Abstract
Many therapies are available for the treatment of relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) after ≥ 2 lines of therapy, albeit with scant evidence on the comparative effectiveness of these therapies. This study used inverse probability of treatment weighting to indirectly compare treatment outcomes of epcoritamab from the EPCORE NHL-1 trial with individual patient data from clinical practice cohorts treated with chemoimmunotherapy (CIT) and novel therapies (polatuzumab-based regimens, tafasitamab-based regimens, and chimeric antigen receptor T-cell [CAR T] therapies) for third-line or later R/R large B-cell lymphoma (LBCL) and DLBCL. In this analysis, epcoritamab demonstrated significantly better response rates and overall survival rates than CIT, polatuzumab-based regimens, and tafasitamab-based regimens. No statistically significant differences in response rates or survival were found for epcoritamab compared with CAR T in R/R LBCL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01594-x.
Keywords: Diffuse large B-cell lymphoma, Electronic health records, Mortality, Non-Hodgkin lymphoma, Proportional-hazards models, Retrospective studies, Propensity score weighting
To the editor
Although current frontline therapies for large B-cell lymphoma (LBCL) have curative potential, 30–40% of patients have refractory disease or relapse following treatment with standard chemoimmunotherapy (CIT) [1]. A number of these patients present with challenging-to-treat disease and suboptimal treatment outcomes [2]. Still, CIT remains the most commonly used therapy among patients with relapsed/refractory (R/R) LBCL and diffuse LBCL (DLBCL) after ≥ 2 lines of therapy (LOTs). Newly approved treatments include chimeric antigen receptor T-cell (CAR T) therapies, polatuzumab vedotin plus bendamustine and rituximab, and tafasitamab plus lenalidomide [3]. Epcoritamab is an off-the-shelf subcutaneous CD3xCD20 bispecific antibody therapy being developed as a core therapy across various lymphoma subtypes. It is currently approved for R/R DLBCL after ≥ 2 LOTs, based on strong efficacy and manageable safety data from EPCORE NHL-1 (NCT03625037) [4–6].
In the absence of head-to-head trials, statistical methods such as inverse probability of treatment weighting (IPTW) can adjust for confounding and assess comparative effectiveness [7]. We report the results of an IPTW analysis comparing outcomes of patients treated with epcoritamab in EPCORE NHL-1 versus clinical practice cohorts receiving third-line or later treatment with CIT or novel therapies from COTA, a US-based electronic health records database. Comparisons were conducted in LBCL or DLBCL depending on each therapy’s US FDA approval indication. Methodological details are in the Supplemental Material.
Epcoritamab versus chemoimmunotherapy
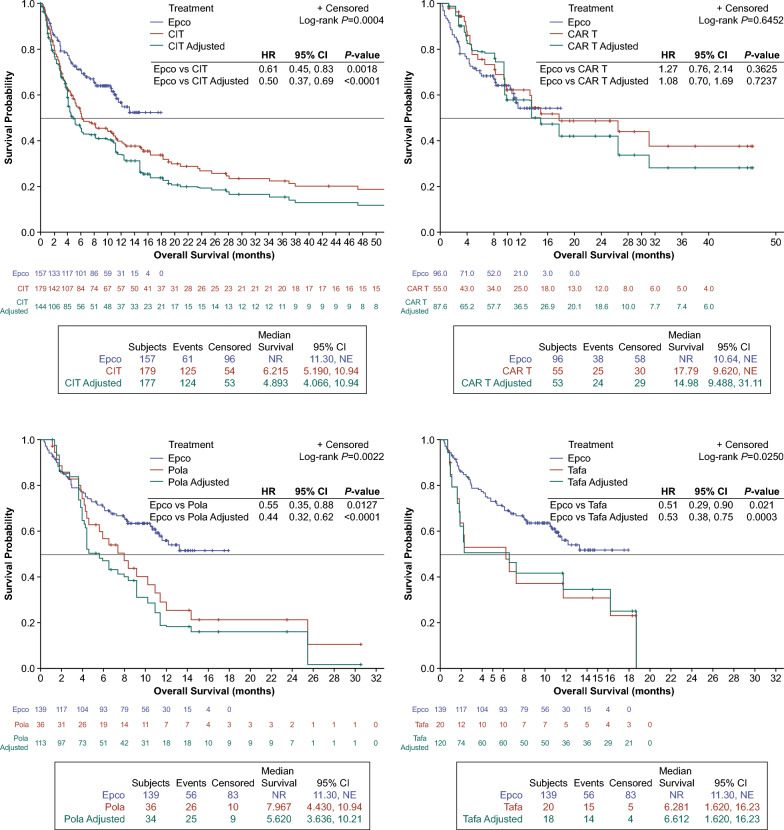
A total of 157 epcoritamab-treated patients with LBCL were compared with 179 CIT-treated patients with LBCL. Median follow-up times were 8.5 months and 5.4 months for the epcoritamab and CIT cohorts, respectively. The adjusted cohorts were balanced on clinical and demographic characteristics: age, sex, previous treatment with CAR T, previous stem cell transplant, number of prior LOTs, primary refractory status, refractory to last LOT status, and time since discontinuation of last LOT (Supplemental Material). After adjustment, complete response (CR) rate was significantly higher with epcoritamab (38.9% [95% CI: 31.2, 46.5]) versus CIT (9.4% [95% CI: 4.7, 14.2]), with an adjusted odds ratio (OR) of 4.1 (95% CI: 2.4, 7.1; P < 0.0001) (Table 1). Median overall survival (OS) was not reached with epcoritamab and was 4.9 months (95% CI: 4.1, 10.9) with CIT, corresponding to an adjusted hazard ratio (HR) of 0.5 (95% CI: 0.4, 0.7; P < 0.0001), indicating significantly better survival with epcoritamab than with CIT (Fig. 1).
Table 1.
Adjusted clinical outcomes of epcoritamab compared with CIT and novel therapies
| Outcome | Treatment after failing ≥ 2 prior LOTs | ||||||
|---|---|---|---|---|---|---|---|
| Epcoritamab | CIT (Adjusted N = 177) | CAR Ta (Adjusted N = 53) | Pola-based regimensb (Adjusted N = 34) | Tafa-based regimensc (Adjusted N = 18) | |||
| LBCL (N = 157) | CAR T-naive LBCL (N = 96) | DLBCL (N = 139) | |||||
| ORR (95% CI) | 63.1% (55.5, 70.6) | 68.8% (59.5, 78.0) | 61.9% (53.8, 69.9) | 41.8% (33.8, 49.9) | 72.0% (62.6, 81.4) | 60.7% (51.7, 69.7) | 34.9% (26.4, 43.4) |
| OR (95% CI) for ORR for epcoritamab vs | 1.5 (1.2, 1.9) P = 0.0004 | 0.95 (0.79, 1.15) P = 0.626 | 1.02 (0.8, 1.2) P = 0.853 | 1.77 (1.3, 2.3) P < 0.0001 | |||
| CR (95% CI) | 38.9% (31.2, 46.5) | 41.7% (31.8, 51.5) | 38.9% (30.8, 47.0) | 9.4% (4.7, 14.2) | 36.5% (26.4, 46.5) | 10.7% (5.1, 16.5) | 11.2% (5.5, 16.8) |
| OR (95% CI) for CR for epcoritamab vs | 4.1 (2.4, 7.1) P < 0.0001 | 1.1 (0.8, 1.6) P = 0.472 | 3.6 (2.0, 6.4) P < 0.0001 | 3.5 (2.0, 6.0) P < 0.0001 | |||
| mPFS (mo) | 4.4 | 5.4 | 4.4 | 2.5 | 5.6 | 3.3 | 1.9 |
| HR (95% CI) for PFS for epcoritamab vs | 0.5 (0.4, 0.6) P < 0.0001 | 0.8 (0.6, 1.1) P = 0.169 | 0.4 (0.3, 0.6) P < 0.0001 | 0.5 (0.4, 0.7) P < 0.0001 | |||
| mOS (mo) | NR | NR | NR | 4.9 | 15.0 | 5.6 | 6.6 |
| HR (95% CI) for OS for epcoritamab vs | 0.5 (0.4, 0.7) P < 0.0001 | 1.1 (0.7, 1.7) P = 0.724 | 0.4 (0.3, 0.6) P < 0.0001 | 0.5 (0.38, 0.8) P = 0.0003 | |||
Two patients from each of the real-world cohorts (CIT, CAR T, pola-based, and tafa-based) were identified as having extreme weights based on the propensity score weighting; thus, these patients were removed from the adjusted analyses
CAR T, chimeric antigen receptor T-cell; CI, confidence interval; CR, complete response; HR, hazard ratio; LOTs, lines of therapy; mOS, median overall survival; mPFS, median progression-free survival; NR, not reached; OR, odds ratio; ORR, overall response rate; pola, polatuzumab; tafa, tafasitamab
aTreatment types: axicabtagene ciloleucel (n = 33), lisocabtagene maraleucel (n = 6), tisagenlecleucel (n = 8), and unknown CAR T (n = 8)
bTreatment types: polatuzumab vedotin plus bendamustine and rituximab (n = 24), polatuzumab vedotin plus bendamustine (n = 3), polatuzumab vedotin plus obinutuzumab (n = 2), and polatuzumab vedotin plus rituximab (n = 8)
cTreatment types: tafasitamab plus lenalidomide (n = 17) and tafasitamab alone (n = 3)
Fig. 1.
Hazard ratios for OS outcomes. Epcoritamab vs A CIT (LBCL); B CAR T (LBCL); C Pola (DLBCL); and D Tafa (DLBCL). CAR T, chimeric antigen receptor T-cell; CI, confidence interval; CIT, chemoimmunotherapy; DLBCL, diffuse large B-cell lymphoma; Epco, epcoritamab; HR, hazard ratio; LBCL, large B-cell lymphoma; NE, not estimable; NR, not reached; OS, overall survival; Pola, polatuzumab; Tafa, tafasitamab
Epcoritamab versus novel therapies
A total of 96 CAR T-naive epcoritamab-treated patients with LBCL were compared with 55 CAR T-treated patients with LBCL. One hundred thirty-nine epcoritamab-treated patients with DLBCL were compared with 37 patients with DLBCL treated with polatuzumab-based regimens and 20 patients with DLBCL treated with tafasitamab-based regimens. Median follow-up times were 10.0, 6.6, and 4.3 months for the CAR T, polatuzumab-based, and tafasitamab-based cohorts, respectively. Polatuzumab-based regimens included polatuzumab combined with other agents including bendamustine, rituximab, obinutuzumab, or bendamustine plus rituximab; tafasitamab-based regimens included tafasitamab with or without lenalidomide. The adjusted cohorts were balanced on clinical and demographic characteristics: age, sex, previous treatment with CAR T, previous stem cell transplant, number of prior LOTs, primary refractory status, refractory to last LOT status, and time since discontinuation of last LOT (Supplemental Material).
The CR rate (95% CI) was 38.9% (30.8, 47.0) with epcoritamab versus 10.7% (5.1, 16.5) with polatuzumab-based and 11.2% (5.5, 16.8) with tafasitamab-based regimens. The adjusted ORs (95% CI) for achieving a CR were 3.6 (2.0, 6.4; P < 0.0001) for epcoritamab versus polatuzumab-based regimens and 3.5 (2.0, 6.0; P < 0.0001) for epcoritamab versus tafasitamab-based regimens (Table 1). The median OS was not reached for epcoritamab and was 5.6 (95% CI: 3.6, 10.2) months for polatuzumab-based regimens and 6.6 (95% CI: 1.6, 16.2) months for tafasitamab-based regimens. Adjusted HRs (95% CI) for OS events were 0.4 (0.3, 0.6; P < 0.0001) for epcoritamab versus polatuzumab-based regimens and 0.5 (0.4, 0.8; P = 0.0003) for epcoritamab versus tafasitamab-based regimens, indicating significantly better survival with epcoritamab (Fig. 1).
The adjusted CR rate (95% CI) with epcoritamab was 41.7% (31.8, 51.5) versus 36.5% (26.4, 46.5) with CAR T; the corresponding adjusted OR (95% CI) for achieving a CR was 1.1 (0.8, 1.6; P = 0.472) (Table 1). Median OS was not reached with epcoritamab and was 15.0 (95% CI: 9.5, 31.1) months with CAR T. The adjusted HR was 1.1 (95% CI: 0.7, 1.7; P = 0.724), indicating no significant difference in survival between epcoritamab and CAR T (Fig. 1).
Conclusions
Epcoritamab demonstrated better efficacy than CIT in R/R LBCL, significantly increasing the likelihood of CR and reducing risk of mortality by half. Compared with polatuzumab-based and tafasitamab-based regimens in R/R DLBCL, epcoritamab significantly increased the likelihood of achieving CR and reduced the risk of mortality by half. No difference in efficacy was found between epcoritamab and CAR T in R/R LBCL. Findings are subject to limitations, including the potential for residual confounding due to unmeasured characteristics and small sample sizes in the real-world novel treatment cohorts.
Supplementary Information
Acknowledgements
Writing and editorial support were provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and funded by Genmab.
Author contributions
Study design: All authors. Study investigator: J.M., A.R., A.I., M.J., T.W. Enrolled patients: n/a. Collection and assembly of data: T.W. Data analysis: A.R., M.J., T.W., A.M., J.Y., A.I. Data interpretation: All authors. Manuscript preparation: M.J., A.M. Manuscript review and revisions: All authors. Final approval of manuscript: All authors.
Funding
Genmab A/S and AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of this article.
Availability of data and materials
De-identified individual participant data collected during the trial will not be available upon request for further analyses by external independent researchers. Aggregated clinical trial data from the trial is provided via publicly accessible study registries/databases as required by law. For more information, please contact ClinicalTrials@genmab.com.
Declarations
Ethical approval and consent to participate
COTA database houses de-identified and secondary data; it is not anticipated that studies using these particular sources present any risk to human subjects. Ethics approval for the NHL-1 trial has been previously published, and this study utilized individual patient-level data from that clinical trial.
Consent for publication
Not applicable.
Competing interests
Allison Rosenthal: Educational Workshop Speaker Role: RMEI, Curio Science, Targeted Oncology, OncLiveU. Javier Munoz: Consulting: Pharmacyclics/AbbVie, Bayer, Gilead/Kite, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, BeiGene, Servier, Novartis, MorphoSys/Incyte, Secura Bio, TG Therapeutics, MEI, Lilly/Loxo; Research Funding: Bayer, Gilead/Kite, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium; Honoraria: Targeted Oncology, OncView, Curio, Kyowa, Physicians’ Education Resource, Seattle Genetics; Speakers Bureau: Gilead/Kite, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, BeiGene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche. Monika Jun, Tongsheng Wang, Alex Mutebi, Fernando Rivas Navarro, Samantha Brodkin, Mariana Sacchi: Genmab: Current Employment. Brian Elliott: Genmab: Current Employment and Stockholder. Shibing Yang: Genmab: Former Employment. Anthony Wang, Kojo Osei-Bonsu, Junhua Yu: AbbVie: Current Employment. Andrew Ip: Honoraria: Pfizer; Speakers Bureau: Seagen; Advisory Board: Secura Bio, AstraZeneca, TG Therapeutics.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–65. 10.1200/JCO.19.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. 10.1182/blood-2017-03-769620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Li LR, Young KH. New agents and regimens for diffuse large B cell lymphoma. J Hematol Oncol. 2020;13:175. 10.1186/s13045-020-01011-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thieblemont C, Phillips T, Ghesquieres H, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41:2238–47. 10.1200/JCO.22.01725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma [press release]. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-epcoritamab-bysp-relapsed-or-refractory-diffuse-large-b-cell. Accessed 10 July 2023.
- 6.Epkinly [package insert]. Plainsboro, NJ, USA: Genmab US, Inc.; 2023.
- 7.Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15:14–20. 10.1093/ckj/sfab158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual participant data collected during the trial will not be available upon request for further analyses by external independent researchers. Aggregated clinical trial data from the trial is provided via publicly accessible study registries/databases as required by law. For more information, please contact ClinicalTrials@genmab.com.