Abstract
Pancreatic islet cells, and in particular insulin-producing beta cells, are centrally involved in the pathogenesis of diabetes mellitus. These cells are of paramount importance for the endocrine control of glycemia and glucose metabolism. In Type 1 Diabetes, islet beta cells are lost due to an autoimmune attack. In Type 2 Diabetes, beta cells become dysfunctional and insufficient to counterbalance insulin resistance in peripheral tissues. Therapeutic agents have been developed to support the function of islet cells, as well as to inhibit deleterious immune responses and inflammation. Most of these agents have undesired effects due to systemic administration and off-target effects. Typically, only a small fraction of therapeutic agent reaches the desired niche in the pancreas. Because islets and their beta cells are scattered throughout the pancreas, access to the niche is limited. Targeted delivery to pancreatic islets could dramatically improve the therapeutic effect, lower the dose requirements, and lower the side effects of agents administered systemically. Targeted delivery is especially relevant for those therapeutics for which the manufacturing is difficult and costly, such as cells, exosomes, and microvesicles. Along with therapeutic agents, imaging reagents intended to quantify the beta cell mass could benefit from targeted delivery. Several methods have been developed to improve the delivery of agents to pancreatic islets. Intraarterial administration in the pancreatic artery is a promising surgical approach, but it has inherent risks. Targeted delivery strategies have been developed based on ligands for cell surface molecules specific to islet cells or inflamed vascular endothelial cells. Delivery methods range from nanocarriers and vectors to deliver pharmacological agents to viral and non-viral vectors for the delivery of genetic constructs. Several strategies demonstrated enhanced therapeutic effects in diabetes with lower amounts of therapeutic agents and lower off-target side effects. Microvesicles, exosomes, polymer-based vectors, and nanocarriers are gaining popularity for targeted delivery. Notably, liposomes, lipid-assisted nanocarriers, and cationic polymers can be bioengineered to be immune-evasive, and their advantages to transport cargos into target cells make them appealing for pancreatic islet-targeted delivery. Viral vectors have become prominent tools for targeted gene delivery. In this review, we discuss the latest strategies for targeted delivery of therapeutic agents and imaging reagents to pancreatic islet cells.
Keywords: Targeted delivery, Pancreatic islet, Beta cells, Diabetes, Type 1 diabetes, Type 2 diabetes, T1D, T2D, Nanocarriers, Cell therapy, Exosomes, Microvesicles
1. Diabetes mellitus, pancreatic islets, and insulin-producing beta cells
In the USA, diabetes mellitus affects more than 9% of the population, is the seventh leading cause of death, and its incidence is increasing (Cowie et al., 2018). Diabetes mellitus manifests clinically with elevation of glucose concentration in the blood – a condition termed hyperglycemia. Chronic hyperglycemia and broad, rapid swings in glycemia cause devastating acute and chronic complications. This disease results from a lack, dysfunction, or insufficiency of pancreatic islet beta cells, the cells that release insulin to control glucose metabolism (Fig. 1) (Cowie et al., 2018). Patients with type 1 diabetes mellitus (T1D) and advanced type 2 diabetes (T2D) require life-long treatment with exogenous insulin to survive. Despite advances in treatment strategies, complications of diabetes mellitus, hypoglycemic unawareness, and severe hypoglycemic episodes still lead to high morbidity and mortality in advanced disease stages.
Fig. 1.
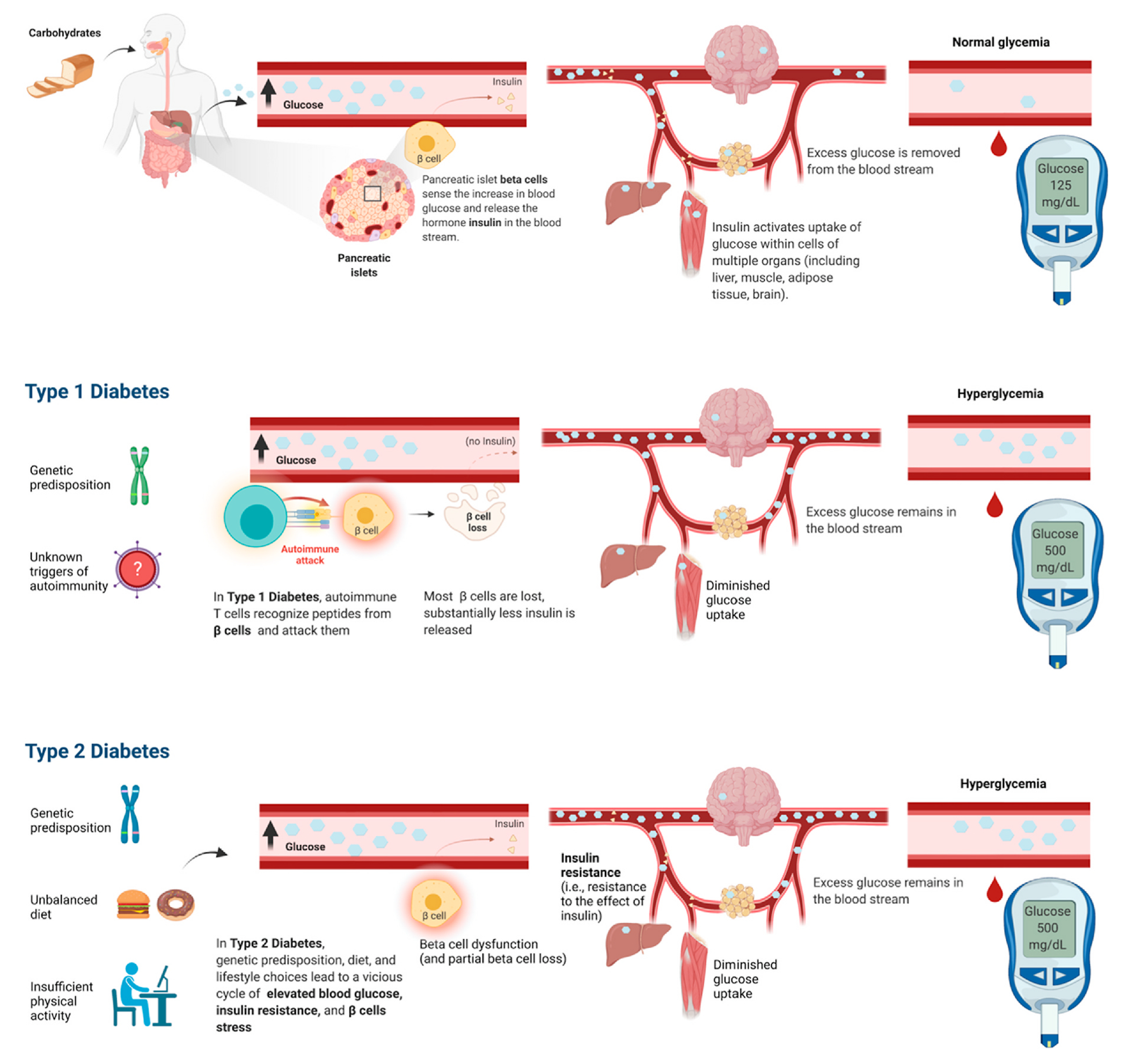
Beta cells control glucose homeostasis via insulin secretion. These cells are centrally involved in Type 1 Diabetes (T1D) and Type 2 Diabetes (T2D).
The various forms of diabetes mellitus, including T1D, T2D, and genetic forms, involve endocrine cells and primarily beta cells with a central role in the hormonal control of glucose metabolism. These endocrine cells reside in the pancreas, clustered in groups of cells that are termed pancreatic islets or islets of Langerhans and that constitute approximately 1–2% of the volume of the pancreas (Abdulreda and Berggren, 2021). The main constituents of pancreatic islets are insulin-secreting beta cells and glucagon-secreting alpha cells.
A rise in blood glucose levels, such as after the ingestion of a meal, stimulates islet beta cells to release the hormone insulin. Insulin has systemic effects, determining glucose utilization for energy and energy storage in various tissues, hence lowering blood glucose levels (Fig. 1).
In certain individuals, an autoimmune attack leads to a progressive loss of beta cells and thus loss of insulin release, resulting in T1D (Fig. 1) (Pugliese, 2014). In T1D patients, the autoimmune attack causes beta cell loss, and the residual beta cells become insufficient to adequately regulate body glycemia, leading to onset of life-threatening T1D (Eisenbarth, 2010). In other individuals, a combination of predisposing genetic factors, improper diet, and sedentary lifestyle determine chronic increase in blood glucose (hyperglycemia), paralleled by the acquisition of resistance to the effect of insulin in peripheral tissues (insulin resistance). Insulin resistance and chronic hyperglycemia impart beta cell stress and determine dysfunction of beta cells, leading to Type 2 Diabetes (T2D) (Fig. 1) (Cowie et al., 2018). In certain individuals, genetic variants impair development and function of pancreatic islet cells and insulin-producing beta cells, leading to genetic forms of diabetes mellitus.
2. Current and emerging therapeutics for diabetes mellitus
The current therapeutic options for diabetes mellitus include insulin therapy, intended to substitute the function of beta cells, and pancreas or pancreatic islet transplantation, intended to replace beta cells (see BOX 1). These therapeutic options have limitations. Insulin therapy has the potential to cause life-threatening iatrogenic hypoglycemia (a dangerous decrease of blood glucose due to the drug); moreover, it provides suboptimal glycemic and metabolic control. Pancreas transplantation has substantial risks of morbidity and mortality. Both pancreas and pancreatic islet transplantation are limited by insufficient donor organ availability and the requirement of chronic immunosuppression - which is associated with side effects and health risks (see BOX 1).
BOX 1. Current therapeutic options for diabetes mellitus, and their limitations.
Agents intended to substitute the function of beta cells: insulin therapy (relevant to: T1D, advanced T2D, certain genetic forms, and other rare forms of diabetes mellitus)
Insulin is the hormone secreted by beta cells in response to increased glucose concentration. Insulin is a century-old pharmacological agent, the go-to agent utilized to substitute the function of beta cells when they are lost (in T1D) or severely dysfunctional and insufficient (in advanced T2D and certain genetic forms of diabetes). A large fraction of patients with advanced diabetes mellitus requires insulin treatment to control glucose metabolism and survive (Cowie et al., 2018). However, this treatment brings risks of life-threatening iatrogenic hypoglycemia and provides suboptimal metabolic control.
Current therapeutic approaches for T1D include daily insulin injections to maintain blood glucose control (Sutton et al., 2014; Reinhart and Panning, 2002; Sands et al., 2015). Exogenous insulin therapy is not a cure for T1D, it is life-long, and after decades of chronic treatment, it can become inadequate.
Replacement of beta cells: transplantation of pancreas and pancreatic islets (relevant to: T1D, advanced T2D, and other forms of diabetes mellitus)
The transplantation of pancreas or isolated pancreatic islets from deceased donors have been performed for decades to control blood glucose and prevent long-term complications of diabetes. The transplantation option is intended for patients with severe forms of diabetes (predominantly ‘brittle’, unstable T1D), forms that are difficult to manage with current pharmacological treatments. Even though whole organ pancreas transplantation is a major surgical procedure, with substantial risks of morbidity and mortality, it has become a common therapeutic intervention for patients with advanced T1D. Pancreas transplantation led to over 80% insulin independence in recipients one year after transplantation (Ferrer-Fabrega and Fernandez-Cruz, 2020; Ichii and Ricordi, 2009; Shapiro et al., 2003). Besides the application in brittle T1D, pancreas transplantation is also offered increasingly to patients who have advanced forms of T2D (10% of pancreas transplants in the U.S.), or who have lost pancreatic endocrine and exocrine function due to trauma, surgery, or chronic pancreatitis. The transplantation of adult pancreatic islets represents a safer alternative to whole pancreas transplantation (Ricordi et al., 2016; Lablanche et al., 2018; Ricordi and Japour, 2019). A successful phase 3 clinical trial of adult islet transplantation built the groundwork for islet cell replacement therapies (Ricordi et al., 2016; Hering et al., 2016).
Pancreas and islet cell transplantation are limited by insufficient donor organ availability and by the requirement of chronic immunosuppression to prevent rejection - a treatment associated with well-described side effects and health risks (Nathan and Group, 2014; Dominguez-Bendala and Ricordi, 2012; Dominguez-Bendala et al., 2012; Mineo et al., 2009; Mehrabi et al., 2014; Ryan et al., 2005).
Looking ahead, a multitude of therapeutic strategies are emerging (see BOX 2). Glucagon-like peptide 1 (GLP-1) Receptor agonists and Dipeptidyl peptidase-4 (DPP-4) inhibitors have been developed for the potentiation of beta cell function. Immunomodulatory agents such as low-dose anti-thymocyte globulin (ATG), low-dose interleukin-2 (IL-2), anti-CD3, and anti-tumor necrosis factor α (TNFα) antibodies are under intense investigation to inhibit T1D autoimmunity against beta cells. Cell therapies are under development for the replacement of beta cells and the inhibition of beta cell autoimmunity. Growth factors and small molecules are under investigation for their ability to stimulate beta cell replication and regeneration (see BOX 2). Notably, many of these emerging strategies aim at exerting effects on beta cells or in the pancreas environment. Hence, targeted delivery strategies for such agents could be highly beneficial.
BOX 2. Emerging therapeutic strategies for diabetes mellitus.
Pharmacological agents for the potentiation of beta cells: (relevant to: T2D, increasing interest in T1D)
Glucagon-like peptide-1 (GLP-1) receptor agonist and related treatments show great promise for the potentiation of beta cell function, both in T2D and T1D. GLP-1 is a hormone released by intestinal cells upon meal ingestion and digestion. Pancreatic beta cells present the GLP-1 receptor on their cell surface. When GLP-1 binds to the GLP-1 receptor on beta cells, insulin secretion is promoted in a glucose-dependent manner. GLP-1 is then rapidly degraded by enzymes such as dipeptidyl peptidase-4 (DPP-4). GLP-1 receptor agonists include molecules such as exenatide, liraglutide, and semaglutide, which activate signaling through the GLP-1 receptor and are more stable than the naturally occurring GLP-1 (Hinnen, 2017; Kielgast et al., 2009). An effect similar to GLP-1 agonists is exerted by DPP-4 inhibitors - molecules that inhibit the breakdown of GLP-1 and thus enable increased signaling through the receptor (Kodera et al., 2014; Gurgel Penaforte-Saboia et al., 2021). While the results of GLP-1 related therapies are robust in the context of T2D (Hinnen, 2017), the results are still far from optimal in T1D. Notably, besides the potentiation of insulin secretion and insulin biosynthesis, GLP-1 agonists can stimulate beta cell proliferation and inhibit beta cell apoptosis (Hinnen, 2017). The off-target effects and side effects of these treatments, primarily gastrointestinal, are connected to the widespread expression of the GLP-1 receptor in intestinal tissues (Tuttle et al., 2018). GLP-1 receptor agonists have been used extensively in T2D and show great promise as future therapies in other forms of diabetes mellitus.
Pharmacological agents for the protection of beta cells from autoimmunity: (relevant to: T1D)
T1D has an autoimmune basis and is characterized by progressive destruction of insulin-producing beta cells (Eisenbarth, 2004). Development of immunomodulatory therapies able to control islet autoimmunity and preserve insulin secretion from the residual beta cell mass at the time of diagnosis is of critical importance (Orlando et al., 2014). Even a partial level of insulin secretion affords protection from chronic complications (Steffes et al., 2003), hypoglycemia and diabetic ketoacidosis (Ludvigsson, 2013), which could all lead to death (Realsen et al., 2012; Castellanos et al., 2020; Weinstock et al., 2013).
In patients with new onset of T1D, treatment with Teplizumab, a humanized anti-CD3 monoclonal antibody, rendered CD8+ T cells exhausted and inactive; remarkably, this correlated with significantly higher beta cell function (C-peptide) at follow-up, an average of 7 years after diagnosis (Perdigoto et al., 2019).
In a different trial in new onset T1D, treatment with low-dose ATG led to a reduction in CD4 T cells: this translated into a slowing of the decline of beta cell function and yielded improvements in metabolic parameters (HbA1c) (Haller et al., 2018). Other immunomodulatory strategies under development aim at inhibiting autoimmunity via stimulation of Regulatory T cells via low dose IL-2 (Dwyer et al., 2016; Rosenzwajg et al., 2020). Additional studies are aimed at inhibiting proinflammatory cytokines such as TNFα. TNFα plays a role in the development and progression of autoimmune T1D and is directly toxic to beta cells. In a trial in new onset T1D, Golimumab (a human monoclonal antibody for TNFα) showed remarkable success, yielding improved endogenous insulin production and lowering exogenous insulin use than placebo (Quattrin et al., 2020). Most of these agents present risks for adverse events related to off-target effects connected with systemic administration. Although no drug has been approved as a disease-modifying therapy for T1D, the inhibition of autoimmunity against beta cells appears attainable.
Cell therapy for the protection of beta cells from autoimmunity: (relevant to: T1D)
Mesenchymal Stem Cells, also called Mesenchymal Stromal Cells (MSCs), are immunomodulatory cells under investigation for the treatment of T1D. MSCs have yielded promising results in clinical trials for autoimmune diseases, inflammatory disorders, and COVID-19 (Dominguez-Bendala et al., 2012; Lanzoni et al., 2020). MSCs can be safely administered intravenously (Dominguez-Bendala and Ricordi, 2012; Dominguez-Bendala et al., 2012; Bassi et al., 2012; Fotino et al., 2010). Results from a clinical study in Sweden showed that a single infusion of autologous Bone Marrow-derived MSCs (BM-MSC) (2.1–3.6 × 106/kg) preserved insulin secretion in patients with new onset T1D (Carlsson et al., 2015). In adult patients with established T1D, a single infusion of Umbilical Cord-derived MSCs (UC-MSC) and autologous BM-derived mononuclear cells (BM-MNCs) led to a range of improvements: improved beta cell function, improved glycemia, and lower insulin requirements at 1 year, compared to baseline and control group (Cai et al., 2016). Hu et al. reported that in patients with new onset T1D, UC-MSC treatment was associated with improvements in glycemic control and beta cell function compared to patients in the control group and compared to pre-UC-MSC infusion values (Hu et al., 2013). Lu et al. reported that UC-MSC treatment was associated with an increase in C-peptide levels in 40.7% of recipients at 1 year (Lu et al., 2021). MSCs represent a promising approach for the treatment of patients with T1D (Hu et al., 2013), with evidence of safety (Li et al., 2021a). Although these results appear very intriguing, controversies still exist in relation to the beneficial effects and risks of MSC (Musial-Wysocka et al., 2019). The mechanisms of action of MSCs is multifaceted and largely obscure. The transplantation of MSCs as live cells leads to a broad range of interactions, which are difficult to explore and that may translate into patient-specific effects and side effects (Avivar-Valderas et al., 2019). Standardization of MSC cell products is very challenging (Kassem, 2004). MSC therapy was not found to be associated with serious side effects, but the safety of MSCs, including their contribution to tumorigenesis in the long term, remains a matter of investigation (Musial-Wysocka et al., 2019).
Cell therapy for the replacement of beta cells (relevant to: T1D, advanced T2D, chronic pancreatitis, and other forms)
Patients with T1D could benefit greatly from islet cell replacement, and patients with T2D could benefit from islet cell supplementation. Pluripotent stem cells represent an exciting source to generate new islet cells and beta cells for replacement. After pioneering work (D’Amour et al., 2006; Kroon et al., 2008; Pagliuca et al., 2014), remarkable progress has been made by employing human pluripotent stem cells (hPSC) to generate islet specific cells for clinical applications (D’Amour et al., 2006). As a source of islet cells, hPSC could solve cell availability issues related to adult islet transplantation and could enable substantially broader application of islet cell replacement (Augsornworawat et al., 2020; Lanzoni and Ricordi, 2021; Agulnick et al., 2015). Recent work has reported scalable differentiation of stem cells (Millman et al., 2016) into beta-like cells that are glucose-responsive and express markers of mature beta cells (Zhu and Chen, 2015).
The first clinical trials using human beta cells from pluripotent stem cells are ongoing (ClinicalTrials.gov NCT number: NCT02239354, NCT02939118, NCT03162926, NCT03163511, NCT04678557) (Pullen, 2018). Transplantation of allogeneic cells requires immunosuppression or some form of immunomodulation. It would be ideal to transition from current immunosuppressive strategies to safer immunomodulation methods, to improve clinical outcomes (Mai et al., 2007). Beta cell replacement from pluripotent stem cells appears attainable, but it will probably require important refinements, such as localized or targeted immunomodulation.
Agents to stimulate beta cell replication and regeneration: (relevant to: T1D, advanced T2D, chronic pancreatitis, and other forms)
It is desirable to stimulate replication and regeneration of beta cells. GLP-1 agonists can stimulate beta cell proliferation and inhibit beta cell apoptosis (Hinnen, 2017). Exendin-4, a GLP-1 agonist, stimulates human beta cell proliferation, but this appears to occur almost exclusively in juvenile, and not in adult, islet beta cells (Dai et al., 2017).
Human pancreatic beta cells can proliferate after treatment with small molecules such as harmine analogs (Wang et al., 2015; Dominguez-Bendala et al., 2016). Hepatocyte-derived secretory SerpinB1 and its mimetic GW311616A also enhance beta cell proliferation (El Ouaamari et al., 2016). Gamma aminobutyric acid (GABA) was found to promote beta cell replication in grafted human islets (Purwana et al., 2014). Although the stimulation of beta cell replication is highly desirable, it may be an uphill battle in patients with advanced T1D, who present a near-complete beta cell loss.
miRNAs - agents affecting islet cell gene expression: (relevant to: T1D, T2D, and other forms)
MicroRNAs (miRNAs) are key regulators of gene expression; miRNAs have been harnessed as therapeutics to impart genes expression changes in islet and vascular cells (He et al., 2017; Zampetaki et al., 2010). A number of miRNAs affect beta cell proliferation and insulin secretion, as reviewed in (Eliasson and Regazzi, 2020). In T2D, miRNA sets can determine a failure in beta cell compensation in response to insulin resistance, and are associated with beta cell dysfunction (Eliasson and Regazzi, 2020). Mir-216, as an example, is a potent modulator of beta cell proliferation: it decreases PTEN expression, which in turn activates beta cell proliferation pathways (Wang et al., 2020). Further developments are needed for enhancement of proliferation and regeneration of beta cells in the pancreas via miRNA manipulation.
Agents for other systemic effects - not directly related to islets or beta cells (relevant to: T1D, T2D, and other forms)
Inhibitors of sodium-glucose cotransporter (SGLT1 and SGLT2) can yield robust improvements in glycemic control for patients with T1D and T2D. SGLT1 inhibitors reduce glucose absorption in the small intestine, whereas SGLT2 inhibitors decrease glucose reabsorption in the kidney. The combination of SGLT1 and SGLT2 inhibitors as an adjunct to insulin therapy appears promising (Sands et al., 2015).
Metformin (Petrie et al., 2017) is an agent broadly utilized in the early stages of T2D. Metformin leads to improved insulin receptor function, increased glucose uptake and utilization, decreased hepatic glucose release, and decreased intestinal absorption of glucose. Hepatic and intestinal absorption is facilitated by Organic cation transporter 1 (OCT1) on the basolateral membrane of enterocytes and hepatocytes (Gong et al., 2012; Foretz et al., 2014). Plasma membrane monoamine transporter (PMAT) expressed on the luminal side of enterocytes has been reported to facilitate metformin intestinal absorption, and the agent subsequently determines a glucose lowering effect by inhibition of gluconeogenesis in the liver (Foretz et al., 2014; Natali and Ferrannini, 2006). Metformin enhances hepatic insulin function by altering lipid homeostasis and inhibiting acetyl Coa carboxylase phosphorylation via AMP- activated protein kinase (AMPK) (Fullerton et al., 2013).
3. Need for targeted delivery of therapeutic agents to pancreatic islets
An array of agents can stimulate beta cell function, modulate beta cell gene expression, or increase beta cell proliferation (see BOX 2). Other agents can inhibit autoimmunity and prevent beta cell loss (see BOX 2). One problem that most of these agents have in common is that systemic administration leads to a range of off-target, undesired side effects. Targeted delivery strategies could improve substantially the effect of therapeutic agents designed to yield effects on beta cells or in the pancreas environment. Delivery of such agents to the pancreatic islet environment could result in an amplified effect for the preservation or regeneration of functional beta cells, with minimal side effects. Innovative approaches, presented in the next paragraphs, are allowing the in vivo targeted delivery of agents to pancreatic islets, beta cells, or inflamed pancreatic islets.
4. Surgical approach for targeted delivery: intra-arterial administration in the pancreatic arteries
The pancreas is perfused from arteries branching from the abdominal aorta. Although pancreatic islets constitute only 1–2% of the pancreatic mass, they are highly vascularized and receive 5–10% of the pancreatic blood flow (Brissova and Powers, 2008). (Brissova and Powers, 2008). The endothelium of pancreatic islets is fenestrated, a feature that facilitates exchanges of solutes (Brissova and Powers, 2008). The body and tail of the pancreas contain most of the islet cells (van Suylichem et al., 1987; Wittingen and Frey, 1974); these regions of the pancreas are perfused by the dorsal pancreatic artery and great pancreatic artery (Wu et al., 2011).
MSCs for diabetes therapy have been successfully infused through the pancreatic arterial system in an attempt to inhibit autoimmune processes and improve islet endocrine function (Cai et al., 2016). In a pilot randomized controlled trial, Cai and colleagues infused Umbilical Cord (UC)-derived MSC and BM-derived stem cells through the dorsal pancreatic artery or its substitute supplying the pancreatic body and tail (Cai et al., 2016). This treatment resulted in improved metabolic profiles in patients with T1D, with a doubling of endogenous C-peptide production, a marker of beta cell function, at 1 year (Cai et al., 2016).
This surgical approach enables a first passage of the therapeutic agent or imaging reagent through the pancreatic vascular bed. It requires a skilled surgeon and is facilitated by imaging (angiography). The surgical procedure requires deep sedation of the patient. Importantly, it bears risks connected with puncturing of the arteries and of the pancreas.
5. Targeting cell surface molecules specific of islet cells
One key goal of targeted delivery strategies for diabetes mellitus is to facilitate accumulation of an agent of interest in pancreatic islet cells. Pancreatic islets contain a wide range of different cell types, characterized by specific expression of cell surface molecules (Dybala and Hara, 2019; RKPHodson, 2018; Cabrera et al., 2006). During pancreatic islet development, CD142+ pancreatic endoderm cells mature into CD200+ and CD318+ endocrine cells (Kelly et al., 2011). Cell-surface markers have been identified for all major cell types of the human pancreas: monoclonal antibodies, such as HIC0, H1C1 and DH1C2/3 clones, are especially useful because of their specificity for pancreatic islet endocrine cells (Dorrell et al., 2016).
Recently, four distinct subtypes of pancreatic islet beta cell have been described, known as Beta 1–4: they have different kinetics of insulin release, and they are distinguished by specific surface marker expression of ST8S1A1 and CD9 (Dorrell et al., 2016). The majority of proinsulin + beta cells appear to be CD9+ (Kelly et al., 2011). These recent observations may open the path to strategies aimed at targeting specific beta cell subtypes, such as to affect specifically mature and functional or immature and dormant beta cells.
The GLP-1 receptor (GLP-1R) represents a major target on the surface of islet beta cells. The GLP-1R receives the signal of GLP-1, an incretin peptide hormone released by small intestinal cells in response to nutrient ingestion, and released also by a subtype of islet alpha cells (Campbell et al., 2020). GLP-1 binds to GLP-1R on the surface of islet beta cells, potentiating glucose-induced insulin secretion, upregulating insulin biosynthesis, and inhibiting apoptosis of beta cells. GLP-1R has been targeted to deliver therapeutic agents and imaging agents to islet cells in vivo, predominantly via conjugation of the GLP-1R ligand exendin-4 to moieties of interest in nanoparticles (Wang et al., 2014) One limitation of GLP-1R targeting is that this molecule is broadly expressed in cells of the gastrointestinal tract, hence the selectivity for islet cells is limited.
Vesicular monoamine transporter 2 (VMAT-2) is a cell-surface molecule responsible for the release of dopamine and serotonin. VMAT-2 is found on both beta cells and neuroendocrine cells (Sheehy et al., 2019). VMAT-2 is highly expressed in pancreatic islet cells (Anlauf et al., 2003). Notably, VMAT-2 has a high specificity fori slet beta cell targeting: VMAT-2 was found to be expressed in insulin-producing beta cells but not in glucagon-, somatostatin-, or pancreatic polypeptide-producing cells (Anlauf et al., 2003). VMAT-2 is present in cell types that store and release monoamines (Anlauf et al., 2003). VMAT2 gene expression may differ in subsets of beta cells (Harris et al., 2008).
The Prostaglandin D2 receptor 2, also called GPR44, is localized into the cell surface, binds Prostaglandin D2, and appears to be almost exclusively restricted to human beta cells (Hellstrom-Lindahl et al., 2016; Lindskog et al., 2012). Zinc transporter 8 (ZnT8) is a zinc transporter related to the beta cell function of insulin secretion. Frequent insulin secretion exposes ZnT8 to the cell surface (Merriman et al., 2018). Ligands and targeting moieties that bind the above cell surface molecules appear to have good specificity for beta cells, and have been utilized for the targeted delivery of agents to beta cells (see Fig. 2, Fig. 3, Table 1). The field of targeted delivery utilizing islet cell-specific and beta cell-specific cell surface marker appears to be largely unexplored; further analyses are warranted.
Fig. 2.
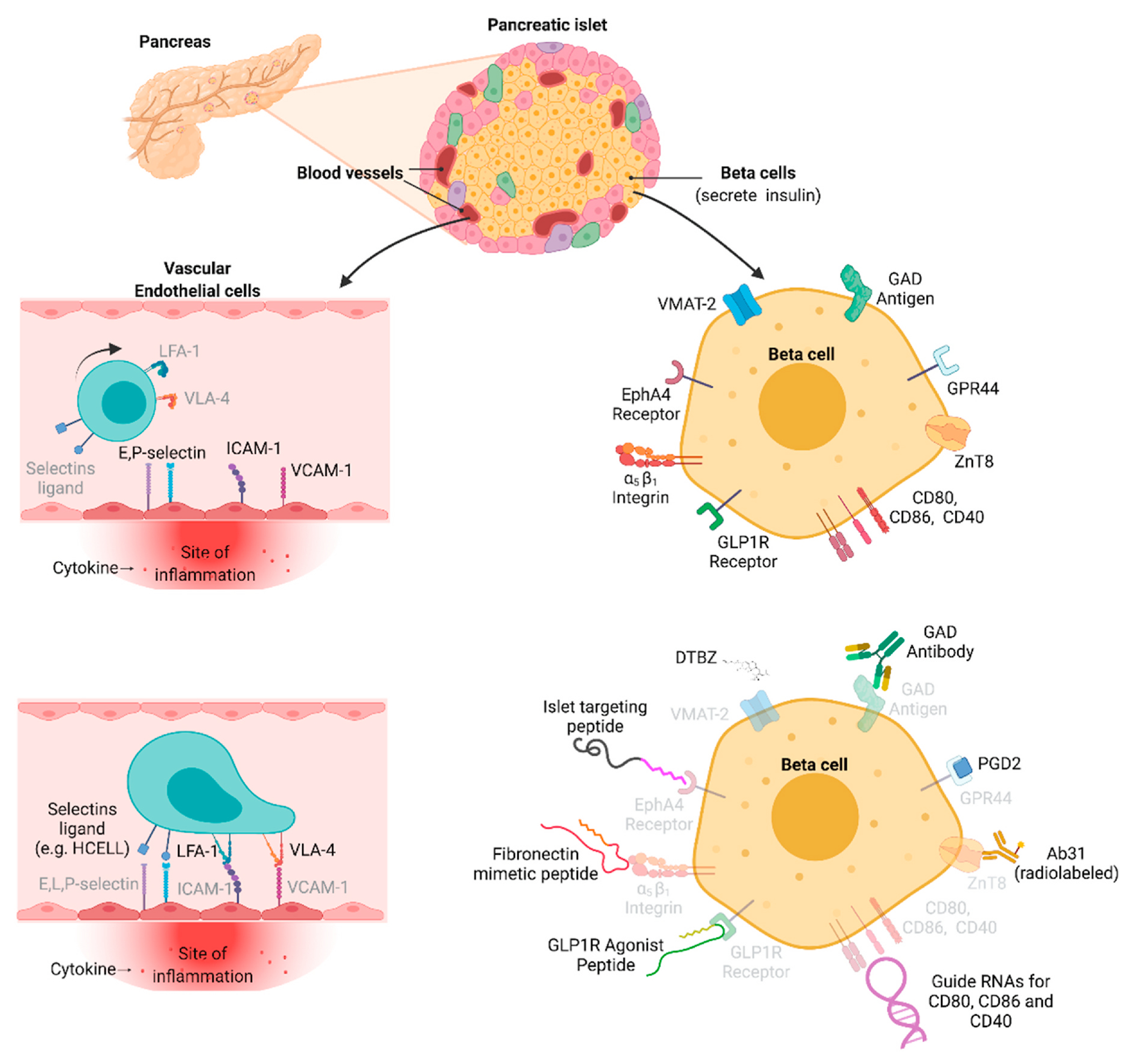
Cell surface molecules and ligands for targeting inflamed vascular endothelial cells and pancreatic islet beta cells.
Fig. 3.
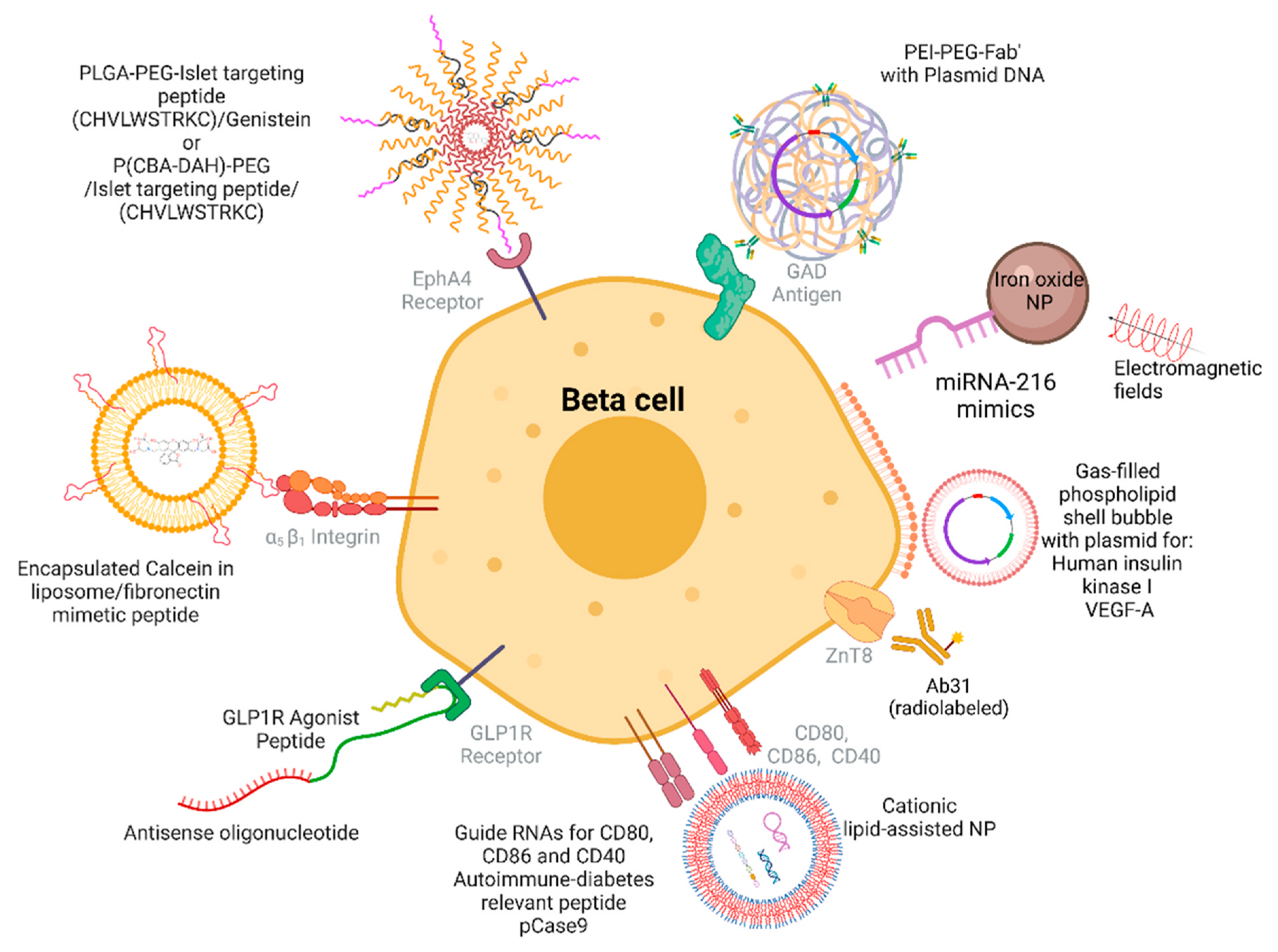
Targeting moieties, nanocarrier structures, and cargos for targeted delivery to beta cells.
Table 1.
Cargos, Vehicles, and Targeting moieties for islet-targeted therapeutic agents.
| Purpose | Cargo | Vehicle | Targeting Moiety/Target/Strategy | Outcome | Reference |
|---|---|---|---|---|---|
| Targeted delivery of an immunosuppressant drug to islet cells | Genistein | PLGA/PEG-COOH | Islet Targeting Peptide (Cyclic) (CHVLWSTRKC)/EphA4 Receptor | 200x increased immunosuppressant effect | Ghosh et al. (2012) |
| Islet beta cells proliferation/Decreased expression of phosphatase and tensin homolog (PTEN) | miR-216 mimics/inhibitors | Iron Oxide Nanoparticles | miR-216 mimics/inhibitors | Decreased PTEN expression and increased beta cell proliferation | Wang et al. (2020) |
| Prevention of autoimmunity to islet components | Autoimmune diabetes relevant peptide/CRISPR-Cas9 plasmid | PLGA/PEG-COOH | Three separate guide RNAs for CD80, CD86 and CD40/CD80, CD86 and CD40 | Prevention of autoimmunity to islet components and inhibition of T1D development | Luo et al. (2020) |
| Delivery of antisense oligonucleotides (ASO) to beta cells for knocking down islet amyloid polypeptide | Antisense oligonucleotides (ASO) | Glucagon Like Peptide 1 Receptor (GLP1R) agonist peptide ligands | GLP1R agonist peptide ligands/GLP1 Receptor | Successful knocking down of islet amyloid polypeptide | Knerr et al. (2021) |
| Polymeric gene delivery system to deliver a plasmid coding for soluble RAE-1γ to the pancreatic islet microvasculature | Soluble Retinoic acid early inducible gene-1 (RAE-1) Plasmid | Poly(cystamine bisacrylamide–diamino hexane) (p(CBA-DAH))/poly(ethylene glycol) (PEG) | CHVLWSTRC Peptide/EphA2 and EphA4 receptors | Protection of beta cells from autoimmune destruction and prevention of type 1 diabetes. |
Blevins et al. (2012)
Joo et al. (2012) |
| 19F MRI imaging of the transplanted human islets | Rhodamine | Perfluorooctylbromide (PFOB) nanoparticles | Non-invasive multimodal cell tracking of human pancreatic islets | Barnett et al. (2011) | |
| Increasing fibronectin production to decrease apoptosis and therefore increase viability of transplanted islets | Calcein | Liposomes | Fibronectin mimetic peptide/α5b1 integrin expressed on pig islets | Targeted liposomes containing calcein was delivered and they shoved improved cell binding and internalized | Atchison et al. (2010) |
| Increasing hexokinase protease I expression, increasing insulin levels in blood and decreasing circulating glucose levels | Plasmids encoding for the genes for Human Insulin and Hexokinase I | Gas-filled Phospholipid Shell Bubbles | Insulin 1 Promoter/beta-cells/Ultrasound triggered delivery | Efficient gene delivery to pancreatic islets with the detection of transgene-encoded proteins for up to 3 weeks after transfection | Chen et al. (2006) |
| Increasing vascular endothelial growth factor A (VEGF-A) expression in transplanted islets to increase vessel density and graft function | Plasmid encoding for the gene for VEGF-A | Gas-filled Phospholipid Shell Bubbles | Ultrasound triggered delivery | Efficient gene delivery of VGEF-A to pancreatic islets was successful, increased vessel density, and improved graft function | Shimoda et al. (2010) |
| Increasing transfection efficiency and gene delivery to islet cells | Plasmid encoding for the gene for luciferase enzyme | PEI-PEG-Anti GAD Fab polymeric gene carrier | Antibody against glutamic acid decarboxylase/glutamic acid decarboxylase antigen | 10-fold transfection efficiency to beta islet cells | Jeong et al. (2005) |
6. Targeting cell surface molecules of inflamed endothelial cells and inflamed pancreatic islets
Because of their function in endocrine hormone release, pancreatic islets are highly vascularized and thus rich of vascular endothelial cells. When pancreatic islets become inflamed, such as during development of T1D and around the time of disease onset (peri-onset period), islet vascular endothelial cells increase expression of adhesion molecules on the luminal surface and recruit immune cells (Figs. 2 and 4). Endothelial cells respond to inflammatory cytokines by overexpressing the adhesion molecules intercellular adhesion molecule 1 (ICAM-1, CD54), E-selectin (CD62E), P-selectin, L-selectins and vascular cell adhesion molecule VCAM-1. These molecules render inflamed endothelial cells “sticky”: they become docking sites for circulating immune cells, endothelial progenitor cells (EPC), and MSC (Linn et al., 1994; Martin et al., 1996; Morgan et al., 2014; Pugliese, 2016; Pezhman et al., 2021; Chen et al., 2020; Phillips et al., 2008; Koh et al., 2000).
Fig. 4.
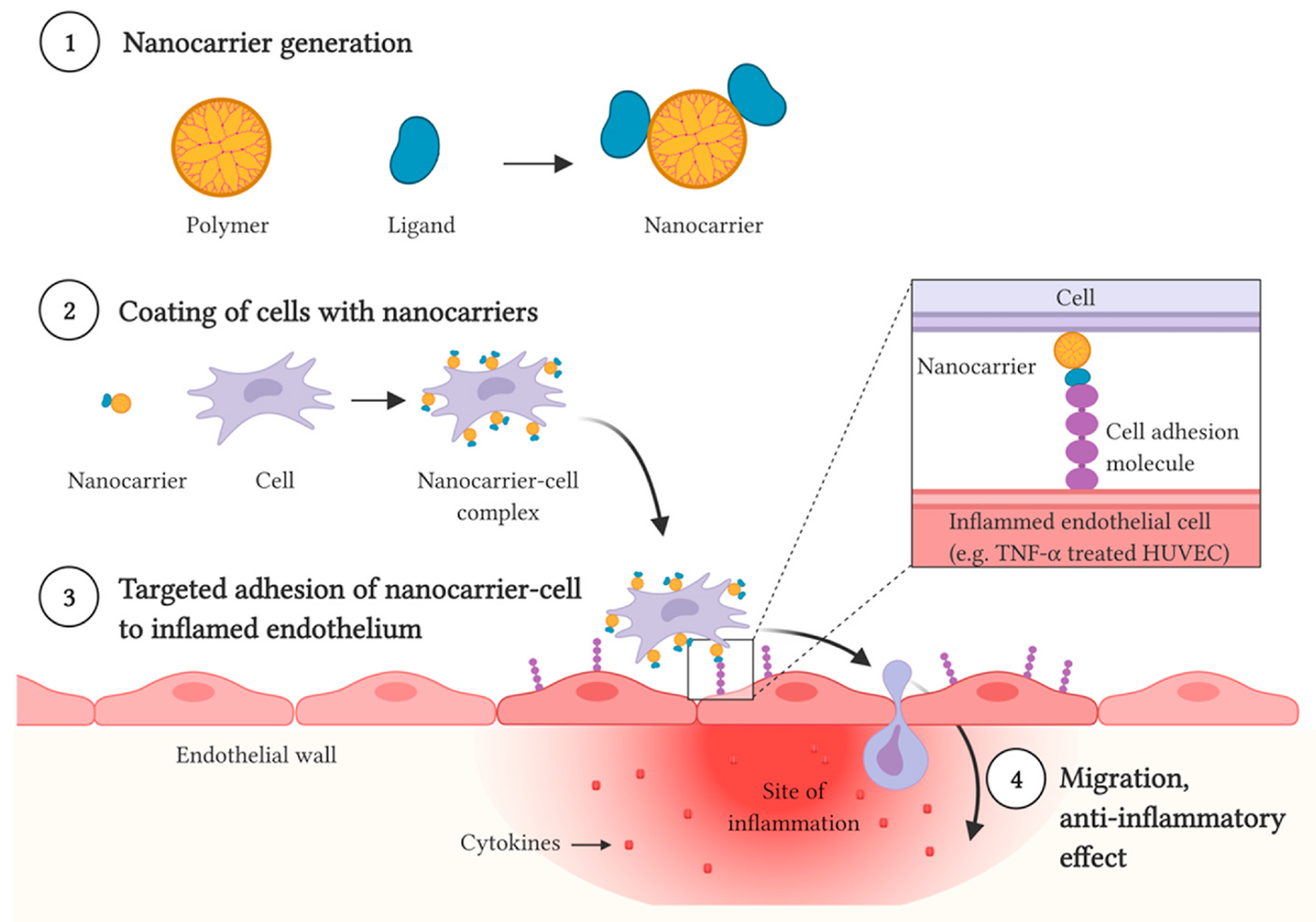
Targeted delivery of cells to inflamed endothelium.
To adhere to inflamed endothelia and initiate extravasation, immune cells present the corresponding ligands, e.g.: Lymphocyte function-associated antigen 1 (LFA-1) for ICAM-1 (Wu et al., 2019), very late antigen-4 (VLA-4) for VCAM-1, and the carbohydrate Sialyl LewisX for E– and L-selectins (Abdi et al., 2015). These binding partnerships have been utilized for the development of targeted delivery systems. The LFA-1:ICAM-1 binding appears of great interest. ICAM-1, a type I transmembrane protein expressed by endothelial cells, is a critical regulator of immune responses: when overexpressed, it facilitates the trans-endothelial migration of T-cell to sites of inflammation. The major binding partner for ICAM-1 is Leukocyte function antigen-1 (LFA-1, CD11a/CD18), a T-cell integrin (Staunton et al., 1990) that enables T cell adhesion to the inflamed endothelium. The LFA-1 alpha subunit I-domain (LFA-1-Id) contains a binding site for ICAM-1 (Lee et al., 1995; Edwards et al., 1995), and could be thus be utilized for targeted delivery. The very late antigen-4 (VLA-4), expressed by multiple immune cells, is the main ligand for VCAM-1 (Gorelik et al., 2012). Multiple studies have employed the VLA-4:VCAM-1 targeting to deliver MSCs to endothelial cells (Majumdar et al., 2003; Ruster et al., 2006; Liu et al., 2018).
Selectins, transmembrane glycoproteins, are adhesion molecules central to the docking and trafficking of immune cells and platelets: E-selectin is found on endothelial cells, L-selectin is found mainly on leukocytes, and P-Selectin is found mainly on platelets and endothelial cells (Bevilacqua and Nelson, 1993). E-selectin is an inducible cell-adhesion molecule that helps localize circulating cells to the inflamed vascular endothelium (Leeuwenberg et al., 1992). Inflammation causes E-selectin to become upregulated on endothelium (Oh et al., 2007; Dedrick et al., 2003). E-selectin binds the P-selectin glycoprotein (CD162), CD44, and Hematopoietic Cell E– and L-selectin ligand (HCELL, a sialofucosylated form of CD44). These are expressed in circulating cells (Lasky, 1992), stem cells, progenitor cells, endothelial progenitor cells (EPC), and hematopoietic cells (Sackstein, 2010). CD162 helps platelet-monocyte complexes and monocytes tether to inflamed endothelium (da Costa Martins et al., 2007). HCELL enables docking of circulating cells to inflamed endothelial cells expressing E-selectin. EPCs express both E-selectin, as well as the E-selectin ligand (Oh et al., 2007). The existing physiological and pathological mechanism that enables the docking of immune cells to inflamed endothelium can thus be utilized to deliver therapeutic cells to target sites.
In Type 1 Diabetes, an islet-specific activation of the endothelium appears to precede overt diabetes and subsequent systemic changes in the endothelium. This is evident in the NOD mouse of T1D. Notably, in this mouse model, ICAM-1 deficiency or blockage of the ICAM-1 and LFA-1 interaction can prevent diabetes (Martin et al., 2001; Yagi et al., 1995). In the presence of a functional ICAM-1 gene, a hyperexpression of ICAM-1 occurs in the islet endothelium of NOD mice, in parallel with insulitis, shortly before and around the time of onset of diabetes (Linn et al., 1994; Martin et al., 1996) and before systemic endothelial activation resulting from hyperglycemia. A similar sequence of events could occur in patients with T1D, as suggested by the observation of inflammatory changes specifically in the islet endothelium appearing in patients with elevated risk of T1D and around the time of initial disease manifestation (Morgan et al., 2014; Pugliese, 2016). Hence, it could be appropriate to target the inflamed islet endothelium in the peri-onset period of T1D. In Type 2 Diabetes, there seems to be more overlap in the timing of inflammatory changes in the endothelium of islets and of the rest of the body (Pezhman et al., 2021), hence this type of targeting could have limited specificity.
During the development of targeted delivery strategies for the inflamed endothelium, the nature of the disease and the stage of disease progression need to be carefully considered to ensure specificity of the targeting and to avoid off-target side effects. Long term hyperglycemia, metabolic syndrome, and entirely unrelated conditions (e.g., trauma) can determine inflammatory changes systemically or determine endothelial activation in specific tissues ofthe body, and these in turn would affect the distribution of ligands engineered to bind inflamed endothelia.
Strategies for the targeted delivery of cells to regions with inflamed endothelia are detailed in section ‘Targeted delivery of therapeutic cells and exosomes’.
7. Nanocarrier-based delivery to islet cells
Nano-sized carriers have been developed to enable targeted delivery of therapeutic agents to pancreatic islets, for the treatment of diabetes mellitus (Fig. 3).
Gosh and colleagues engineered nanoparticles by utilizing a peptide that binds preferentially to islet microvasculature (CHVLWSTRKC), connected to a PLGA-b-PEG-COOH block copolymer, and incorporating the anti-inflammatory agent genistein in the nascent nanoparticles. With this strategy, the team observed that the engineered nanoparticles enabled a 3-fold stronger binding to pancreatic islet endothelial cells, and this binding led to a 200-fold increase in the immunosuppressive effect of genistein (Ghosh et al., 2012).
In other studies, iron oxide nanoparticles have been utilized to deliver the microRNA miR-216a, a potent modulator of beta cell proliferation: the treatment of diabetic mice with miR-216a mimics, conjugated with iron oxide nanoparticles, led to potentiation of beta cell proliferation and beta cell function (Wang et al., 2020; Luo et al., 2020).
Other studies have utilized low molecular weight and biodegradable polymers, to facilitate gene delivery. The application of low molecular weight and biodegradable polymers is advantageous for multiple reasons: these polymers are easy to manufacture; they have low immunogenicity; their chemical composition and their in vivo degradation can be tightly controlled, which can reduce cytotoxicity (Chen et al., 2020). Gene delivery with polymers targeting the islet microvasculature has provided protection of beta cells from autoimmune destruction. The targeted delivery of nucleic acids through a variety of polymers enabled the maintenance of sequence integrity and the halting of diabetes mellitus progression in animal models. Microspheres were engineered to deliver antisense RNAs for CD80, CD86 and CD40, intended to silence the expression of these costimulatory molecules on dendritic cells in the NOD mouse model of autoimmune diabetes; this treatment enabled prevention and reversal of autoimmune diabetes (Phillips et al., 2008). Recently, the codelivery of guide RNAs for CD80, CD86, and CD40, an autoimmune diabetes relevant peptide (2.5mi), and CRISPR-Cas9 through cationic lipid assisted poly (ethylene glycol)-b-poly(lactide--co-glycolide) (PEG-PLGA) nanoparticles (CLAN) has prevented islet cell autoimmunity by restoring immune tolerance and induced long-term protection of islet beta cells (Luo et al., 2020).
Immune regulatory pathways may be harnessed to prevent autoimmune insulitis: regulatory T-cells (T regs) produce the molecules interleukin-10 (IL-10) and interleukin-4 (IL-4) to modulate immune functions. In the NOD mouse model, the biodegradable polymer polyaminobutyl glycolic acid (PAGA) was used to deliver a plasmid encoding IL-10 and concurrently protect it from DNase-I degradation (Koh et al., 2000; Kim, 2011). This treatment enabled prevention of the onset of autoimmune diabetes in a majority of recipient NOD mice (Koh et al., 2000; Kim, 2011). A water soluble lipopolymer (WSLP) nanocarrier was engineered to deliver a plasmid encoding for IL-4 with rat insulin promoter [WSLP-(pRIP-IL4)]. The WSLP-pRIP-IL4 enabled pancreas beta-cell specific and glucose responsive expression of IL-4. This system demonstrated no systemic side effects along with high protection of plasmid DNA from DNase I, and was proposed for use for the prevention of autoimmune diabetes (Lee et al., 2001a). Treatment with combined delivery of IL-4 and IL-10 gene plasmid DNA using PAGA in NOD mice resulted in prevention of autoimmune diabetes; in treated animals, 75% of islets remained intact compared to only less than 3% in control animals (Ko et al., 2001).
Glutamic acid decarboxylase (GAD) is a major autoantigen in T1D. Silencing of the expression of GAD by antisense DNA in NOD mice enabled protection of islet beta-cells from autoimmunity and inhibition of progression of diabetes. The delivery of antisense expression plasmid, complexed with polymeric gene carrier of PEG-grafted poly(lysine) (PEG-g-PLL), to GAD-producing cells determined repression of GAD autoantigen expression specifically in pancreatic beta cells (Lee et al., 2001b). This system demonstrated that repression of GAD expression using AS-GAD/PEG-g-PLL may not have any effect on normal expression of GAD in other organs nor have any side effects on normal function of the pancreas.
Yamada et al. targeted pancreatic beta cells via a nanocarrier system composed of sphingomyelin, phosphatidyl choline, and cholesterol, to deliver nucleic acids. To increase insulin secretion, the team knocked down the expression of miR-375 with an antisense RNA in MIN6 pancreatic beta-like cells. This knockdown led to increased insulin secretion via enhanced expression of Pdk1 and Mtpn (Yamada et al., 2014).
Interactions between Fas (CD95) and its ligand (FasL) activate multiple signaling pathways leading to the T cell-mediated beta cell apoptosis. Suppression of Fas (CD95) expression in beta cells by a complex of Fas silencing small interfering RNA (siRNA) has been studied by Kim’s group (Jeong et al., 2010). The application of siRNA in clinical settings is challenging due to its highly negative charged backbone, that results in a low intracellular uptake by a negatively charged plasma membrane and a fast degradation by extracellular enzymes. Jeong and colleagues developed an islet cell targeting polymeric gene carrier by conjugating anti-GAD Fab’ fragment to PEI via a PEG linker (PEI--PEG-Fab’). This carrier enabled an increase in the specificity of transfection to beta cells, that was dependent on the binding of the beta cell specific GAD (Jeong et al., 2005). The application of a cationic polymer-based nanocarrier of polyethylenimine (PEI) in the delivery of Fas siRNA lead to benefits in the NOD mouse model of diabetes (Jeong et al., 2010). Another pathway that has been successfully harnessed is that of the activating receptor natural killer group 2 member D (NKG2D), which is involved in the progression of T1D, and its ligand retinoic acid early inducible gene-1 (RAE-1). The RAE-1 ligand is present in prediabetic islets of NOD mice, and autoreactive CD8+ T cells infiltrating the pancreas express the receptor NKG2D. Kim and colleagues engineered a delivery system with plasmid coding for the islet microvasculature, via targeting the EphA2 and EphA4 receptors. This system was intended to deliver the soluble ligand to NKG2D (sRAE-1y) and protect beta cells from autoimmune destruction. In that study, the bioreducible cationic polymer poly(cystamine bisacrylamide–diamino hexane) (p(CBA-DAH)), poly(ethylene glycol) (PEG), and the targeting peptide to the EphA2 and EphA4 receptors were used to deliver the sRAE-1y in the pancreas of NOD mice. This resulted in a significant improvement in the degree of insulitis and lower infiltration of CD8+ T-cells in the treated NOD mice (Blevins et al., 2012; Joo et al., 2012).
Nanocarriers have also been developed to improve islet vascularization and increase insulin production. To improve vascularization, Shimoda and colleagues developed a method based on ultrasound triggered delivery (ultrasound-targeted microbubble destruction) for plasmid gene delivery of vascular endothelial growth factor (VEGF)-A to islet cells. This method was applied in a model of islet transplantation, leading to increased vessel density and improved function of transplanted islets (Shimoda et al., 2010). To increase insulin production and decrease glycemia, Chen et al. engineered phospholipid shell bubbles to deliver plasmids encoding genes for human insulin and hexokinase I to pancreatic beta cells (Chen et al., 2006). Nanoparticles have emerged as a promising vehicle to improve islet cell transplant outcomes. Following islet cell transplantation, islet cell viability could be protected by liposomes containing calcein to increase fibronectin production and decrease apoptosis (Atchison et al., 2010).
Even though nanocarriers offer several potential advantages through targeted delivery of therapeutic agents, the application of nanoparticles can determine side effects and toxicity; the nanoparticles might aggregate or agglomerate in vivo, which could result in unforeseen side effects (Patra et al., 2018). Unfortunately, there is limited information regarding the toxicity of newly designed nanocarriers. Further research on the safety and efficacy of nanocarriers is warranted.
8. Targeted delivery for gene editing
Gene editing with tools such as CRISPR-Cas9 is opening exciting avenues for therapy of genetic forms of diabetes. Genetic variants involved in pancreatic islet cell and beta cell development can cause diabetes. Diabetes-causing variants can occur in the gene Wolfram syndrome 1 (WFS1). With CRISPR-Cas9, one such genetic variant has been successfully corrected via gene editing (Maxwell et al., 2020). Targeted delivery of all gene editing tools in a single vector, such as a non-integrating lentiviral vector, encoding guide RNA and donor DNA, could allow for delivery of the CRISPR-Cas9 components to islet cells (Uchida et al., 2021). CRISPR-Cas9 delivery can be further optimized by transient endonuclease function and gene correction in the target tissue (Uchida et al., 2021).
Gene transfer using recombinant adeno-associated viral (AAV) vectors has delivered clinical successes, but islets are notoriously difficult to target. A chimeric AAV capsid has been generated to facilitate AAV-based transduction to human islet cells and human embryonic stem cell-derived beta cells (Pekrun et al., 2019). While this field of investigation is in its infancy, its potential for therapy appears remarkable.
9. Targeted delivery of therapeutic cells and exosomes
MSCs have immunomodulatory, anti-inflammatory, and pro-regenerative properties. MSCs have yielded remarkable therapeutic benefits in early-phase trials for the treatment of T1D, T2D, and in the context of islet transplantation (see BOX 2) (Huang et al., 2010). In a majority of applications, MSCs are delivered via systemic intravascular administration. When administered intravenously, most cells become trapped in organs such as the liver, lung, spleen, and kidneys - that function as filters for MSCs. Only a small fraction of cells reaches the target tissues. Local MSC administration in the region of the pancreas has challenges, including difficult accessibility and risk of damaging the pancreas via surgical tools. For applications in diabetes mellitus, especially in the context of T1D, one option could be to deliver MSC specifically to inflamed endothelia in pancreatic islets. For such goal, ICAM-1, VCAM-1, E– and L-selectins are candidate targets. At sites of inflammation, vascular endothelial cells hyperexpress ICAM-1, VCAM-1, E–, P- and L-selectins on the luminal surface, determining recruitment of immune cells (Fig. 2). As stated before, to adhere to inflamed endothelia and initiate extravasation, immune cells present the corresponding ligands: LFA-1 for ICAM-1 (Wu et al., 2019), VLA-4 for VCAM-1 (Dedrick et al., 2003; Gorelik et al., 2012), Sialyl LewisX for E– and L-selectins (Abdi et al., 2015).
When MSC are stimulated with interleukin-1beta (IL-1B), they increase their expression of LFA-1 and adhere more efficiently to inflamed Human Umbilical Vein Endothelial Cells (HUVEC) through LFA-1/ICAM-1interaction (Wu et al., 2019). Although pretreatment of MSCs with factors such as IL-1B may increase MSC migration and adhesion to the site of inflammation, this method generates a harsh microenvironment and modifies the function of MSCs - which may limit such application (Guo et al., 2018; Lin et al., 2015). An alternative strategy to enhance MSCs applications is the targeting of cell surface markers of the inflamed endothelium via engineere nanocarriers (Cuesta-Gomez et al., 2021).
Nanocarriers consisting of functionalized dendrimers may increase the adhesion of cells to inflamed endothelia (Fig. 4). Liu et al. engineered nanocarriers to enable targeted delivery of MSCs to sites with inflamed endothelium. The team generated nanocarriers by combining acetylated, fifth generation, polyamido amine (PAMAM) dendrimers with soluble E-selectin (sE-sel) as the targeting moiety (Liu et al., 2016). MSCs coated with this dendrimer sE-sel complex can be captured at the microcirculatory bed of the inflamed target site. The coated MSCs underwent extravasation and efficiently migrated into the target inflamed tissue (Liu et al., 2016). Coating with nanocarriers could thus potentiate the localized immunomodulatory, pro-angiogenic, and pro-regenerative effects of MSCs (Liu et al., 2016).
It is important to note that dendrimers present positively charged groups that can lead to toxicity in vivo. Acetylation is a highly effective strategy to abate such toxicity (Mishra et al., 2019).
To increase delivery of MSC to inflamed islets, Abdi et al. engineered MSC via exofucosylation with the enzyme fucosyltransferase, to generate the carbohydrate Sialyl LewisX (Abdi et al., 2015). This enzymatic modification of the MSC cell surface led to the generation of the Sialyl LewisX on CD44, yielding HCELL. The modified HCELL+ MSC, exhibited a three-fold increase in islet infiltration. Administration of HCELL+ MSCs resulted in durable reversal of hyperglycemia in NOD mice, whereas only transient reversal was observed following administration of HCELL− MSCs (Abdi et al., 2015).
MSCs appear to have great potential as a therapeutic agent for T1D and T2D. However, it is important to consider the limitations and potential side effects arising from MSC-based cell therapy. Several factors can affect the MSCs application as a therapeutic agent such as the method of culture, cell heterogeneity, cell dose. MSC are living cells, hence standardizing products made with MSC is difficult. After transplantation in the allogenic setting, the cells will have numerous interactions with cells and tissue of the recipients: the effect of these interactions may vary greatly in different recipients. MSCs are considered hypoimmunogenic, but they can elicit an immune response in the recipient, potentially leading to sensitization and rejection (Avivar-Valderas et al., 2019). The long-term effects of MSC therapy still need to be explored in depth via long term follow-up in recipients (Kassem, 2004). The specific culture methods affect MSC phenotype, differentiation, and immunomodulatory effect (Yang et al., 2018). Long term culture can also increase the probability of malignant transformation. There is controversy on MSCs’ pro-tumor and anti-tumor effects: reports suggest that MSC treatment could increase tumor progression via secretion of proangiogenic factors (Pan et al., 2014). The potential negative effects of MSC-based cell therapy have been discussed in other reviews in detail (Musial-Wysocka et al., 2019)(Li et al., 2021b).
Exosomes, small bilayer vesicles derived from eukaryotic cells, present a promising delivery platform (Barile and Vassalli, 2017). Exosomes exhibit low toxicity, structural stability, and cargo loading ability at a nanoscale (Fu et al., 2020). As natural carriers of functional proteins and regulatory nucleic acids, exosomes have emerged as a novel therapeutic tool to deliver RNA and DNA for the treatment of T1D (Pang et al., 2020). Human bone marrow mesenchymal stem cells (hBM-MSC) and their exosomes have been reported to promote islet function and inhibit immune rejection (Wen et al., 2016). Mahato and colleagues found that hBM-MSC-derived exosomes delivered a plasmid encoding anti-miR-375 and shFas to downregulate miR-375 and Fas to improve islet function in NOD mice (Wen et al., 2016). MSC-derived exosomes are emerging as a viable therapeutic strategy for diabetes, and could improve islet cell transplantation outcomes (Keshtkar et al., 2020). Exosomes derived from UC-MSC led to improvements in viability, survival, and function of islets transplanted in hypoxic conditions (Nie et al., 2018). Beta cell-derived exosomes play a central role in preserving islet architecture and vascularization (Sun et al., 2019). Jia et al. reported that beta cell-derived exosomes promote islet cell angiogenesis leading to improved glucose tolerance and increased insulin production in STZ-induced diabetic mice (Sun et al., 2019). Although the matter of islet cell targeting was not investigated in depth, it is possible that beta cell-derived exosomes could bind natural targets preferentially in the islet environment.
Although exosomes appear promising as agents for beta cells- and diabetes-targeted therapies, caution should be exercised with these agents. Future research in extracellular vesicles merits that different isolation procedures are unified, gender and age matching are considered, and other metabolic conditions are carefully analyzed in the selection of donors. Exosomes have a risk of biological contamination and can transport infectious agents. Moreover, they can affect target and non-target cells in a variety of ways. Caution should be exercised to ensure that exosomes or other extracellular vesicles (EV) are not derived from cancer cells. EV’s derived from cancer cells have been shown to transfer miRNAs and oncoproteins which may result in oncogenic effects (Andre et al., 2002; Valadi et al., 2007). Exosomes derived from human Umbilical Cord MSC were shown to promote growth of lung adenocarcinoma cells via transmission of miR-410 - which suppresses the expression of PTEN, a tumor suppressor gene (Dong et al., 2018). Exosomes derived from human Bone Marrow MSC increase VEGF and CXCR4 expression in a dose-dependent manner, leading to tumor growth and angiogenesis in vivo (Zhu et al., 2012). Further investigation of MSC-derived and islet-cell derived exosomes are of great interest for the delivery of agents to islet cells and for the advancement of therapies for diabetes.
In summary, MSC and exosomes are emerging as therapeutic agents for diabetes mellitus. Coating of MSC with functionalized nanocarriers has enabled targeted delivery to inflamed endothelia. Enzymatic modification of glycoproteins in the MSC cell surface has been successfully applied for the same goal. Exosomes may be amenable to similar modifications. Based on the cell derivation, exosomes could deliver cargos of interest and could bind preferentially to target islet or vascular cells. A substantial amount of work will be required to address the open issues related to these biological agents, that range from limited knowledge of the mechanism of effect, to difficult standardization, to unknown long-term safety. Nevertheless, these agents appear as excellent candidates for islet-targeted strategies for diabetes mellitus.
10. Imaging of beta cells in vivo with ligands and nanoparticles
Beside the need for targeted delivery of therapeutic agents to pancreatic islets, there is a need for the targeted delivery of imaging reagents. The latter would enable the development of noninvasive imaging methods to quantify the beta cell mass. Such methods could be extremely useful for monitoring the progression of diabetes mellitus and the response to treatments. The pancreatic beta cell mass is severely depleted in T1D as well as T2D. Concerning T1D, it is believed that beta cell loss occurs long before diabetes diagnosis, as suggested by tests using indirect parameters (Velikyan and Eriksson, 2020). To determine beta cell function, blood glucose, HbA1c, insulin, and C-peptide levels are analyzed in blood plasma. However, none of these directly measures beta cell mass.
Two possible targets for the non-invasive imaging of pancreatic beta cell mass are GLP-1R and ZnT8. As previously discussed, GLP-1R is abundantly expressed on the surface of pancreatic beta cell, and it can be targeted with the GLP-1 mimetic Exendin-4. ZnT8 is also expressed in beta cells, mainly in insulin granules. ZnT8 becomes exposed in the surface of beta cells at the time of insulin release. ZnT8 expression seems to be confined to pancreatic islet beta cells. Antibodies have been generated to target ZnT8 on the cell surface. One antibody, Ab31, targeting loop 2 of ZnT8, appears to be useful for imaging beta cell mass (Eriksson et al., 2018). Ab31 was radiolabeled with iodine-125 ([125I] Ab31) and compared with Exendin-4, radiolabeled with the same iodine-125 ([125I] Exendin-4). In vivo, [125I]Exendin-4 showed superior targeting ability when compared to [125I]Ab31, possibly because GLP-1R is constitutively present onto the cell surface, whereas ZnT8 is most frequently residing in its intracellular location in beta cells. GLP-1R was thus found to be a promising target for human beta cell imaging. In studies in the NOD mouse model of autoimmune diabetes, probes engineered with Exendin-4 conjugated with magnetic oxide-based nanoparticle (MN-exendin-4) demonstrated decrease binding in diabetic mice, correctly indicating beta cell loss (Wang et al., 2014). Future studies focused on affinity, ligand miniaturization, and radionuclide selection will be required to improve beta cell imaging (Eriksson et al., 2018).
Another cell surface molecule with high specificity for beta cells is VMAT-2. A selective ligand for VMAT-2, dihydrotetrabenazine (DTBZ), has been utilized to build radiotracers such as [18F]-DTBZ and [11C]-DTBZ for imaging via positron emission tomography (PET). PET imaging for [11C]-DTBZ was tested for the measurement of beta cell mass in patients with long-standing T1D (Goland et al., 2009). The results suggested that [11C]-DTBZ PET could allow quantification of VMAT2 binding in the human pancreas, but the beta cell mass appeared to be overestimated. For this approach to be successful, current limitations with PET imaging need to be overcome (Alavi and Werner, 2018), and measurement of non-displaceable tracer uptake need to be refined to enable quantification of the specific binding to beta cells (Naganawa et al., 2018).
Other strategies under investigation have utilized perfluorocarbon nanoparticles carrying Rhodamine to non-invasively track transplanted human islets through MRI imaging (Barnett et al., 2011). Among the possible MRI contrast probes, iron oxide nanoparticles have important advantages and are commonly used in the clinical setting (Wang et al., 2014).
These observations represent important steps towards a reliable imaging of beta cell mass, but further refinements are needed.
11. Conclusion
Diabetes affects more than 9% of the population and is the seventh leading cause of death. Current therapeutic strategies have substantial limitations and do not correct the root causes of the disease at the level of pancreatic islets. Emerging therapeutic strategies for the treatment of diabetes are increasingly centered around pancreatic islet cells, islet beta cells, and their microenvironment. In the context of T1D, remarkable results were obtained with novel agents that counteract beta cell autoimmunity, such as Teplizumab (anti-CD3), Golimumab (anti-TNFα), ATG (anti-Thymocyte Globulin), and Mesenchymal Stem Cells (MSC). In the context of T2D, GLP-1 mimetics for the potentiation of beta cell function have radically improved the treatment paradigm. Targeting these therapeutic agents specifically to pancreatic islets appears of great importance, because it could maximize therapeutic benefits while avoiding off-target side effects. The targeted delivery of agents to pancreatic islets could result in preservation or regeneration of functional beta cells with minimal side effects. A multitude of targeted delivery strategies have harnessed ligands for cell surface molecules specific to islet cells and have shown great promise to improve therapeutic outcomes. Among the many islet cell surface molecules chosen as targets, GLP-1R has been utilized frequently, but VMAT-2, GPR44 and ZnT8 appear to have excellent specificity for islet beta cells and should thus be investigated in depth. Engineered nanoparticles and nanocarriers are emerging as viable options to enable targeted delivery of therapeutic agents and cells to inflamed islet endothelia. Targeting inflamed endothelia appears as an intriguing strategy, especially in the context of T1D, but the window of applicability could be limited to the peri-onset period - when such inflammation occurs specifically in islets. MSCs and their exosomes have yielded remarkable benefits in animal models of diabetes, ranging from prevention to correction of disease. Modification of MSCs or of their exosomes to deliver them to inflamed islet endothelia appears as a very promising strategy. Further investigations of nanocarrier-targeted delivery of therapeutic agents in animal models are required to determine homing, therapeutic effects, and toxicity.
Along with the need for islet-targeted therapeutic strategies, there is an urgent need of imaging methods to quantify the mass of insulin producing beta cells – which is severely depleted in Type 1 Diabetes mellitus and partially depleted or dysfunctional in Type 2 Diabetes. The targeted delivery of imaging reagents to islet beta cells, via ligands for cell surface molecules such as GLP-1R and VMAT2, could enable beta cell mass quantification and the development of improved therapeutic protocols.
Studies focused on safety and efficacy of targeted delivery to the islet environment are warranted, to enable translation of the most promising interventions to clinical trials, and ultimately to effective therapies for diabetes.
Acknowledgments
E.Z. would like to thank the University of Miami Department of Chemistry and the University of Miami Department of Biochemistry and Molecular Biology.
S.D. and S.K.D. would like to thank NINDS (1R21NS118427) and NHLBI (R01 HL149452) for funding support. S.D. thanks the Miller School of Medicine of the University of Miami for the Lucille P. Markey Chair in Biochemistry and Molecular Biology.
L.S., C.R., and G.L. would like to thank the Diabetes Research Institute Foundation (DRIF) for supporting this study.
The authors would like to thank Andrea Cordoba, Samantha Verling, Clarissa Victoria Ruiz, and Gabriela Suzel Martinez for the critical review of the manuscript. Figures were created with BioRender.com.
Glossary
- T1D
Type 1 Diabetes
- T2D
Type 2 Diabetes
- MSC
Mesenchymal Stem Cells
- UC-MSC
Umbilical Cord-derived Mesenchymal Stem Cells
- BM-MSC
Bone Marrow-derived Mesenchymal Stem Cells
- GLP-1R
Glucagon-like peptide 1 Receptor
- ICAM-1
Intercellular Adhesion Molecule 1
- VCAM-1
Vascular Cell Adhesion Molecule 1
- VMAT-2
Vesicular Monoamine Transporter 2
- GPR44
G protein coupled receptor 44 (Prostaglandin D2 receptor 2)
- ZnT8
Zinc transporter 8
- LFA-1
Lymphocyte function-associated antigen 1
- VLA-4
Very Late Antigen-4
- HCELL
Hematopoietic Cell E–and L-selectin ligand
- DTBZ
dihydrotetrabenazine
- PET
Positron Emission Tomography
- MRI
Magnetic Resonance Imaging
References
- Abdi R, Moore R, Sakai S, Donnelly CB, Mounayar M, Sackstein R, 2015. HCELL expression on murine MSC licenses pancreatotropism and confers durable reversal of autoimmune diabetes in NOD mice. Stem Cell. 33 (5), 1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulreda MH, Berggren PO, 2021. The pancreatic islet: a micro-organ in control. CellR4 Repair Replace Regen Reprogram 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. , 2015. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem. Cells Transl. Med 4 (10), 1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A, Werner TJ, 2018. Futility of attempts to detect and quantify beta cells by PET imaging in the pancreas: why it is time to abandon the approach. Diabetologia 61 (12), 2512–2515. [DOI] [PubMed] [Google Scholar]
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. , 2002. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360 (9329), 295–305. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Eissele R, Schafer MK, Eiden LE, Arnold R, Pauser U, et al. , 2003. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J. Histochem. Cytochem 51 (8), 1027–1040. [DOI] [PubMed] [Google Scholar]
- Atchison NA, Fan W, Papas KK, Hering BJ, Tsapatsis M, Kokkoli E, 2010. Binding of the fibronectin-mimetic peptide, PR_b, to alpha5beta1 on pig islet cells increases fibronectin production and facilitates internalization of PR_b functionalized liposomes. Langmuir 26 (17), 14081–14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsornworawat P, Maxwell KG, Velazco-Cruz L, Millman JR, 2020. Single-cell transcriptome profiling reveals beta cell maturation in stem cell-derived islets after transplantation. Cell Rep. 32 (8), 108067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avivar-Valderas A, Martin-Martin C, Ramirez C, Del Rio B, Menta R, Mancheno-Corvo P, et al. , 2019. Dissecting Allo-sensitization after local administration of human allogeneic adipose mesenchymal stem cells in perianal fistulas of Crohn’s disease patients. Front. Immunol 10, 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile L, Vassalli G, 2017. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol. Ther 174, 63–78. [DOI] [PubMed] [Google Scholar]
- Barnett BP, Ruiz-Cabello J, Hota P, Ouwerkerk R, Shamblott MJ, Lauzon C, et al. , 2011. Use of perfluorocarbon nanoparticles for non-invasive multimodal cell tracking of human pancreatic islets. Contrast Media Mol. Imaging 6 (4), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS, et al. , 2012. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 61 (10), 2534–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua MP, Nelson RM, 1993. Selectins. J. Clin. Invest 91 (2), 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins KS, Jeong JH, Ou M, Brumbach JH, Kim SW, 2012. EphA2 targeting peptide tethered bioreducible poly(cystamine bisacrylamide-diamino hexane) for the delivery of therapeutic pCMV-RAE-1gamma to pancreatic islets. J. Contr. Release 158 (1), 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Powers AC, 2008. Revascularization of transplanted islets: can it be improved? Diabetes 57 (9), 2269–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A, 2006. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. U. S. A 103 (7), 2334–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, et al. , 2016. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care 39 (1), 149–157. [DOI] [PubMed] [Google Scholar]
- Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. , 2020. Human islets contain a subpopulation of glucagon-like peptide-1 secreting alpha cells that is increased in type 2 diabetes. Mol. Metabol 39, 101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K, 2015. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 64 (2), 587–592. [DOI] [PubMed] [Google Scholar]
- Castellanos L, Tuffaha M, Koren D, Levitsky LL, 2020. Management of diabetic ketoacidosis in children and Adolescents with type 1 diabetes mellitus. Paediatr Drugs 22 (4), 357–367. [DOI] [PubMed] [Google Scholar]
- Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, et al. , 2006. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc. Natl. Acad. Sci. U. S. A 103 (22), 8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Huang PK, Law WC, Chu CH, Chen NT, Lo LW, 2020. Biodegradable polymers for gene-delivery applications. Int. J. Nanomed 15, 2131–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, et al. , 2018. Diabetes in America. [PubMed] [Google Scholar]
- Cuesta-Gomez N, Graham GJ, Campbell JDM, 2021. Chemokines and their receptors: predictors of the therapeutic potential of mesenchymal stromal cells. J. Transl. Med 19 (1), 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, et al. , 2006. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol 24 (11), 1392–1401. [DOI] [PubMed] [Google Scholar]
- da Costa Martins P, Garcia-Vallejo JJ, van Thienen JV, Fernandez-Borja M, van Gils JM, Beckers C, et al. , 2007. P-selectin glycoprotein ligand-1 is expressed on endothelial cells and mediates monocyte adhesion to activated endothelium. Arterioscler. Thromb. Vasc. Biol 27 (5), 1023–1029. [DOI] [PubMed] [Google Scholar]
- Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, et al. , 2017. Age-dependent human beta cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J. Clin. Invest 127 (10), 3835–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick RL, Bodary S, Garovoy MR, 2003. Adhesion molecules as therapeutic targets for autoimmune diseases and transplant rejection. Expet Opin. Biol. Ther 3 (1) , 85–95. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bendala J, Ricordi C, 2012. Present and future cell therapies for pancreatic beta cell replenishment. World J. Gastroenterol 18 (47), 6876–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C, 2012. Concise review: mesenchymal stem cells for diabetes. Stem. Cells Transl. Med 1 (1), 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bendala J, Lanzoni G, Klein D, Alvarez-Cubela S, Pastori RL, 2016. The human endocrine pancreas: new insights on replacement and regeneration. Trends Endocrinol. Metabol 27 (3), 153–162. [DOI] [PubMed] [Google Scholar]
- Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, et al. , 2018. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 9 (2), 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, et al. , 2016. Human islets contain four distinct subtypes of beta cells. Nat. Commun 7, 11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer CJ, Ward NC, Pugliese A, Malek TR, 2016. Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr. Diabetes Rep 16 (6), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybala MP, Hara M, 2019. Heterogeneity of the human pancreatic islet. Diabetes 68 (6), 1230–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CP, Champe M, Gonzalez T, Wessinger ME, Spencer SA, Presta LG, et al. , 1995. Identification of amino acids in the CD11a I-domain important for binding of the leukocyte function-associated antigen-1 (LFA-1) to intercellular adhesion molecule-1 (ICAM-1). J. Biol. Chem 270 (21), 12635–12640. [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS, 2004. Prediction of type 1 diabetes: the natural history of the prediabetic period. Adv. Exp. Med. Biol 552, 268–290. [PubMed] [Google Scholar]
- Eisenbarth GS, 2010. Banting Lecture 2009: an unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes 59 (4), 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ouaamari A, Dirice E, Gedeon N, Hu J, Zhou JY, Shirakawa J, et al. , 2016. SerpinB1 promotes pancreatic beta cell proliferation. Cell Metabol. 23 (1), 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Regazzi R, 2020. Micro(RNA) management and mismanagement of the islet. J. Mol. Biol 432 (5), 1419–1428. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Korsgren O, Selvaraju RK, Mollaret M, de Boysson Y, Chimienti F, et al. , 2018. Pancreatic imaging using an antibody fragment targeting the zinc transporter type 8: a direct comparison with radio-iodinated Exendin-4. Acta Diabetol. 55 (1), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Fabrega J, Fernandez-Cruz L, 2020. Exocrine drainage in pancreas transplantation: complications and management. World J. Transplant 10 (12), 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Poliak M, Viollet B, 2014. Metformin: from mechanisms of action to therapies. Cell Metabol. 20 (6), 953–966. [DOI] [PubMed] [Google Scholar]
- Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A, 2010. Bone marrow-derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev. Diabet. Stud 7 (2), 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Wang Y, Xia X, Zheng JC, 2020. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact 100261. [Google Scholar]
- Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, et al. , 2013. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med 19 (12), 1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Kanapathipillai M, Korin N, McCarthy JR, Ingber DE, 2012. Polymeric nanomaterials for islet targeting and immunotherapeutic delivery. Nano Lett. 12 (1), 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, et al. , 2009. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J. Nucl. Med 50 (3), 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE, 2012. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenetics Genom. 22 (11) , 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, et al. , 2012. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology 265 (1), 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YC, Chiu YH, Chen CP, Wang HS, 2018. Interleukin-1beta induces CXCR3-mediated chemotaxis to promote umbilical cord mesenchymal stem cell transendothelial migration. Stem Cell Res. Ther 9 (1), 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel Penaforte-Saboia J, Couri CEB, Vasconcelos Albuquerque N, Lauanna Lima Silva V, Bitar da Cunha Olegario N, Oliveira Fernandes V., et al. , 2021. Emerging roles of dipeptidyl peptidase-4 inhibitors in delaying the progression of type 1 diabetes mellitus. Diabetes Metab Syndr Obes 14, 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, et al. , 2018. Low-dose anti-thymocyte globulin (ATG) preserves beta-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 41 (9), 1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A, 2008. VMAT2 gene expression and function as it applies to imaging beta-cell mass. J. Mol. Med. (Berl.) 86 (1), 5–16. [DOI] [PubMed] [Google Scholar]
- He Y, Ding Y, Liang B, Lin J, Kim TK, Yu H, et al. , 2017. A systematic study of dysregulated MicroRNA in type 2 diabetes mellitus. Int. J. Mol. Sci 18 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Danielsson A, Ponten F, Czernichow P, Korsgren O, Johansson L, et al. , 2016. GPR44 is a pancreatic protein restricted to the human beta cell. Acta Diabetol. 53 (3), 413–421. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, et al. , 2016. Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39 (7), 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen D, 2017. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 30 (3), 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, et al. , 2013. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr. J 60 (3), 347–357. [DOI] [PubMed] [Google Scholar]
- Huang Y, Chen P, Zhang CB, Ko GJ, Ruiz M, Fiorina P, et al. , 2010. Kidney-derived mesenchymal stromal cells modulate dendritic cell function to suppress alloimmune responses and delay allograft rejection. Transplantation 90 (12), 1307–1311. [DOI] [PubMed] [Google Scholar]
- Ichii H, Ricordi C, 2009. Current status of islet cell transplantation. J. Hepatobiliary Pancreat. Surg 16 (2), 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JH, Lee M, Kim WJ, Yockman JW, Park TG, Kim YH, et al. , 2005. Anti-GAD antibody targeted non-viral gene delivery to islet beta cells. J. Contr. Release 107 (3), 562–570. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Kim SH, Lee M, Kim WJ, Park TG, Ko KS, et al. , 2010. Non-viral systemic delivery of Fas siRNA suppresses cyclophosphamide-induced diabetes in NOD mice. J. Contr. Release 143 (1), 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo WS, Jeong JH, Nam K, Blevins KS, Salama ME, Kim SW, 2012. Polymeric delivery of therapeutic RAE-1 plasmid to the pancreatic islets for the prevention of type 1 diabetes. J. Contr. Release 162 (3), 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassem M, 2004. Mesenchymal stem cells: biological characteristics and potential clinical applications. Cion Stem Cell 6 (4), 369–374. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, et al. , 2011. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol 29 (8), 750–756. [DOI] [PubMed] [Google Scholar]
- Keshtkar S, Kaviani M, Sarvestani FS, Ghahremani MH, Aghdaei MH, Al-Abdullah IH, et al. , 2020. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI J 19, 1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielgast U, Holst JJ, Madsbad S, 2009. Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr. Diabetes Rev 5 (4), 266–275. [DOI] [PubMed] [Google Scholar]
- Kim SW, 2011. Polymeric gene delivery for diabetic treatment. Diabetes Metab. J 35 (4), 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr L, Prakash TP, Lee R, Drury Iii WJ, Nikan M, Fu W, et al. , 2021. Glucagon like peptide 1 receptor agonists for targeted delivery of antisense oligonucleotides to pancreatic beta cell. J. Am. Chem. Soc 143 (9), 3416–3429. [DOI] [PubMed] [Google Scholar]
- Ko KS, Lee M, Koh JJ, Kim SW, 2001. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol. Ther 4 (4), 313–316. [DOI] [PubMed] [Google Scholar]
- Kodera R, Shikata K, Takatsuka T, Oda K, Miyamoto S, Kajitani N, et al. , 2014. Dipeptidyl peptidase-4 inhibitor ameliorates early renal injury through its anti-inflammatory action in a rat model of type 1 diabetes. Biochem. Biophys. Res. Commun 443 (3), 828–833. [DOI] [PubMed] [Google Scholar]
- Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW, 2000. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 7 (24), 2099–2104. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. , 2008. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol 26 (4), 443–452. [DOI] [PubMed] [Google Scholar]
- Lablanche S, Vantyghem MC, Kessler L, Wojtusciszyn A, Borot S, Thivolet C, et al. , 2018. Islet transplantation versus insulin therapy in patients with type 1 diabetes with severe hypoglycaemia or poorly controlled glycaemia after kidney transplantation (TRIMECO): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 6 (7), 527–537. [DOI] [PubMed] [Google Scholar]
- Lanzoni G, Ricordi C, 2021. Transplantation of stem cell-derived pancreatic islet cells. Nat. Rev. Endocrinol 17 (1), 7–8. [DOI] [PubMed] [Google Scholar]
- Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez R, Kouroupis D, et al. , 2020. Umbilical cord mesenchymal stem cells for COV1D-19 ARDS: a double blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med [Google Scholar]
- Lasky LA, 1992. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 258 (5084), 964–969. [DOI] [PubMed] [Google Scholar]
- Lee JO, Rieu P, Arnaout MA, Liddington R, 1995. Crystal structure of the A domain from the alpha subunit of integrin CR3 (CD11b/CD18). Cell 80 (4), 631–638. [DOI] [PubMed] [Google Scholar]
- Lee M, Han S, Ko KS, Kim SW, 2001a. Cell type specific and glucose responsive expression of interleukin-4 by using insulin promoter and water soluble lipopolymer. J. Contr. Release 75 (3), 421–429. [DOI] [PubMed] [Google Scholar]
- Lee M, Han SO, Ko KS, Koh JJ, Park JS, Yoon JW, et al. , 2001b. Repression of GAD autoantigen expression in pancreas beta-Cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol. Ther 4 (4), 339–346. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg JF, Smeets EF, Neefjes JJ, Shaffer MA, Cinek T, Jeunhomme TM, et al. , 1992. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 77 (4), 543–549. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, et al. , 2021a. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res. Ther 12 (1), 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Mills Z, Zheng Z, 2021b. Novel cell sources for bone regeneration. MedComm 2 (2), 145–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Zu CH, Yang CC, Tsai PJ, Shyu JF, Chen CP, et al. , 2015. IL-1beta-Induced mesenchymal stem cell migration involves MLCK activation via PKC signaling. Cell Transplant. 24 (10), 2011–2028. [DOI] [PubMed] [Google Scholar]
- Lindskog C, Korsgren O, Ponten F, Eriksson JW, Johansson L, Danielsson A, 2012. Novel pancreatic beta cell-specific proteins: antibody-based proteomics for identification of new biomarker candidates. J. Proteonomics 75 (9), 2611–2620. [DOI] [PubMed] [Google Scholar]
- Linn T, Strate C, Federlin K, Papaccio G, 1994. Intercellular adhesion molecule-1 (ICAM-1) expression in the islets of the non-obese diabetic and low-dose streptozocin-treated mouse. Histochemistry 102 (4), 317–321. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Daftarian P, Kovalski L, Wang B, Tian R, Castilla DM, et al. , 2016. Directing and potentiating stem cell-mediated angiogenesis and tissue repair by cell surface E-selectin coating. PLoS One 11 (4), e0154053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen JX, Zhang XW, Sun Q, Yang L, Liu A, et al. , 2018. Chemokine receptor 7 overexpression promotes mesenchymal stem cell migration and proliferation via secreting Chemokine ligand 12. Sci. Rep 8 (1), 204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lu J, Shen SM, Ling Q, Wang B, Li LR, Zhang W, et al. , 2021. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res. Ther 12 (1), 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J, 2013. C-peptide in diabetes diagnosis and therapy. Front Biosci (Elite Ed) 5, 214–223. [DOI] [PubMed] [Google Scholar]
- Luo YL, Liang LF, Gan YJ, Liu J, Zhang Y, Fan YN, et al. , 2020. An all-in-one nanomedicine consisting of CRISPR-Cas9 and an autoantigen peptide for restoring specific immune tolerance. ACS Appl. Mater. Interfaces 12 (43), 48259–48271. [DOI] [PubMed] [Google Scholar]
- Mai G, del Rio ML, Tian J, Ramirez P, Buhler L, Rodriguez-Barbosa JI, 2007. Blockade of the PD-1/PD-1L pathway reverses the protective effect of anti-CD40L therapy in a rat to mouse concordant islet xenotransplantation model. Xenotransplantation 14 (3), 243–248. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. , 2003. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci 10 (2), 228–241. [DOI] [PubMed] [Google Scholar]
- Martin S, Hibino T, Faust A, Kleemann R, Kolb H, 1996. Differential expression of ICAM-1 and LFA-1 versus L-selectin and VCAM-1 in autoimmune insulitis of NOD mice and association with both Th1- and Th2-type infiltrates. J. Autoimmun 9 (5), 637–643. [DOI] [PubMed] [Google Scholar]
- Martin S, van den Engel NK, Vinke A, Heidenthal E, Schulte B, Kolb H, 2001. Dominant role of intercellular adhesion molecule-1 in the pathogenesis of autoimmune diabetes in non-obese diabetic mice. J. Autoimmun.17 (2), 109–117. [DOI] [PubMed] [Google Scholar]
- Maxwell KG, Augsornworawat P, Velazco-Cruz L, Kim MH, Asada R, Hogrebe NJ, et al. , 2020. Gene-edited human stem cell-derived beta cells from a patient with monogenic diabetes reverse preexisting diabetes in mice. Sci. Transl. Med 12 (540). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi A, Golriz M, Adili-Aghdam F, Hafezi M, Ashrafi M, Morath C, et al. , 2014. Expanding the indications of pancreas transplantation alone. Pancreas 43 (8), 1190–1193. [DOI] [PubMed] [Google Scholar]
- Merriman C, Huang Q, Gu W, Yu L, Fu D, 2018. A subclass of serum anti-ZnT8 antibodies directed to the surface of live pancreatic beta-cells. J. Biol. Chem 293 (2), 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman JR, Xie C, Van Dervort A, Gurtler M, Pagliuca FW, Melton DA, 2016. Corrigendum: generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat. Commun 7, 12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo D, Pileggi A, Alejandro R, Ricordi C, 2009. Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care 32 (8), 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Yadav N, Saraogi GK, Tambuwala MM, Giri N, 2019. Dendrimer based nanoarchitectures in diabetes management: an overview. Curr. Pharmaceut. Des 25 (23), 2569–2583. [DOI] [PubMed] [Google Scholar]
- Morgan NG, Leete P, Foulis AK, Richardson SJ, 2014. Islet inflammation in human type 1 diabetes mellitus. IUBMB Life 66 (11), 723–734. [DOI] [PubMed] [Google Scholar]
- Musial-Wysocka A, Kot M, Majka M, 2019. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 28 (7), 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganawa M, Lim K, Nabulsi NB, Lin SF, Labaree D, Ropchan J, et al. , 2018. Evaluation of pancreatic VMAT2 binding with active and inactive enantiomers of [(18)F]FP-DTBZ in healthy subjects and patients with type 1 diabetes. Mol. Imag. Biol 20 (5), 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali A, Ferrannini E, 2006. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia 49 (3), 434–441. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Group DER, 2014. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 37 (1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W, Ma X, Yang C, Chen Z, Rong P, Wu M, et al. , 2018. Human mesenchymal-stem-cells-derived exosomes are important in enhancing porcine islet resistance to hypoxia. Xenotransplantation 25 (5), e12405. [DOI] [PubMed] [Google Scholar]
- Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, et al. , 2007. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 110 (12), 3891–3899. [DOI] [PubMed] [Google Scholar]
- Orlando G, Gianello P, Salvatori M, Stratta RJ, Soker S, Ricordi C, et al. , 2014. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes 63 (5), 1433–1444. [DOI] [PubMed] [Google Scholar]
- Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. , 2014. Generation of functional human pancreatic beta cells in vitro. Cell 159 (2), 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Fouraschen SM, de Ruiter PE, Dinjens WN, Kwekkeboom J, Tilanus HW, et al. , 2014. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp. Biol. Med 239 (1), 105–115. [DOI] [PubMed] [Google Scholar]
- Pang H, Luo S, Xiao Y, Xia Y, Li X, Huang G, et al. , 2020. Emerging roles of exosomes in T1DM. Front. Immunol 11, 593348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. , 2018. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol 16 (1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekrun K, De Alencastro G, Luo QJ, Liu J, Kim Y, Nygaard S, et al. , 2019. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight 4 (22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, et al. , 2019. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62 (4), 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, et al. , 2017. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 5 (8), 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezhman L, Tahrani A, Chimen M, 2021. Dysregulation of leukocyte trafficking in type 2 diabetes: mechanisms and potential therapeutic avenues. Front. Cell Dev. Biol 9, 624184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B, Nylander K, Harnaha J, Machen J, Lakomy R, Styche A, et al. , 2008. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes 57 (6), 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, 2014. Advances in the etiology and mechanisms of type 1 diabetes. Discov. Med 18 (98), 141–150. [PubMed] [Google Scholar]
- Pugliese A, 2016. Insulitis in the pathogenesis of type 1 diabetes. Pediatr. Diabetes 17 (Suppl. 22), 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen LC, 2018. Stem cell-derived pancreatic progenitor cells have now been transplanted into patients: report from IPITA 2018. Am. J. Transplant 18 (7), 1581–1582. [DOI] [PubMed] [Google Scholar]
- Purwana I, Zheng J, Li X, Deurloo M, Son DO, Zhang Z, et al. , 2014. GABA promotes human beta-cell proliferation and modulates glucose homeostasis. Diabetes 63 (12), 4197–4205. [DOI] [PubMed] [Google Scholar]
- Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, et al. , 2020. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N. Engl. J. Med 383 (21), 2007–2017. [DOI] [PubMed] [Google Scholar]
- Realsen J, Goettle H, Chase HP, 2012. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol. Therapeut 14 (12), 1149–1154. [DOI] [PubMed] [Google Scholar]
- Reinhart L, Panning CA, 2002. Insulin glargine: a new long-acting insulin product. Am. J. Health Syst. Pharm 59 (7), 643–649. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Japour A, 2019. Transplanting islet cells can fix brittle diabetes. Why isn’t it available in the U.S.? CellR4 Repair Replace. Regen Reprogram 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, et al. , 2016. National institutes of health-sponsored clinical islet transplantation consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes 65 (11), 3418–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rkp Benninger, Hodson DJ, 2018. New understanding of beta-cell heterogeneity and in situ islet function. Diabetes 67 (4), 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzwajg M, Salet R, Lorenzon R, Tchitchek N, Roux A, Bernard C, et al. , 2020. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a Phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 63 (9), 1808–1821. [DOI] [PubMed] [Google Scholar]
- Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, et al. , 2006. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 108 (12), 3938–3944. [DOI] [PubMed] [Google Scholar]
- Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. , 2005. Five-year follow-up after clinical islet transplantation. Diabetes 54 (7), 2060–2069. [DOI] [PubMed] [Google Scholar]
- Sackstein R, 2010. Directing stem cell trafficking via GPS. Methods Enzymol. 479, 93–105. [DOI] [PubMed] [Google Scholar]
- Sands AT, Zambrowicz BP, Rosenstock J, Lapuerta P, Bode BW, Garg SK, et al. , 2015. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in type 1 diabetes. Diabetes Care 38 (7), 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering B, 2003. Edmonton’s islet success has indeed been replicated elsewhere. Lancet 362 (9391), 1242. [DOI] [PubMed] [Google Scholar]
- Sheehy DF, Quinnell SP, Vegas AJ, 2019. Targeting type 1 diabetes: selective approaches for new therapies. Biochemistry 58 (4), 214–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Chen S, Noguchi H, Matsumoto S, Grayburn PA, 2010. In vivo non-viral gene delivery of human vascular endothelial growth factor improves revascularisation and restoration of euglycaemia after human islet transplantation into mouse liver. Diabetologia 53 (8), 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staunton DE, Dustin ML, Erickson HP, Springer TA, 1990. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61 (2), 243–254. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Sibley S, Jackson M, Thomas W, 2003. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26 (3), 832–836. [DOI] [PubMed] [Google Scholar]
- Sun Y, Mao Q, Shen C, Wang C, Jia W, 2019. Exosomes from beta-cells alleviated hyperglycemia and enhanced angiogenesis in islets of streptozotocin-indueed diabetic mice. Diabetes Metab Syndr Obes 12, 2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton G, Minguet J, Ferrero C, Bramlage P, 2014. U300, a novel long-acting insulin formulation. Expet Opin. Biol. Ther 14 (12), 1849–1860. [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Brosius FC 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, et al. , 2018. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol. Dial. Transplant 33 (11), 1950–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Drysdale CM, Nassehi T, Gamer J, Yapundich M, DiNicola J, et al. , 2021. Cas9 protein delivery non-integrating lentiviral vectors for gene correction in sickle cell disease. Mol Ther Methods Clin Dev 21, 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9 (6), 654–659. [DOI] [PubMed] [Google Scholar]
- van Suylichem PT, Pasma A, Wolters GH, van Schilfgaarde R, 1987. Microscopic aspects of the structure and collagen content of the pancreas from the perspective of islet isolation. Transplant. Proc 19 (5), 3958–3959. [PubMed] [Google Scholar]
- Velikyan I, Eriksson O, 2020. Advances in GLP-1 receptor targeting radiolabeled agent development and prospective of theranostics. Theranostics 10 (1), 437–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Yoo B, Yang J, Zhang X, Ross A, Pantazopoulos P, et al. , 2014. GLP-1R-targeting magnetic nanoparticles for pancreatic islet imaging. Diabetes 63 (5), 1465–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Alvarez-Perez JC, Felsenfeld DP, Liu H, Sivendran S, Bender A, et al. , 2015. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med 21 (4), 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Liu Q, Zhao H, Bishop JO, Zhou G, Olson LK, et al. , 2020. miR-216a-targeting theranostic nanoparticles promote proliferation of insulin-secreting cells in type 1 diabetes animal model. Sci. Rep 10 (1), 5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock RS, Xing D, Maahs DM, Michels A, Rickels MR, Peters AL, et al. , 2013. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J. Clin. Endocrinol. Metab 98 (8), 3411–3419. [DOI] [PubMed] [Google Scholar]
- Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI, 2016. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J. Contr. Release 238, 166–175. [DOI] [PubMed] [Google Scholar]
- Wittingen J, Frey CF, 1974. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann. Surg 179 (4), 412–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZX, Yang XZ, Cai JQ, Liao LM, Yang L, Lin YN, et al. , 2011. Digital subtraction angiography and computed tomography angiography of predominant artery feeding pancreatic body and tail. Diabetes Technol. Therapeut 13 (5), 537–541. [DOI] [PubMed] [Google Scholar]
- Wu TY, Liang YH, Wu JC, Wang HS, 2019. Interleukin-1beta enhances umbilical cord mesenchymal stem cell adhesion ability on human umbilical Vein endothelial cells via LFA-1/ICAM-1 interaction. Stem Cell. Int 2019, 7267142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi N, Yokono K, Amano K, Nagata M, Tsukamoto K, Hasegawa Y, et al. , 1995. Expression of intercellular adhesion molecule 1 on pancreatic beta-cells accelerates beta-cell destruction by cytotoxic T-cells in murine autoimmune diabetes. Diabetes 44 (7), 744–752. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Tabata M, Yasuzaki Y, Nomura M, Shibata A, Ibayashi Y, et al. , 2014. A nanocarrier system for the delivery of nucleic acids targeted to a pancreatic beta cell line. Biomaterials 35 (24), 6430–6438. [DOI] [PubMed] [Google Scholar]
- Yang YK, Ogando CR, Wang See C., Chang TY, Barabino GA, 2018. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther 9 (1), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. , 2010. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res 107 (6), 810–817. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Chen F, 2015. pH-responsive drug-delivery systems. Chem. Asian J 10 (2), 284–305. [DOI] [PubMed] [Google Scholar]
- Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. , 2012. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 315 (1), 28–37. [DOI] [PubMed] [Google Scholar]


