Abstract
Background
To investigate the peripapillary retinal nerve fibre layer (RNFL) thickness changes and analyse factors associated with visual recovery of G11778A Leber hereditary optic neuropathy (LHON) patients.
Methods
Patients diagnosed with G11778A LHON between July 2017 and December 2020 in Tongji hospital were included in this follow-up study. Patients were grouped according to disease duration. Variations in the RNFL thickness in each quadrant at different disease stages were characterised using optical coherence tomography. According to the absence or presence of significant visual acuity improvements, LHON patients of disease duration ≥ 6 months were divided into two groups. A bivariate logistic regression model was constructed to analyse the potential factors associated with spontaneous visual recovery.
Results
This study included 56 G11778A LHON patients (112 eyes) and 25 healthy controls (50 eyes), with a mean follow-up of 5.25 ± 1.42 months. All quadrants and mean RNFL thicknesses of LHON patients first increased and then decreased, except for the temporal RNFL. As the disease progressed, RNFL thinning slowed; however, gradual RNFL thinning occurred. Logistic regression revealed that baseline best corrected visual acuity was related to spontaneous visual recovery of LHON patients with disease duration ≥ 6 months.
Conclusion
The pattern of RNFL involvement could be helpful in the differential diagnosis of LHON and other optic neuropathies. LHON patients with better vision are more likely to experience some degree of spontaneous visual acuity recovery after the subacute phase.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-024-03625-0.
Keywords: Leber hereditary optic neuropathy, Retinal nerve fibre layer, Retinal ganglion cell, Clinical follow-up study, Spontaneous visual acuity recovery
Introduction
Leber hereditary optic neuropathy (LHON) is an inherited mitochondrial disease characterised by acute or subacute painless vision loss [1]. The pathogenesis of LHON is characterised by mitochondrial DNA (mtDNA) mutations, which lead to dysfunction of the complex I subunits of the mitochondrial respiratory chain, impairing mitochondrial respiration, and increasing the production of reactive oxygen species [2]. Retinal ganglion cells are highly vulnerable to mitochondrial dysfunction, which promotes apoptosis and axonal degeneration, ultimately leading to optic atrophy [2]. Although the m.11778G > A mutation is the most common cause of LHON worldwide, it is well known to be a severe mutation, with < 25% patients achieving some degree of vision recovery [3, 4]. Therapeutic strategies for LHON remain limited, although a subgroup of LHON patients treated with idebenone has shown some improvement [5]. Intravitreal gene therapy has also yielded promising results [6].
Visual outcomes are generally poor, with most patients worsening to at least 20/200 visual acuity (VA) in both eyes. After reaching a nadir within 6 months, visual loss is generally permanent [7]. Some patients with LHON have some degree of visual recovery [3, 4]; however, the factors associated with a better visual prognosis remain ambiguous. It has been reported that LHON patients with thicker RNFL in the atrophic phase (disease duration ≥ 6 months) were more likely to show some degree of visual recovery in the period after the nadir, suggesting that RNFL thickness could be a potential clinical indicator to predict visual recovery in LHON patients [8]. In addition, the age at the onset of vision loss could be another important predictor of visual outcomes in LHON [4, 9].
Currently, most studies have evaluated LHON progression by detecting differences in RNFL thickness. We aimed to investigate the peripapillary RNFL thickness changes and analyse the factors associated with visual recovery of G11778A LHON patients and ultimately provide valuable clues regarding the factors influencing visual recovery outcomes.
Methods
Study participants
A total of 56 (112 eyes) G11778A LHON patients were enrolled in this follow-up study between July 2017 and December 2020. The patients were divided into three groups (< 6, 6–12, and > 12 months) according to the duration of the disease at the time of inclusion in the study and were followed up at intervals of 3 ± 2 months, with a total of two follow-up visits. Changes in the RNFL thickness in each quadrant at different disease stages were measured and compared using optical coherence tomography (OCT).
All the participants provided informed consent. This study was approved by the Ethics Committee of Tongji Hospital at Tongji Medical College and conducted in strict accordance with the regulations of the Declaration of Helsinki (TJ-IRB20180316). Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Inclusion criteria
Patients diagnosed with LHON based on clinical symptoms and signs, with genetic testing indicating m.11778G > A/MT-ND4 mutation; age, 6–65 years; without significant visual improvement within 3 months or since the onset of the disease; have not used any drug within 3 months or since the onset of the disease; and provided informed consent and were willing to participate in the ophthalmic examinations and follow-up, were included. A change in best-corrected visual acuity (BCVA) ≥ 0.3 of the logarithm of the minimal angle of resolution (LogMAR) was defined as visual improvement.
Exclusion criteria
Patients with glaucoma and high myopia as well as those with retinal and optic nerve diseases other than LHON were excluded. Additional exclusion criteria were previous treatment with gene therapy product, smokers and heavy drinkers as previously described [10]. The time point at which uncorrectable vision loss or visual dysfunction occurred was considered the time point of onset for recording the disease course.
Study grouping
According to the international consensus statement, G11778A LHON patients were grouped as follows: subacute stage, within 6 months of onset; dynamic stage, 6–12 months from onset; and chronic stage, > 12 months from onset [11]. Age- and sex-matched healthy individuals were recruited in the healthy control group and included those with BCVA > 20/25, refractive errors < 6 dioptres sphere and 2 dioptres cylinder, intraocular pressure < 21 mmHg, and lack of systemic or central nervous system diseases.
An improvement in BCVA of ≥ 0.3 LogMAR during follow-up was used as a criterion for the presence of visual recovery, and G11778A LHON patients with a disease duration of ≥ 6 months were categorised into the visual recovery and visual non-recovery groups.
Instrumentation and procedures
The ophthalmic examination procedure was explained to the patients beforehand, and they were instructed to cooperate during the examination. All OCT scans were performed in a dark room by experienced operators. RNFL thickness in LHON patients was monitored using Spectralis® HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). The RNFL thicknesses in the superior, inferior, temporal, and nasal quadrants and 360° averages were recorded. Participants with pupil diameters < 2 mm required pupil dilation. Repeated measurements were required to ensure good image quality. Spectralis® HRA + OCT software automatically analysed all OCT data. The VA test was based on BCVA, and the eye chart was a 2.5-m standard logMAR chart (Star Kang Medical Technology Co., Ltd., Wenzhou, China). During the measurements, the patients were 2.5 m away from the VA chart, and the data were measured and recorded by the same physician. All patients were examined thrice to confirm changes in VA, and the mean value was considered the final VA.
Statistical analysis
All data are expressed as mean ± standard deviation and were analysed using SPSS (SPSS V.21.0; IBM Corp., Armonk, NY). RNFL thicknesses in all groups were compared using a one-way analysis of covariance (ANCOVA), with sex and age as covariates, followed by the Bonferroni post-hoc test for pairwise comparisons. One-way ANOVA was performed for comparisons between multiple groups, and Fisher’s least significant difference test was performed for two-by-two comparisons between groups. Nonparametric tests were used to compare baseline and subsequent follow-up observations between the visual recovery and non-recovery groups. Binary logistic regression analysis was used to analyse the correlation between temporal RNFL thickness, age, age of onset, and baseline BCVA at the time of inclusion in the study and VA recovery in G11778A LHON patients (disease duration ≥ 6 months). Receiver operating characteristic (ROC) curve analysis was performed to evaluate the discriminatory ability of baseline BCVA. Statistical significance was set at P < 0.05.
Results
Study population characteristics and follow-up period
There were two follow-up visits with an interval of 3 ± 2 months (Supplemental Table 1). The study included 56 G11778A LHON patients (52 men and 4 women; n = 112 eyes). The mean follow-up time was 5.25 months. The follow-up time was 5.04 ± 1.51 months for patients in the acute phase, 4.90 ± 1.30 months for patients in the dynamic phase, and 5.54 ± 1.39 months for patients in the chronic phase. On inclusion in the study, the mean age of LHON patients was 20.23 ± 7.47 years (range: 11–40 years), and the mean disease duration was 42.06 ± 65.34 months (range: 1–314 months). There were 25 normal controls (23 men and 2 women; n = 50 eyes), with a mean age of 21.16 ± 4.40 years (range: 9–27 years). There was no significant difference between LHON patients and normal controls in terms of age (P = 0.49, Student’s t-test) or sex (P = 1.00, chi-square test).
Changes in RNFL thickness within each LHON subgroup
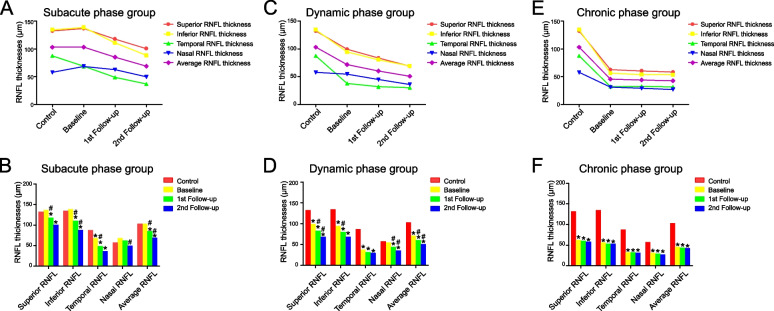
Figure 1 and Supplemental Table 2 show the changes in RNFL thickness in each quadrant for healthy controls and G11778A LHON patients with different disease durations during the follow-up period. Generally, except for the temporal RNFL, all quadrants and mean RNFL thicknesses of LHON patients first increased and then decreased. As the disease progressed, RNFL thinning slowed; however, there was still a tendency for gradual RNFL thinning (Fig. 1).
Fig. 1.
The changes in RNFL thicknesses of each quadrant and the 360˚ averages for the healthy controls and G11778A LHON patients of different disease duration groups during follow-up period. Line diagram (A) and bar diagram (B) showed that the temporal RNFL thickness of LHON patients in the subacute phase group (disease duration < 6 months) was already significantly thinner than that of normal controls at the time of enrollment (P < 0.01), while RNFL thickness in the remaining quadrants and the average RNFL thickness were thicker than that of normal controls with no significant difference. By the 1st follow-up, subacute phase group showed reductions in RNFL thickness of each quadrant and the average thickness compared to baseline values, with significant decrease in RNFL thickness in temporal quadrant (P < 0.01), superior quadrant (P = 0.03), inferior quadrant (P < 0.01), and the average (P < 0.01). By the 2nd follow-up, patients in the subacute phase group showed further reductions in RNFL thickness of each quadrant and the average thickness compared to the measurements at the 1st follow-up visit; the decrease in RNFL thickness in the inferior quadrant (P = 0.02), nasal quadrant (P = 0.02) and the average (p = 0.01) reached significant differences. Line diagram (C) and bar diagram (D) showed that dynamic phase group (disease duration = 6–12 months) had significant decrease in RNFL thickness of each quadrant and the average thickness compared with normal controls, except the RNFL thickness in nasal quadrant (P = 1.00). RNFL thickness of each quadrant and the average thickness were ultimately significantly thinner than that of normal controls during follow-up (P < 0.01). Line diagram (E) and bar diagram (F) showed that all quadrants and the mean RNFL in the chronic phase group (disease duration > 12 months) were already significantly thinner than those in the normal control group at the time of enrollment (P < 0.01). And as the disease progressed, there was still a trend for further thinning of the RNFL. *P < 0.05 when compared with normal control. #P < 0.05 when compared with previous measurements
The temporal RNFL thickness of LHON patients in the subacute phase group was already significantly lower than that of normal controls at the time of enrolment (P < 0.01), whereas the RNFL thickness in the remaining quadrants and the average RNFL thickness were higher than those of normal controls, although no significant difference was observed. By the first follow-up, patients in the subacute phase group showed reductions in RNFL thickness in each quadrant and average thickness compared to baseline values, with a significant decrease in RNFL thickness in the temporal (P < 0.01), superior (P = 0.03), and inferior (P < 0.01) quadrants, and average thickness (P < 0.01). Moreover, the RNFL thinning was more severe in the inferior RNFL (from 138.73 µm to 110.77 µm) than in the superior RNFL (from 136.65 µm to 117.81 µm). By the second follow-up, patients in the subacute phase group showed further reductions in the RNFL thickness of each quadrant and the average thickness compared to the measurements at the first follow-up visit; the decreases in RNFL thickness in the inferior quadrant (P = 0.02), nasal quadrant (P = 0.02), and average thickness (P = 0.01) were significantly different (Supplemental Table 2) (Fig. 1A and B).
LHON patients in the dynamic-phase group showed a significant decrease in RNFL thickness in each quadrant and average thickness compared to normal controls, except for RNFL thickness in the nasal quadrant (P = 1.00). The RNFL thickness of each quadrant and average thickness were significantly lower than those of the normal controls during follow-up (P < 0.01) (Fig. 1C and D). All quadrants and the mean RNFL in the chronic-phase group were significantly thinner than those in the normal control group at the time of enrolment (P < 0.01). As the disease progressed, there was still a trend toward further thinning of the RNFL (Fig. 1E and F).
Comparison of RNFL thickness between the visual recovery and visual non-recovery groups in LHON patients (disease duration ≥ 6 months)
Based on the observation of a BCVA improvement of ≥ 0.3 LogMAR during follow-up as a criterion for identifying visual recovery, G11778A LHON patients (disease duration ≥ 6 months) were categorised into visual recovery (BCVA improvement of ≥ 0.3 LogMAR; n = 16 eyes) and visual non-recovery (n = 70 eyes) groups. The follow-up time was 5.31 ± 1.54 months for patients in the visual recovery group and 5.29 ± 1.41 months for patients in the visual non-recovery group, with no significant difference found between the two groups (P = 0.946). In the vision recovery group, vision recovery was observed in the chronic phase in most of the LHON patients (93.75%). In particular, there was no significant difference in sex (P = 0.643), age at onset (P = 0.933), age (P = 0.322), superior RNFL thickness (P = 0.138), inferior RNFL thickness (P = 0.069), temporal RNFL thickness (P = 0.209), nasal RNFL thickness (P = 0.094), mean RNFL thickness (P = 0.087), visual field index (VFI; P = 0.789), mean defect (MD; P = 0.846), and BCVA (P = 0.050) between the two groups (Table 1).
Table 1.
Comparison of the baseline between the visual recovery group and visual non-recovery group in LHON patients with disease duration ≥ 6 months
| Visual recovery group (BCVA improvement of ≥ 0.3 LogMAR; n = 16 eyes) | Visual non-recovery group (BCVA improvement of < 0.3 LogMAR; n = 70 eyes) | P-value | |||||
|---|---|---|---|---|---|---|---|
| Sex (M/F) | 15/1 | 63/7 | 0.643 | ||||
| Age at onset (years) | 17.44 | ± | 6.65 | 16.66 | ± | 5.47 | 0.933 |
| Age (years) | 23.75 | ± | 8.93 | 21.06 | ± | 7.19 | 0.322 |
| Disease duration (months) | 73.38 | ± | 72.93 | 49.70 | ± | 69.48 | 0.026 |
| Superior RNFL thickness (μm) | 59.94 | ± | 10.64 | 78.97 | ± | 36.69 | 0.138 |
| Inferior RNFL thickness (μm) | 54.06 | ± | 11.25 | 73.13 | ± | 32.10 | 0.069 |
| Temporal RNFL thickness (μm) | 29.50 | ± | 6.70 | 35.16 | ± | 13.33 | 0.209 |
| Nasal RNFL thickness (μm) | 29.81 | ± | 9.11 | 41.53 | ± | 22.65 | 0.094 |
| Average RNFL thickness (μm) | 43.38 | ± | 5.92 | 57.17 | ± | 22.45 | 0.087 |
| VFI (%) | 23.07 | ± | 26.38 | 25.29 | ± | 26.43 | 0.789 |
| MD (dB) | -25.63 | ± | 7.88 | -24.58 | ± | 9.07 | 0.846 |
| BCVA (LogMAR) | 1.81 | ± | 0.42 | 1.56 | ± | 0.45 | 0.050 |
RNFL Retinal nerve fiber layer, BCVA Best-corrected visual acuity, MD Mean deviation, VFI Visual field index
Subsequently, the baseline values of RNFL thickness, mean RNFL thickness, VFI, MD, and BCVA for each quadrant in the vision recovery group were compared with their follow-up values when VA recovery was observed. Significant differences were observed in MD (P = 0.047) and BCVA (P < 0.001), whereas no significant differences were observed in superior (P = 0.108), inferior (P = 0.755), temporal (P = 0.937), nasal (P = 0.950), and mean RNFL thickness (P = 0.206) or VFI (P = 0.059) (Table 2). In addition, the follow-up values of RNFL thickness, mean RNFL thickness, VFI, MD, and BCVA for each quadrant in the vision recovery group when they showed vision recovery were compared with the second follow-up values of the vision non-recovery group. Significant differences were identified in BCVA (P = 0.027); whereas superior (0.474), inferior (P = 0.312), temporal (P = 0.392), nasal (P = 0.969), and mean visual field RNFL thicknesses (P = 0.399); VFI (P = 0.805); and MD (P = 0.653) did not show significant differences (Supplemental Table 3).
Table 2.
Comparison of follow-up measurements at baseline and at the time of the observation of visual recovery in the visual recovery group of LHON patients (disease duration ≥ 6 months)
| Baseline of vision recovery group (n = 16 eyes) | Measurements at follow-up when vision restoration occurred in the LHON vision recovery group (n = 16 eyes) | P-value | |||||
|---|---|---|---|---|---|---|---|
| Superior RNFL thickness (μm) | 59.94 | ± | 10.64 | 56.19 | ± | 9.84 | 0.108 |
| Inferior RNFL thickness (μm) | 54.06 | ± | 11.25 | 54.38 | ± | 8.11 | 0.755 |
| Temporal RNFL thickness (μm) | 29.50 | ± | 6.70 | 29.75 | ± | 6.97 | 0.937 |
| Nasal RNFL thickness (μm) | 29.81 | ± | 9.11 | 29.94 | ± | 7.22 | 0.950 |
| Average RNFL thickness (μm) | 43.38 | ± | 5.92 | 42.56 | ± | 5.80 | 0.206 |
| VFI (%) | 23.07 | ± | 26.38 | 27.63 | ± | 27.78 | 0.059 |
| MD (dB) | -25.63 | ± | 7.88 | -23.90 | ± | 8.65 | 0.047 |
| BCVA (LogMAR) | 1.81 | ± | 0.42 | 1.45 | ± | 0.47 | 0.000 |
RNFL Retinal nerve fiber layer, BCVA Best-corrected visual acuity, MD Mean deviation, VFI Visual field index
Binary logistic regression analysis and ROC curve of factors associated with visual recovery in LHON patients (disease duration ≥ 6 months)
Binary logistic regression analysis was performed to evaluate the effects of age, age at onset, temporal RNFL thickness, and baseline BCVA on visual recovery in G11778A LHON patients (disease duration ≥ 6 months. The modified Hosmer–Lemshow goodness-of-fit chi-square test statistic was 11.838 (P = 0.159 > 0.05), suggesting that the multivariable models were of good fit. The analysis revealed a significant correlation between baseline BCVA at the time of patient enrolment (P = 0.040) and visual recovery in LHON patients (disease duration ≥ 6 months), whereas there was no correlation between temporal RNFL thickness (P = 0.121), age (P = 0.232), or age at onset (P = 0.232) with visual recovery in these patients (Table 3).
Table 3.
Binary logistic regression analysis of factors associated with visual recovery in LHON patients (disease duration ≥ 6 months)
| P-value | OR | 95% CI for Exp (B) | |||
|---|---|---|---|---|---|
| Age | 0.232 | 1.059 | 0.964 | - | 1.164 |
| Age at onset | 0.232 | 0.918 | 0.798 | - | 1.056 |
| BCVA | 0.040 | 4.544 | 1.074 | - | 19.213 |
| Temporal RNFL thickness | 0.121 | 0.939 | 0.867 | - | 1.017 |
RNFL Retinal nerve fiber layer, BCVA Best-corrected visual acuity
The ROC curves of baseline BCVA were constructed to evaluate the predictive value for visual recovery in LHON patients (disease duration ≥ 6 months). The areas under the ROC curve were 0.654 (95% confidence interval [CI], 0.522–0.584) versus visual recovery. The cut-off point of the ROC curve was 1.85 (sensitivity of 62.5% and specificity of 61.4%) for live births, making the cut-off a potential overall indicator of visual recovery in LHON patients (disease duration ≥ 6 months) (Fig. 2).
Fig. 2.
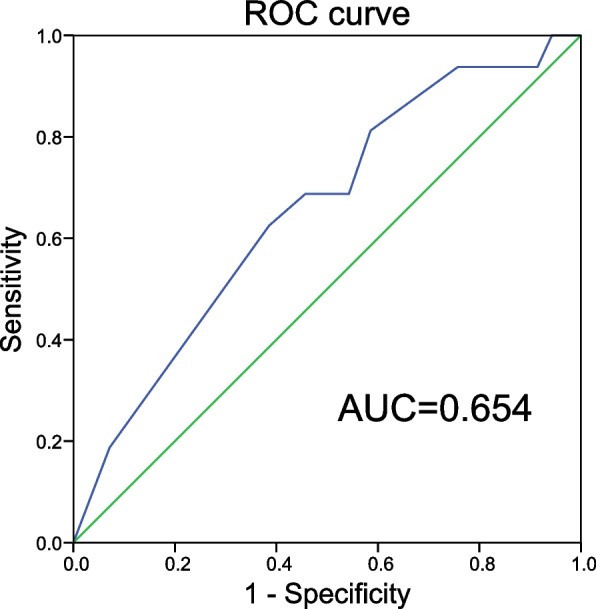
The receiver operator characteristic curve (ROC) curves of baseline BCVA for the evaluation of the predictive value for visual recovery in LHON patients (disease duration ≥ 6 months)
Discussion
This research followed 56 G11778A LHON patients (112 eyes) and selected 25 healthy individuals (50 eyes) matched for age and sex as controls. By comparing the changes in RNFL thickness in each quadrant over time in patients with different disease durations, this study identified characteristic RNFL thickness changes. Consistent with previous cross-sectional research [12], this study further validated the characterisation of RNFL thickness alterations over the course of LHON disease progression in its natural history. Thinning of the RNFL started from the temporal side, followed by the inferior and superior quadrants, and finally the nasal RNFL (Supplemental Table 2 and Fig. 1). This is consistent with the pattern observed in previous studies on the natural history of LHON [13, 14] and provides support for the differential diagnosis of LHON and other optic neuropathies [15–19].
G11778A LHON patients in the subacute phase group had significantly thinner temporal RNFL at enrolment, whereas the RNFL in the rest of the quadrants and the mean RNFL were thicker than those of individuals in the control group, although the difference was not significant (Fig. 1). RNFL thinning started on the temporal side, which is mainly formed by papillomacular-bundle (PMB), and as the disease progressed, the most pronounced RNFL thinning was observed on the temporal side (Supplemental Table 2 and Fig. 1). As the PMB, characterized by high-energy demands and low-energy production, is the preferred neural axons to be damaged in LHON patients [20, 21]. As the disease progressed, at the first follow-up, RNFL thickness in all quadrants of the subacute-stage group further decreased relative to the baseline values, with significant differences in temporal (P < 0.01), superior (P = 0.03), inferior (P < 0.01), and mean (P < 0.01) RNFL thicknesses (Fig. 1). The degree of thinning was more severe in the inferior RNFL (from 138.73 µm to 110.77 µm) than that in the superior RNFL (from 136.65 µm to 117.81 µm) (Supplemental Table 2), suggesting that alterations in the inferior RNFL may occur earlier than those in the superior RNFL during LHON disease progression, which need to be further illustrated. At the second follow-up, all quadrant and mean RNFL thicknesses were further reduced in the subacute-phase group relative to the measurements at the first follow-up, with significant changes in the inferior (P = 0.02), nasal (P = 0.02), and mean (P = 0.01) RNFL thicknesses (Fig. 1).
G11778A LHON patients in the dynamic-phase group were included in the study with significantly lower RNFL thickness in all quadrants compared to normal controls (P < 0.01), except on the nasal side (P = 1.00). During follow-up, the RNFL thickness in all quadrants was significantly lower than that in normal controls (P < 0.01) (Supplemental Table 2 and Fig. 1). The chronic-phase group had significantly thinner quadrants and mean RNFL than the control group on enrolment (P < 0.01). RNFL thinning slowed as the disease progressed but continued to progress slowly (Fig. 1). Taken together, the changes in RNFL thickness after the onset of LHON showed a certain pattern, with the temporal RNFL thinning first, followed by the inferior and superior RNFL, and eventually the nasal RNFL (Fig. 1). This confirms the findings of a previous cross-sectional study [12] and is consistent with the patterns observed in previous studies [13, 22].
LHON patients have a certain possibility of spontaneous visual recovery, and the identification of relevant factors could help evaluate the prognosis of these patients. Some studies have suggested that changes in RNFL thickness can be examined to evaluate disease progression; however, there is limited information on the factors associated with the spontaneous recovery of VA in LHON patients. Previous studies have reported that the visual acuity of LHON patients gradually stabilizes and reaches the nadir after 6 months of onset [8, 23]. G11778A LHON patients with a disease duration of ≥ 6 months were categorised into the visual recovery and visual non-recovery groups in our study. Previous findings by our research team revealed that the VFI, baseline BCVA, and age in LHON patients might be associated with VA recovery after rAAV2-ND4 gene therapy [10, 24]. Piero Barboni et al. have found that RNFL thickness was significantly greater in all quadrants of the LHON population presenting with vision restoration than that in the LHON population without vision restoration. The authors accordingly propose that patients with a thicker RNFL are more likely to show some degree of visual recovery after the visual nadir [8]. Our study compared the baseline RNFL in G11778A LHON patients (disease duration ≥ 6 months) with and without visual recovery and no significant difference was observed (Table 1). Moreover, there was no significant difference between the RNFL thickness at the time when LHON patients showed visual recovery and their baseline level upon inclusion in the study (Table 2). In addition, no differences were observed in RNFL thickness at the time of visual recovery in this group compared with the results of the second follow-up in those who did not experience visual recovery (Supplemental Table 3). These results suggested that RNFL thickness in LHON patients with disease duration ≥ 6 months may not correlate with visual recovery. Previous studies have demonstrated that younger age at onset is associated with greater likelihood of VA recovery [4, 25]. However, some studies have shown that VA prognosis is also poor in patients with young age at onset [26]. In our study, we did not find a significant younger age at onset in the group of patients with visual recovery compared with those without visual recovery (Table 1). In contrast, binary logistic regression analysis revealed a correlation between baseline BCVA and visual recovery in LHON patients with a disease duration ≥ 6 months (Table 3 and Fig. 2), a finding that is corroborated by our previous studies on the factors associated with visual recovery after rAAV2-ND4 gene therapy [10]. Our study revealed a significant association between baseline BCVA and the degree of VA recovery after gene therapy in LHON patients [10, 24]. Taken together, our findings suggest that patients with better vision are more likely to experience some degree of VA recovery after the subacute phase than those with poor vision. RNFL thickness was not significantly associated with improvement in VA. In the vision recovery group, vision recovery was observed in the chronic phase in most of the LHON patients (93.75%). Most cases of visual recovery (25.00%) occurred within 2 years after onset in this study. Interestingly, there were LHON patients in our study who were observed to have vision recovery 22.3 years later after onset. This finding emphasizes the importance of providing LHON patients with long-term follow-up care and guidance for late vision improvement. A proposed explanation for the visual recovery is remyelination of optic nerve axons [3]. Notably, the recovery mechanism is unclear in patients who exhibit spontaneous visual acuity recovery and need to be further explored.
This study has some limitations due to the retrospective nature of the study design and the relatively small sample size of G11778A LHON patients (n = 56). Because of the the rarity of patients with LHON, we included data from both eyes in the study. Also, we have taken into account the delays since the start of the LHON. The disease duration of both eyes was recorded separately and grouped accordingly. Our conclusion needs to be further confirmed by large-sample prospective studies. Ganglion cell thickness provides better reflection of atrophic changes in the optic nerve during the asymptomatic and early stages of LHON. RNFL thickness, on the other hand, provides reliable information on disease progression during the middle and late stages of the LHON disease duration (3–12 months) [27]. These aspects should be considered in the design of future studies.
Nevertheless, our study confirmed the patterns of RNFL involvement in LHON and revealed that baseline BCVA was related to the visual recovery in LHON patients after the subacute phase. The changes in RNFL thickness after the onset of LHON showed a certain pattern, with the temporal RNFL thinning first, followed by the inferior and superior RNFL, and eventually the nasal RNFL, which provides clues for the differential diagnosis of LHON and other optic neuropathies. In addition, Our study revealed that baseline BCVA was related to spontaneous visual recovery of LHON patients. Better BCVA at baseline predicting better outcome during nature history. Patients with worse BCVA are advised to seek treatment earlier and more actively.
Supplementary Information
Abbreviations
- RNFL
Peripapillary retinal nerve fibre layer
- LHON
Leber hereditary optic neuropathy
- mtDNA
Mitochondrial DNA
- VA
Visual acuity
- BCVA
Best-corrected visual acuity
- LogMAR
The logarithm of the minimal angle of resolution
- ANCOVA
One-way analysis of covariance
- ROC
Receiver operating characteristic
Authors’ contributions
TZ, BL, and HJ designed the study and obtained funding. DW collected the data.
LS-L, HY, ML-C, JJ-Y, HL-L, and NM analysed the data. DW drafted the manuscript. All authors approved the final version of the manuscript.
Funding
This study was supported by Hubei Province Natural Science Foundation of China (2022CFB893) and the Guangdong Provincial Natural Science Foundation of China (2023A1515010382).
Availability of data and materials
The data that support the findings of this study are available from the authors but restrictions apply to the availability of these data, (research related to these data is still in process and these studies are not yet publicly released), and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission from Wuhan Children’s Hospital.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
All the participants provided informed consent. This study was approved by the Ethics Committee of Tongji Hospital at Tongji Medical College and conducted in strict accordance with the regulations of the Declaration of Helsinki (TJ-IRB20180316).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hong Jie, Email: 2479899372@qq.com.
Bin Li, Email: libin-12@163.com.
Tao Zhang, Email: zhangtao@tjh.tjmu.edu.cn.
References
- 1.Lam BL, Feuer WJ, Schiffman JC, Porciatti V, Vandenbroucke R, Rosa PR, Gregori G, Guy J. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy : preparation for gene therapy clinical trial. JAMA Ophthalmol. 2014;132:428–36. 10.1001/jamaophthalmol.2013.7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coussa RG, Merat P, Levin LA. Propagation and selectivity of axonal loss in Leber hereditary optic neuropathy. Sci Rep. 2019;9:6720. 10.1038/s41598-019-43180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spruijt L, Kolbach DN, de Coo RF, Plomp AS, Bauer NJ, Smeets HJ, de Die-Smulders CE. Influence of mutation type on clinical expression of Leber hereditary optic neuropathy. Am J Ophthalmol. 2006;141:676–82. 10.1016/j.ajo.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Stone EM, Newman NJ, Miller NR, Johns DR, Lott MT, Wallace DC. Visual recovery in patients with Leber’s hereditary optic neuropathy and the 11778 mutation. J Clin Neuroophthalmol. 1992;12:10–4. [PubMed] [Google Scholar]
- 5.Klopstock T, Yu-Wai-Man P, Dimitriadis K, Rouleau J, Heck S, Bailie M, Atawan A, Chattopadhyay S, Schubert M, Garip A, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134:2677–86. 10.1093/brain/awr170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J, Zhang Y, Liu H, Wang D, Du Y, Tian Z, Li X, Yang S, Pei H, Wan X, et al. Seven-year follow-up of gene therapy for Leber’s hereditary optic neuropathy. Ophthalmology. 2020;127:1125–7. 10.1016/j.ophtha.2020.02.023 [DOI] [PubMed] [Google Scholar]
- 7.Barboni P, Savini G, Valentino ML, La Morgia C, Bellusci C, De Negri AM, Sadun F, Carta A, Carbonelli M, Sadun AA, et al. Leber’s hereditary optic neuropathy with childhood onset. Invest Ophthalmol Vis Sci. 2006;47:5303–9. 10.1167/iovs.06-0520 [DOI] [PubMed] [Google Scholar]
- 8.Barboni P, Savini G, Valentino ML, Montagna P, Cortelli P, De Negri AM, Sadun F, Bianchi S, Longanesi L, Zanini M, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112:120–6. 10.1016/j.ophtha.2004.06.034 [DOI] [PubMed] [Google Scholar]
- 9.Mashima Y, Kigasawa K, Shinoda K, Wakakura M, Oguchi Y. Visual prognosis better in eyes with less severe reduction of visual acuity one year after onset of Leber hereditary optic neuropathy caused by the 11,778 mutation. BMC Ophthalmol. 2017;17:192. 10.1186/s12886-017-0583-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Li X, Yuan J, Tian Z, Liu H, Wang D, Li B. Prognostic factors for visual acuity in patients with Leber’s hereditary optic neuropathy after rAAV2-ND4 gene therapy. Clin Experiment Ophthalmol. 2019;47:774–8. 10.1111/ceo.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carelli V, Carbonelli M, de Coo IF, Kawasaki A, Klopstock T, Lagreze WA, La Morgia C, Newman NJ, Orssaud C, Pott JWR, et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuroophthalmol. 2017;37:371–81. 10.1097/WNO.0000000000000570 [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Liu HL, Du YY, Yuan J, Li X, Tian Z, Zhou H, Wang S, Song L, Sun J, et al. Characterisation of thickness changes in the peripapillary retinal nerve fibre layer in patients with Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2021;105:1166–71. 10.1136/bjophthalmol-2020-316573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barboni P, Carbonelli M, Savini G, Ramos Cdo V, Carta A, Berezovsky A, Salomao SR, Carelli V, Sadun AA. Natural history of Leber’s hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–7. 10.1016/j.ophtha.2009.07.026 [DOI] [PubMed] [Google Scholar]
- 14.Hwang TJ, Karanjia R, Moraes-Filho MN, Gale J, Tran JS, Chu ER, Salomao SR, Berezovsky A, Belfort R Jr, Moraes MN, et al. Natural history of conversion of Leber’s hereditary optic neuropathy: a prospective case series. Ophthalmology. 2017;124:843–50. 10.1016/j.ophtha.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Bennett JL. Optic neuritis. Continuum. 2019;25:1236–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenaers G, Hamel C, Delettre C, Amati-Bonneau P, Procaccio V, Bonneau D, Reynier P, Milea D. Dominant optic atrophy. Orphanet J Rare Dis. 2012;7:46. 10.1186/1750-1172-7-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun BY, Rizzo JF 3rd. Dominant optic atrophy and Leber’s hereditary optic neuropathy: update on clinical features and current therapeutic approaches. Seminars Pediatr Neurol. 2017;24:129–34. 10.1016/j.spen.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 18.Margolin E, Blair K, Shemesh A. Toxic and Nutritional Optic Neuropathy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed]
- 19.Kim US, Jurkute N, Yu-Wai-Man P. Leber Hereditary optic neuropathy-light at the end of the tunnel? Asia-Pac J Ophthalmol. 2018;7:242–5. [DOI] [PubMed] [Google Scholar]
- 20.Savini G, Barboni P, Valentino ML, Montagna P, Cortelli P, De Negri AM, Sadun F, Bianchi S, Longanesi L, Zanini M, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–31. 10.1016/j.ophtha.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 21.Sadun AA, Win PH, Ross-Cisneros FN, Walker SO, Carelli V. Leber’s hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc. 2000;98:223–32 Discussion 232-225. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Huang H, Wei S, Qiu H, Gong Y, Li H, Dai Y, Jiang Z, Liu Z. Characterization of retinal nerve fiber layer thickness changes associated with Leber’s hereditary optic neuropathy by optical coherence tomography. Exp Ther Med. 2014;7:483–7. 10.3892/etm.2013.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber’s hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(Pt 2):319–37. 10.1093/brain/118.2.319 [DOI] [PubMed] [Google Scholar]
- 24.Liu HL, Yuan JJ, Zhang Y, Tian Z, Li X, Wang D, Du YY, Song L, Li B. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol. 2020;98:e730–3. 10.1111/aos.14379 [DOI] [PubMed] [Google Scholar]
- 25.Salmaggi A, Carrara F, Zeviani M. Remarkable recovery of visual function in a patient with Leber’s optic neuropathy and multiple mutations of mitochondrial DNA. Int J Neurosci. 1994;77:261–6. 10.3109/00207459408986036 [DOI] [PubMed] [Google Scholar]
- 26.Leo-Kottler B, Christ-Adler M. Leber optic neuropathy in women and children. Ophthalmologe. 1999;96:698–701. 10.1007/s003470050479 [DOI] [PubMed] [Google Scholar]
- 27.Balducci N, Savini G, Cascavilla ML, La Morgia C, Triolo G, Giglio R, Carbonelli M, Parisi V, Sadun AA, Bandello F, et al. Macular nerve fibre and ganglion cell layer changes in acute Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2016;100:1232–7. 10.1136/bjophthalmol-2015-307326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors but restrictions apply to the availability of these data, (research related to these data is still in process and these studies are not yet publicly released), and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission from Wuhan Children’s Hospital.
No datasets were generated or analysed during the current study.