid and alpha-ketoisocaproic acid. Biochim Biophys Acta. 1976 Jul 21;437(2):345–353. doi: 10.1016/0304-4165(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M., Harrison D. E., Ashcroft S. J. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. 1984 Nov 29-Dec 5Nature. 312(5993):446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Bunce J., Lowry M., Hansen S. E., Hedeskov C. J. The effect of sugars on (pro)insulin biosynthesis. Biochem J. 1978 Aug 15;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J. Glucoreceptor mechanisms and the control of insulin release and biosynthesis. Diabetologia. 1980 Jan;18(1):5–15. doi: 10.1007/BF01228295. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hammonds P., Harrison D. E. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986 Oct;29(10):727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Hughes S. J. Protein phosphorylation in the regulation of insulin secretion and biosynthesis. Biochem Soc Trans. 1990 Feb;18(1):116–118. doi: 10.1042/bst0180116. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Sanchez-Pescador R. Sequence of a cDNA encoding Syrian hamster preproinsulin. Diabetes. 1984 Mar;33(3):297–300. doi: 10.2337/diab.33.3.297. [DOI] [PubMed] [Google Scholar]
- Boam D. S., Clark A. R., Docherty K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem. 1990 May 15;265(14):8285–8296. [PubMed] [Google Scholar]
- Boam D. S., Docherty K. A tissue-specific nuclear factor binds to multiple sites in the human insulin-gene enhancer. Biochem J. 1989 Nov 15;264(1):233–239. doi: 10.1042/bj2640233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstedt J., Chan S. J. Direct effect of glucose on the preproinsulin mRNA level in isolated pancreatic islets. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1383–1389. doi: 10.1016/0006-291x(82)91267-0. [DOI] [PubMed] [Google Scholar]
- Bucchini D., Ripoche M. A., Stinnakre M. G., Desbois P., Lorès P., Monthioux E., Absil J., Lepesant J. A., Pictet R., Jami J. Pancreatic expression of human insulin gene in transgenic mice. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2511–2515. doi: 10.1073/pnas.83.8.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles S., Tamagawa T., Henquin J. C. A single mechanism for the stimulation of insulin release and 86Rb+ efflux from rat islets by cationic amino acids. Biochem J. 1982 Nov 15;208(2):301–308. doi: 10.1042/bj2080301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cordle S. R., Whelan J., Henderson E., Masuoka H., Weil P. A., Stein R. Insulin gene expression in nonexpressing cells appears to be regulated by multiple distinct negative-acting control elements. Mol Cell Biol. 1991 May;11(5):2881–2886. doi: 10.1128/mcb.11.5.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe D. T., Tsai M. J. Mutagenesis of the rat insulin II 5'-flanking region defines sequences important for expression in HIT cells. Mol Cell Biol. 1989 Apr;9(4):1784–1789. doi: 10.1128/mcb.9.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Walker M. D., Barr P. J., Rutter W. J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5' flanking elements. Science. 1985 Nov 22;230(4728):912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- Efrat S., Hanahan D. Bidirectional activity of the rat insulin II 5'-flanking region in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):192–198. doi: 10.1128/mcb.7.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Surana M., Fleischer N. Glucose induces insulin gene transcription in a murine pancreatic beta-cell line. J Biol Chem. 1991 Jun 15;266(17):11141–11143. [PubMed] [Google Scholar]
- Fromont-Racine M., Bucchini D., Madsen O., Desbois P., Linde S., Nielsen J. H., Saulnier C., Ripoche M. A., Jami J., Pictet R. Effect of 5'-flanking sequence deletions on expression of the human insulin gene in transgenic mice. Mol Endocrinol. 1990 May;4(5):669–677. doi: 10.1210/mend-4-5-669. [DOI] [PubMed] [Google Scholar]
- Garcia S. D., Jarrousse C., Rosselin G. Biosynthesis of proinsulin and insulin in newborn rat pancreas. Interaction of glucose, cyclic AMP, somatostatin, and sulfonylureas on the (3H) leucine incorporation into immunoreactive insulin. J Clin Invest. 1976 Jan;57(1):230–243. doi: 10.1172/JCI108264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J. M., Permutt M. A. Glucose regulated insulin biosynthesis in isolated rat pancreatic islets is accompanied by changes in proinsulin mRNA. Diabetes Res. 1985 Mar;2(2):71–75. [PubMed] [Google Scholar]
- Giddings S. J., Chirgwin J., Permutt M. A. Effects of glucose on proinsulin messenger RNA in rats in vivo. Diabetes. 1982 Jul;31(7):624–629. doi: 10.2337/diab.31.7.624. [DOI] [PubMed] [Google Scholar]
- Gold G., Qian R. L., Grodsky G. M. Insulin biosynthesis in HIT cells. Effects of glucose, forskolin, IBMX, and dexamethasone. Diabetes. 1988 Feb;37(2):160–165. doi: 10.2337/diab.37.2.160. [DOI] [PubMed] [Google Scholar]
- Gold G., Walker M. D., Edwards D. L., Grodsky G. M. Biosynthetic regulation of endogenous hamster insulin and exogenous rat insulin II in transfected HIT cells. Diabetes. 1988 Nov;37(11):1509–1514. doi: 10.2337/diab.37.11.1509. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guest P. C., Bailyes E. M., Rutherford N. G., Hutton J. C. Insulin secretory granule biogenesis. Co-ordinate regulation of the biosynthesis of the majority of constituent proteins. Biochem J. 1991 Feb 15;274(Pt 1):73–78. doi: 10.1042/bj2740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds P., Schofield P. N., Ashcroft S. J. Glucose regulates preproinsulin messenger RNA levels in a clonal cell line of simian virus 40-transformed B cells. FEBS Lett. 1987 Mar 9;213(1):149–154. doi: 10.1016/0014-5793(87)81481-3. [DOI] [PubMed] [Google Scholar]
- Hammonds P., Schofield P. N., Ashcroft S. J., Sutton R., Gray D. W. Regulation and specificity of glucose-stimulated insulin gene expression in human islets of Langerhans. FEBS Lett. 1987 Oct 19;223(1):131–137. doi: 10.1016/0014-5793(87)80523-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Hughes S. J., Chalk J. G., Ashcroft S. J. The role of cytosolic free Ca2+ and protein kinase C in acetylcholine-induced insulin release in the clonal beta-cell line, HIT-T15. Biochem J. 1990 Apr 1;267(1):227–232. doi: 10.1042/bj2670227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. J., Christie M. R., Ashcroft S. J. Potentiators of insulin secretion modulate Ca2+ sensitivity in rat pancreatic islets. Mol Cell Endocrinol. 1987 Apr;50(3):231–236. doi: 10.1016/0303-7207(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Hwung Y. P., Gu Y. Z., Tsai M. J. Cooperativity of sequence elements mediates tissue specificity of the rat insulin II gene. Mol Cell Biol. 1990 Apr;10(4):1784–1788. doi: 10.1128/mcb.10.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Edlund T., Moss J. B., Rutter W. J., Walker M. D. A mutational analysis of the insulin gene transcription control region: expression in beta cells is dependent on two related sequences within the enhancer. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8819–8823. doi: 10.1073/pnas.84.24.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Thor S., Norberg T., Ohlsson H., Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990 Apr 26;344(6269):879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- Karlsson O., Walker M. D., Rutter W. J., Edlund T. Individual protein-binding domains of the insulin gene enhancer positively activate beta-cell-specific transcription. Mol Cell Biol. 1989 Feb;9(2):823–827. doi: 10.1128/mcb.9.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B. J. An apparent inhibition of insulin biosynthesis resulting from inhibition of transport of neutral amino acids by arginine. Diabetologia. 1977 Jan;13(1):77–82. doi: 10.1007/BF00996331. [DOI] [PubMed] [Google Scholar]
- Lin B. J., Haist R. E. Effects of some modifiers of insulin secretion on insulin biosynthesis. Endocrinology. 1973 Mar;92(3):735–742. doi: 10.1210/endo-92-3-735. [DOI] [PubMed] [Google Scholar]
- Lin B. J., Haist R. E. Insulin biosynthesis: effects of carbohydrates and related compounds. Can J Physiol Pharmacol. 1969 Sep;47(9):791–801. doi: 10.1139/y69-133. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Gonzalez G. A., Yamamoto K. K. Regulation of cAMP-inducible genes by CREB. Trends Neurosci. 1990 May;13(5):184–188. doi: 10.1016/0166-2236(90)90045-c. [DOI] [PubMed] [Google Scholar]
- Nielsen D. A., Welsh M., Casadaban M. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. I. Effects of glucose and cyclic AMP on the transcription of insulin mRNA. J Biol Chem. 1985 Nov 5;260(25):13585–13589. [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H., Edlund T. Sequence-specific interactions of nuclear factors with the insulin gene enhancer. Cell. 1986 Apr 11;45(1):35–44. doi: 10.1016/0092-8674(86)90535-0. [DOI] [PubMed] [Google Scholar]
- Permutt M. A. Effect of glucose on initiation and elongation rates in isolated rat pancreatic islets. J Biol Chem. 1974 May 10;249(9):2738–2742. [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. II. Effect of glucose on ribonucleic acid synthesis in isolated rat islets. J Biol Chem. 1972 Feb 25;247(4):1200–1207. [PubMed] [Google Scholar]
- Philippe J., Missotten M. Functional characterization of a cAMP-responsive element of the rat insulin I gene. J Biol Chem. 1990 Jan 25;265(3):1465–1469. [PubMed] [Google Scholar]
- Philippe J. Structure and pancreatic expression of the insulin and glucagon genes. Endocr Rev. 1991 Aug;12(3):252–271. doi: 10.1210/edrv-12-3-252. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden R. F., Skośkiewicz M. J., Howie K. B., Russell P. S., Goodman H. M. Regulation of human insulin gene expression in transgenic mice. 1986 May 29-Jun 4Nature. 321(6069):525–528. doi: 10.1038/321525a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J., Ishii S., Seino Y., Imamoto F., Imura H. Negative regulation of human insulin gene expression by the 5'-flanking region in non-pancreatic cells. FEBS Lett. 1989 Apr 10;247(1):41–45. doi: 10.1016/0014-5793(89)81236-0. [DOI] [PubMed] [Google Scholar]
- Walker M. D., Edlund T., Boulet A. M., Rutter W. J. Cell-specific expression controlled by the 5'-flanking region of insulin and chymotrypsin genes. Nature. 1983 Dec 8;306(5943):557–561. doi: 10.1038/306557a0. [DOI] [PubMed] [Google Scholar]
- Welsh M., Brunstedt J., Hellerström C. Effects of D-glucose, L-leucine, and 2-ketoisocaproate on insulin mRNA levels in mouse pancreatic islets. Diabetes. 1986 Feb;35(2):228–231. doi: 10.2337/diab.35.2.228. [DOI] [PubMed] [Google Scholar]
- Welsh M. Glucose regulation of insulin gene expression. Diabete Metab. 1989 Nov-Dec;15(6):367–371. [PubMed] [Google Scholar]
- Welsh M., Nielsen D. A., MacKrell A. J., Steiner D. F. Control of insulin gene expression in pancreatic beta-cells and in an insulin-producing cell line, RIN-5F cells. II. Regulation of insulin mRNA stability. J Biol Chem. 1985 Nov 5;260(25):13590–13594. [PubMed] [Google Scholar]
- Welsh M., Scherberg N., Gilmore R., Steiner D. F. Translational control of insulin biosynthesis. Evidence for regulation of elongation, initiation and signal-recognition-particle-mediated translational arrest by glucose. Biochem J. 1986 Apr 15;235(2):459–467. doi: 10.1042/bj2350459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M., Weber T., Wrange O., Nielsen D. A., Matthieu M., Steiner D. F. Regulation of insulin gene expression by dexamethasone, Ca2+ and a phorbol ester. Biomed Biochim Acta. 1988;47(4-5):299–303. [PubMed] [Google Scholar]
- Welsh N., Welsh M., Steiner D. F., Hellerström C. Mechanisms of leucine- and theophylline-stimulated insulin biosynthesis in isolated rat pancreatic islets. Biochem J. 1987 Aug 15;246(1):245–248. doi: 10.1042/bj2460245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan J., Poon D., Weil P. A., Stein R. Pancreatic beta-cell-type-specific expression of the rat insulin II gene is controlled by positive and negative cellular transcriptional elements. Mol Cell Biol. 1989 Aug;9(8):3253–3259. doi: 10.1128/mcb.9.8.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]


