Abstract
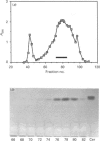
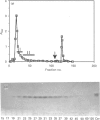
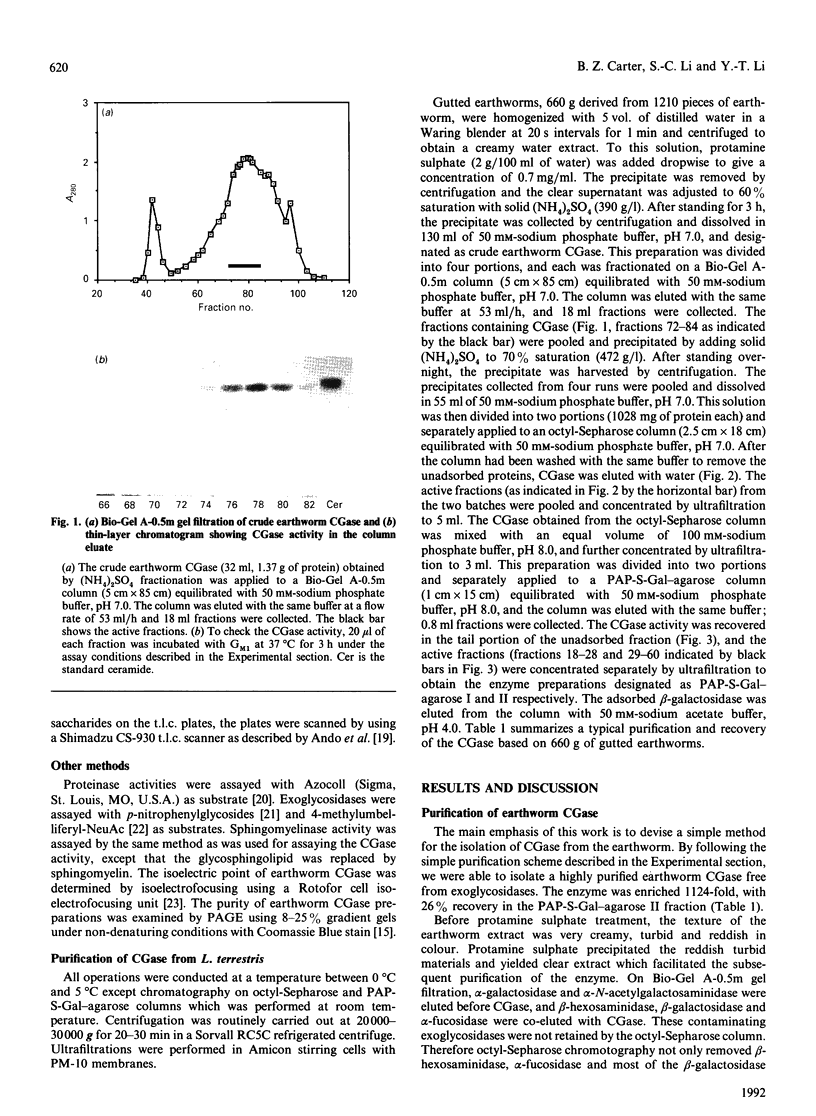
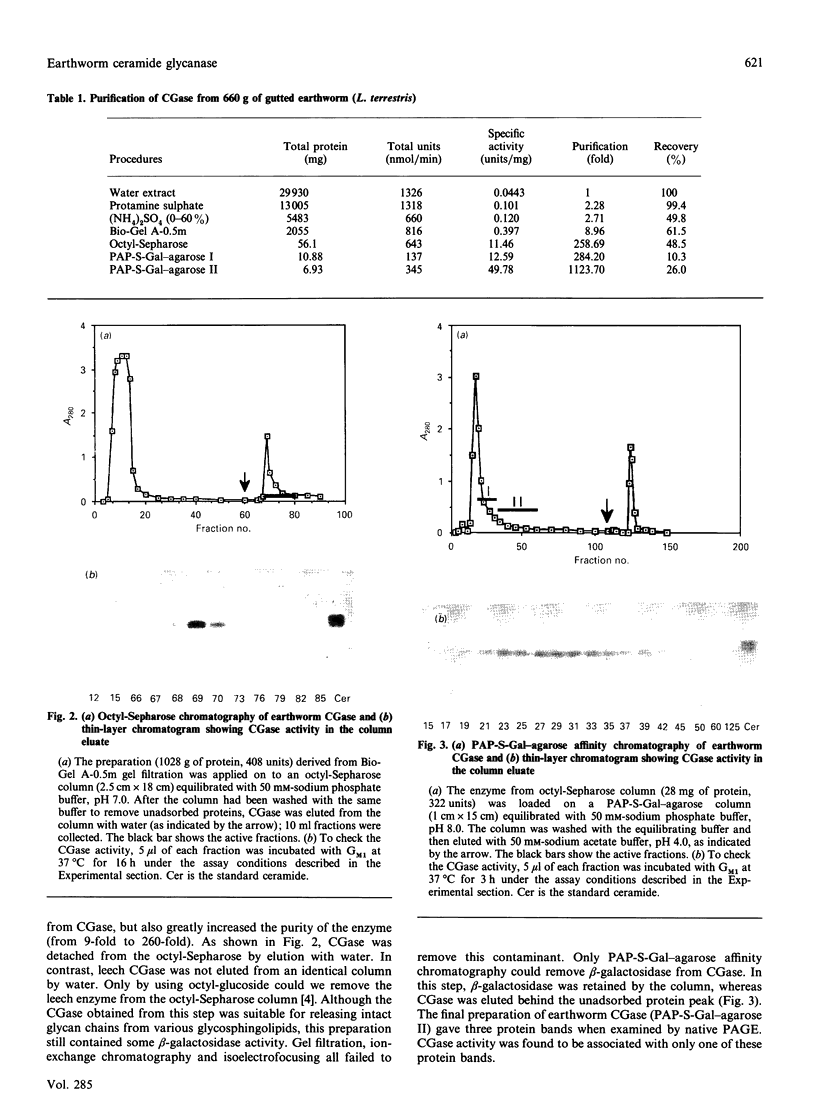
Ceramide glycanase (CGase) is an enzyme that cleaves the linkage between the sugar chain and the ceramide. To make this enzyme readily available, we have developed a simple method for preparing it from the earthworm, Lumbricus terrestris. The method involves Bio-Gel A-0.5m, octyl-Sepharose and p-aminophenylthiogalactoside-agarose column chromatography. By gel filtration, the molecular mass of earthworm CGase was found to be 43.7 kDa. With ganglioside GM1 as substrate, the optimal pH of this enzyme was found to be between pH 3.5 and 4.0. Earthworm CGase hydrolyses glycolipids only in the presence of a detergent. Among various bile salts tested, sodium cholate was found to be the most effective in stimulating the hydrolysis of GM1 by this enzyme. Earthworm CGase released intact glycan chains from various glycosphingolipids in which the glycan chain is linked to the ceramide through a beta-glucosyl linkage. It also detached glycan chains from lactosyldialkylglycerol and alkyl-beta-lactosides.
Full text
PDF
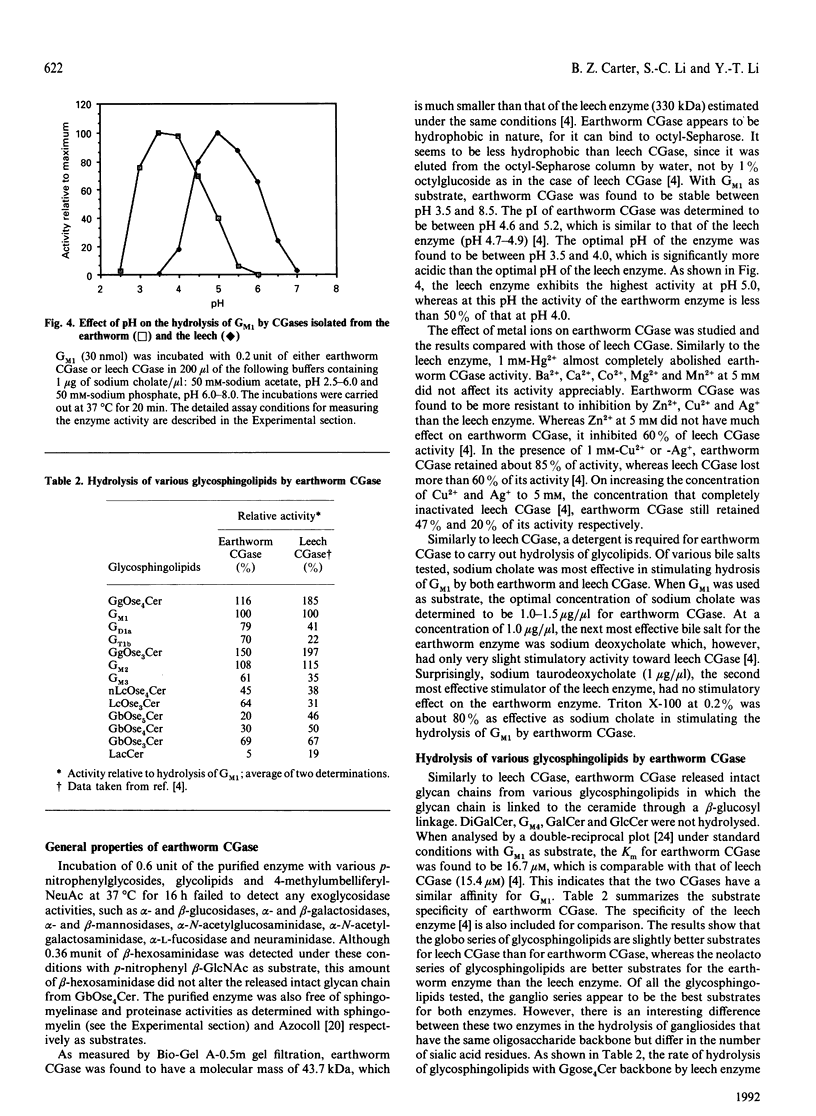

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Chang N. C., Yu R. K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal Biochem. 1978 Sep;89(2):437–450. doi: 10.1016/0003-2697(78)90373-1. [DOI] [PubMed] [Google Scholar]
- Hansson G. C., Li Y. T., Karlsson H. Characterization of glycosphingolipid mixtures with up to ten sugars by gas chromatography and gas chromatography-mass spectrometry as permethylated oligosaccharides and ceramides released by ceramide glycanase. Biochemistry. 1989 Aug 8;28(16):6672–6678. doi: 10.1021/bi00442a021. [DOI] [PubMed] [Google Scholar]
- Ito M., Yamagata T. A novel glycosphingolipid-degrading enzyme cleaves the linkage between the oligosaccharide and ceramide of neutral and acidic glycosphingolipids. J Biol Chem. 1986 Oct 25;261(30):14278–14282. [PubMed] [Google Scholar]
- Ito M., Yamagata T. Purification and characterization of glycosphingolipid-specific endoglycosidases (endoglycoceramidases) from a mutant strain of Rhodococcus sp. Evidence for three molecular species of endoglycoceramidase with different specificities. J Biol Chem. 1989 Jun 5;264(16):9510–9519. [PubMed] [Google Scholar]
- Kitamikado M., Ito M., Li Y. T. Isolation and characterization of a keratan sulfate-degrading endo-beta-galactosidase from Flavobacterium keratolyticus. J Biol Chem. 1981 Apr 25;256(8):3906–3909. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. C., Chien J. L., Wan C. C., Li Y. T. Occurrence of glycosphingolipids in chicken egg yolk. Biochem J. 1978 Aug 1;173(2):697–699. doi: 10.1042/bj1730697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. C., DeGasperi R., Muldrey J. E., Li Y. T. A unique glycosphingolipid-splitting enzyme (ceramide-glycanase from leech) cleaves the linkage between the oligosaccharide and the ceramide. Biochem Biophys Res Commun. 1986 Nov 26;141(1):346–352. doi: 10.1016/s0006-291x(86)80375-8. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Ishikawa Y., Li S. C. Occurrence of ceramide-glycanase in the earthworm, Lumbricus terrestris. Biochem Biophys Res Commun. 1987 Nov 30;149(1):167–172. doi: 10.1016/0006-291x(87)91619-6. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Li S. C., Dawson G. Anomeric structure of ceramide digalactoside isolated from the kidney of a patient with Fabry's disease. Biochim Biophys Acta. 1972 Jan 27;260(1):88–92. [PubMed] [Google Scholar]
- McKibbin J. M. The composition of the glycolipids in dog intestine. Biochemistry. 1969 Feb;8(2):679–685. doi: 10.1021/bi00830a033. [DOI] [PubMed] [Google Scholar]
- Moore G. L. Use of azo-dye-bound collagen to measure reaction velocities of proteolytic enzymes. Anal Biochem. 1969 Oct 15;32(1):122–127. doi: 10.1016/0003-2697(69)90111-0. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Handa S. Coomassie brilliant blue staining of lipids on thin-layer plates. Anal Biochem. 1984 Nov 1;142(2):406–410. doi: 10.1016/0003-2697(84)90484-6. [DOI] [PubMed] [Google Scholar]
- Potier M., Mameli L., Bélisle M., Dallaire L., Melançon S. B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979 Apr 15;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Renou J. P., Giziewicz J. B., Smith I. C., Jarrell H. C. Glycolipid membrane surface structure: orientation, conformation, and motion of a disaccharide headgroup. Biochemistry. 1989 Feb 21;28(4):1804–1814. doi: 10.1021/bi00430a057. [DOI] [PubMed] [Google Scholar]
- Shimamura M., Hayase T., Ito M., Rasilo M. L., Yamagata T. Characterization of a major neutral glycolipid in PC12 cells as III3Gal alpha-globotriaosylceramide by the method for determining glycosphingolipid saccharide sequence with endoglycoceramidase. J Biol Chem. 1988 Aug 25;263(24):12124–12128. [PubMed] [Google Scholar]
- Svennerholm L., Månsson J. E., Li Y. T. Isolation and structural determination of a novel ganglioside, a disialosylpentahexosylceramide from human brain. J Biol Chem. 1973 Jan 25;248(2):740–742. [PubMed] [Google Scholar]
- Zhou B., Li S. C., Laine R. A., Huang R. T., Li Y. T. Isolation and characterization of ceramide glycanase from the leech, Macrobdella decora. J Biol Chem. 1989 Jul 25;264(21):12272–12277. [PubMed] [Google Scholar]