Abstract
Background
The prognosis nutritional index (PNI) and the systemic inflammatory immunological index (SII) are characteristic indicators of the nutritional state and the systemic inflammatory response, respectively. However, there is an unknown combined effect of these indicators in the clinic. Therefore, the practicality of using the SII-PNI score to predict prognosis and tumor response of locally advanced gastric cancer (LAGC) following chemotherapy was the main focus of this investigation.
Methods
We retrospectively analyzed 181 patients with LAGC who underwent curative resection after neoadjuvant chemotherapy in a prospective study (NCT01516944). We divided these patients into tumour regression grade(TRG) 3 and non-TRG3 groups based on tumor response (AJCC/CAP guidelines). The SII and PNI were assessed and confirmed the cut-off values before treatment. The SII-PNI values varied from 0 to 2, with 2 being the high SII (≥ 471.5) as well as low PNI (≤ 48.6), a high SII or low PNI is represented by a 1 and neither is represented by a 0, respectively.
Results
51 and 130 samples had TRG3 and non-TRG3 tumor responses respectively. Patients with TRG3 had substantially higher SII-PNI scores than those without TRG3 (p < 0.0001). Patients with greater SII-PNI scores had a poorer prognosis (p < 0.0001). The SII-PNI score was found to be an independent predictor of both overall survival (HR = 4.982, 95%CI: 1.890-10.234, p = 0.001) and disease-free survival (HR = 4.763, 95%CI: 1.994–13.903, p = 0.001) in a multivariate analysis.
Conclusion
The clinical potential and accuracy of low-cost stratification based on SII-PNI score in forecasting tumor response and prognosis in LAGC is satisfactory.
Keywords: Systemic inflammatory immune index, Prognostic nutrition index, Chemotherapy response, Prognosis, Locally advanced gastric cancer
Introduction
Gastric cancer has the 5th highest incidence and 3rd highest fatality rates globally, which is common and frequently presenting as malignancy in gastrointestinal tumors [1]. Nearly half of the deaths occurred in China, and most patients were diagnosed as locally advanced [2, 3]. Previous studies have found that surgical resection may be the optimal treatment for locally advanced gastric cancer (LAGC) [4, 5]. Growing evidence have demonstrated treatment strategies for patients with LAGC, neoadjuvant chemotherapy (NACT) has become an increasingly recognized treatment modality [6, 7]. This is mainly due to NACT decreasing tumour stage and reducing tumour volume, improving the rate of complete surgical resection and prolonging survival. The effectiveness and safety of neoadjuvant chemotherapy in LAGC have also been confirmed in numerous prospective studies, most notably with XELOX(capecitabine combined with oxaliplatin) and SOX(oxaliplatin combined with S-1) chemotherapy regimens [8–10]. There is a previous prospective study of ours also reached similar conclusions [11].
Nevertheless, studies have found that neoadjuvant chemotherapy cannot benefit all patients, with approximately 40% of patients with LAGC being unresponsive to chemotherapy. As a result, neoadjuvant chemotherapy may led to immunocompromised and delayed surgery in these patients, while increasing the economic burden. Few markers are now available to forecast the outcome of patients with LAGC who have received neoadjuvant treatment. Previous studies have screened patients for potential sensitivity to neoadjuvant chemotherapy based on age, clinical Tumor Node Metastasis(TNM) stage, Eastern Cooperative Oncology Group (ECOG) score and preoperative body mass index(BMI) [12], however, the predictive power of these metrics is limited. The gold standard to assess chemosensitivity to neoadjuvant chemotherapy is the tumour regression grade(TRG), but this can only be performed after surgical resection, when patients may not be able to prevent neoadjuvant chemotherapy tolerance or surgical complications [13]. Therefore, in clinical practice, the goal is to find a marker that can precisely accurately screen patients who have a therapeutic response to XELOX and SOX chemotherapy regimens to avoid unnecessary preoperative treatment.
Previous research has shown a significant association between the development of gastric cancer and the peripheral blood response (neutrophils, platelets, lymphocytes, etc.) to inflammation [14, 15]. Neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) are characteristic inflammation-based indicators, which are currently applied for prognostic evaluation of various malignancies and prediction of neoadjuvant chemotherapy efficacy [16, 17]. In contrast, the systemic inflammatory immunity index (SII) was first proposed by Hu in 2014 [18], which combines neutrophil, lymphocyte and platelet counts to reflect systemic inflammation and immune status more comprehensively than NLR and PLR [19–21]. The SII is correlated with the effectiveness as well as a poor prognosis of neoadjuvant chemotherapy for many cancers [22–25]. The prognosis nutritional index (PNI) is an indicator that thoroughly takes into account albumin concentration and lymphocyte count, which can indicate the immunological and nutritional metabolism state [26]. Presently, PNI can predict a patient’s prognosis for cancers of the pancreas [27], non-small cell lung [28], and stomach [29] and is a predictor of susceptibility to neoadjuvant chemotherapy according to a number of studies. Previous studies have examined the impact of single indicators of SII and PNI on clinical outcomes, we have achieved more favorable results in two small prospective studies in which the combined SII and PNI scoring system was used for the first time to assess sensitivity and prognosis after conversion therapy [30] and immunotherapy [31] respectively. Nevertheless, it is unclear whether the SII-PNI scoring system would be a superior indicator of the prognosis and effectiveness of first-line chemotherapy in LAGC patients.
Therefore, we sought to explore whether SII-PNI score is a superior indicator for predicting chemotherapy sensitivity and prognosis in patients with LAGC undergoing it as well as to find the optimal cut-off value for predicting survival and the clinicopathological changes to neoadjuvant chemotherapy in this study.
Materials and methods
Participants
The 459 LAGC patients who were undergoing neoadjuvant chemotherapy at the Fourth Hospital of Hebei Medical University between January 2011 and May 2016 were enrolled in this study, which is prospective randomized research (NCT01516944). Following were the criteria for inclusion: (I) gastric cancer was identified by pathological evidence; (II) preoperative abdominal computed tomography (CT) staging for T2-T4N + M0; (III) age ≥ 18 years; (IV) no other treatment had been given to the tumor before neoadjuvant chemotherapy; (V) the Eastern Cooperative Oncology Group (ECOG) activity status score was ≤ 2 points, with the tolerance of cardiac, pulmonary and renal function to chemotherapy; (VI) no preoperative associated infections or acute and chronic inflammatory reactions. The following were the exclusion requirements: (I) residual tumor cells around the edges of the surgery (R1/R2 resection); (II) preoperative co-infection resulted in unexpected blood test findings; (III) history of other tumors or hematologic disorders; (VI) complete clinical data were not available; (V) number of neoadjuvant chemotherapy cycles not according to study design (2 cycles of SOX/XELOX chemotherapy). All patients in this study have signed the informed consent, and have been authorized by the Ethics Committee of the Fourth Hospital of Hebei Medical University (approval number: 20111214029).
Experimental measurements
Peripheral venous blood samples were collected from all patients while fasting 1 week before neoadjuvant chemotherapy and 1 week before surgery, respectively. The methods mentioned in previous studies were used for analyzing peripheral blood neutrophil, lymphocyte, and platelet counts [30, 31]. The precise procedure is as follows: counting and analyzing neutrophils, platelets, and lymphocytes using an automatic hematology analyzer (Beckman Coulter LH750), and measuring albumin levels using an automatic hematology analyzer (Beckman Coulter AU5800). According to its definition, the SII = P×N/L, where P, N, and L, respectively, stand for platelet, neutrophil, and lymphocyte counts, respectively [18]. Furthermore, albumin plus 5 times the lymphocyte level is used to determine PNI, the specific calculation formula is: PNI = albumin + 5*L [32]. We defined the optimal cut-off value higher than SII and lower than PNI as 2 points, and the optimal cut-off value higher than SII, or lower than the optimal cut-off value of PNI is defined as 1 point, and the optimal cut-off value is lower than SII, and higher than the best PNI The cut-off value is defined as 0 points.
Neoadjuvant chemotherapy regimens
In this study, 236 (51.42%) of the 459 LAGC patients randomly assigned to neoadjuvant chemotherapy regimens received XELOX chemotherapy, while the remaining 223 (48.58%) received SOX chemotherapy. The chemotherapeutic protocol for XELOX was as follows: intravenous administration of oxaliplatin 130 mg/m2 on day 1; followed by continuous oral capecitabine, 1000 mg/m2 for 14 days, while the SOX chemotherapy regimen was intravenous oxaliplatin 130 mg/m2 on day 1; and orally administered S-1,40 mg/m2, twice daily for 14 days. Every subjects underwent preoperative neoadjuvant chemotherapy for 2 rounds followed by a repeat whole abdomen enhanced CT scan. In addition, patients with imaging assessment of effective neoadjuvant chemotherapy underwent surgery 3 weeks following the conclusion of two-stage of neoadjuvant chemotherapy with XELOX/SOX according to the Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Management of Gastric Cancer [33]. The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 served as the framework for assessing toxicity, which is graded on a scale from 0 to 5: Grade 0 indicating no adverse events (AEs), Grade 1 for mild AEs, Grade 2 for moderate AEs, Grade 3 for severe AEs, Grade 4 for life-threatening or disabling AEs, and Grade 5 for death.
Assessment of treatment response
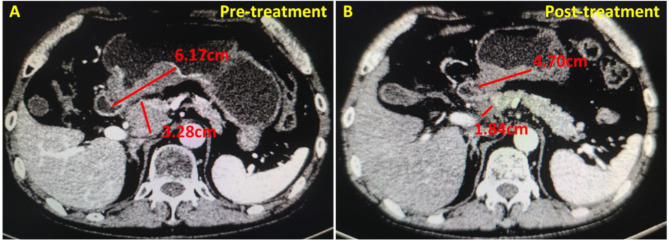
In our study, all initial visits for gastric cancer underwent abdominal CT scanning to assess tumor diameter, and all patients were given 800–1000 ml of drinking water orally 30 min prior to CT scanning (except for patients with combined pyloric obstruction). At the same time, intramuscular injection of scopolamine butylbromide was given to reduce or inhibit gastrointestinal peristalsis, so that the gastric lumen could be fully dilated and the illusion of thickening of the gastric wall could be eliminated or alleviated, and the lesions could be better localized and observed. In this way, we can accurately identify the target lesion by CT and measure the longest diameter and thickness of the tumor, so that we can accurately determine the change in efficacy before and after neoadjuvant therapy. In this study, we selected the layer with the largest lesion in the axial image of gastric cancer patients to measure the longest diameter of the tumor, and we also needed to record the perigastric lymph node lesions with a short diameter of > 1.5 cm, and the measurements were done by a senior radiologist (Fig. 1). The sum of the diameters of all target lesions at baseline (longest diameter for non-lymph node lesions, shortest axis for lymph node lesions) was the basis for subsequent evaluation comparisons. We took the average of the three measurements as the final measurement to compare and judge the efficacy before and after neoadjuvant therapy.
Fig. 1.
Changes in tumor diameter and lymph node short diameter at the maximal level of axial images in LAGC patients before and after neoadjuvant therapy. (A) before neoadjuvant therapy, the tumor diameter measured at the maximal level of axial images was 6.17 cm, and the short diameter of lymph nodes was 3.28 cm, resulting in a diameter of all target lesions of 9.45 cm at baseline; (B) after neoadjuvant therapy, the tumor diameter measured at the maximal level of axial images was 4.70 cm, and the lymph node short diameter was 1.84 cm, which represented a reduction of 2.91 cm in the diameter of all target lesions compared with the initial diagnosis, with a reduction rate of 30.8% (2.91/9.45), and thus the efficacy was evaluated as PR
Three weeks following the completion of two cycles of preoperative neoadjuvant chemotherapy, the efficacy and resectability of the tumors were assessed via computed tomography (CT). Evaluation of tumor response adhered to the guidelines set forth by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, categorizing responses into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Specifically, CR is defined as the disappearance of all target lesions. PR is defined as at least a 30% decrease in the sum of diameters of target lesions compared to the baseline sum. PD is defined as at least a 20% increase in the sum of diameters of target lesions compared to the baseline sum. SD is defined as neither sufficient decrease to qualify as PR nor sufficient increase to qualify as PD. The objective response rate (ORR) was determined by the percentage of patients exhibiting CR and PR, whereas the disease control rate (DCR) encompassed the percentage of patients showing CR, PR, or SD.
All pathological sections from patients undergoing radical surgical resection were analyzed by two pathologists and the tumor regression response was graded using the guidelines set out by the TRG grading scale (AJCC/CAP guidelines) [34]. The absence of any remaining tumor cells on several consecutive sections when viewed under a microscope is defined as TRG 0. TRG 1 is described as the presence of a few discrete tumor cell clusters visible underneath the plasma membrane. The discovery of fibrosis and residual tumor cell debris within the tumor lesion is considered TRG2. TRG3 is identified as having extremely low to nonfibrotic and a stable cell population in the lesion. We divided responses into TRG 3 and non-TRG 3 categories in this study, with TRG2, TRG1, and TRG0 considered non-TRG3.
Follow-up investigation
The time from enrollment in the study and the participant’s death from cancer-related causes or last communication was referred to as overall survival (OS). The period of time from the start of randomization to a relapse or a disease progression that results in death is known as disease-free survival (DFS). A total abdominal enhanced CT examination was recommended for each patient after surgery, with a 3-month interval for the first three years and a 6-month interval for the fourth and fifth years. Follow-up was conducted by telephone communication, outpatient and inpatient treatment.
Statistical analysis
In this study, we analyzed statistical with SPSS 21.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 8.01 (GraphPad Software, San Diego, California). For continuous data having a normal distribution (mean ± SD), the Shapiro-Wilk test was used to assess normality; for continuous variables without a normal distribution, the median (interquartile range) test was applied. The Mann-Whitney U-test or independent t-test was used to compare the groups. To identify the ideal cut-off values for SII and PNI, receiver operating characteristic (ROC) curves were used to separate TRG3 patients from non-TRG3 patients. The predictive power of each predictor could then be compared using the area under the ROC curve (AUC) value. Kaplan-Meier curves were constructed for survival analysis. Univariate and multifactorial analyses were performed using Cox risk regression models to identify the poor prognostic variables. Relative risks were assessed by hazard ratios (HR) and 95% confidence intervals (CI). We ascertained the relationship between PNI and SII using Spearman correlation analysis. Statistical significance was determined to be a p-value < 0.05.
Results
Demographic data and tumor features of patients
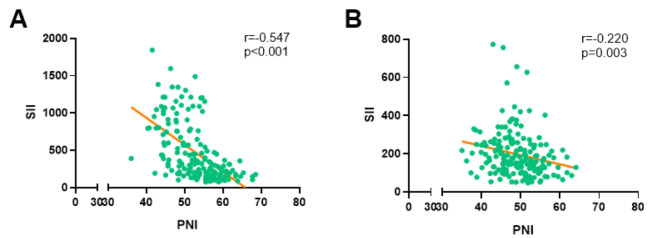
The 181 individuals with LAGC in all participated in this research, including 134 male (74.03%) and 47 female (25.97%) (Fig. 2). Table 1 lists the patients’ clinical characteristics as well as the outcomes of laboratory tests. Patients’ ages ranged from 44 to 75 when they received their diagnoses with a median age of 59. The average BMI of the whole group was 27.8 ± 9.2Kg/m2, and there were 152 patients with ASA-PS grade I, 29 with grade II, and no further grade. All patients underwent two courses of neoadjuvant chemotherapy, 94 (51.93%) of whom received neoadjuvant chemotherapy with the XELOX regimen, and the remaining 87 (48.07%) received the SOX regimen. All patients underwent radical D2 surgical resection, including 69 (38.12%) cases of distal subtotal gastrectomy, 92 (50.83%) cases of total gastrectomy, and 20 (11.05%) cases of proximal subtotal gastrectomy. In addition, nearly 2/3 (120/181) of the patients underwent laparoscopic resection, and the remaining patients (61/181) underwent laparotomy. The mean SII and PNI before neoadjuvant chemotherapy were 467.3 ± 405.8 (ranging from 75.1 to 2247.0) and 52.9 ± 6.1 (ranging from 36.0 to 68.5), respectively, and a strong negative association around them (r=-0.547, p < 0.0001; Fig. 3A). And after neoadjuvant treatment SII and PNI were 197.8 ± 120.5 (ranging from 47.5 to 773.2) and 49.0 ± 5.5 (ranging from 35.0 to 64.1) respectively, which also showed a significant negative connection (r=-0.220, p < 0.0001; Fig. 3B).
Fig. 2.
Flow chart of patient enrollment and exclusion
Table 1.
The clinical characteristics of 181 patients with locally advanced gastric cancer
| Characteristics | Case (%) | Mean (SD) | Range |
|---|---|---|---|
| Gender | |||
| Male | 134(74.03) | ||
| Female | 47(25.97) | ||
| Age (years) | 59.0 ± 8.9 | 44–75 | |
| ≤ 60.0 | 92(50.83) | ||
| >60.0 | 89(49.17) | ||
| BMI (Kg/m 2 ) | 27.8 ± 9.2 | 18.7–37.6 | |
| ≤ 25 | 89(49.16) | ||
| >25 | 92(50.83) | ||
| ASA-PS | |||
| I | 152(83.70) | ||
| II | 29(16.30) | ||
| ECOG performance status | |||
| 0 | 164(90.61) | ||
| 1 | 17(9.39) | ||
| Tumor size (cm) | 5.8 ± 1.6 | 2.1–9.6 | |
| < 5.0 | 54(29.83) | ||
| ≥ 5.0 | 127(70.17) | ||
| Lesion site | |||
| Up 1/3 | 76(41.99) | ||
| Middle 1/3 | 25(13.81) | ||
| Low 1/3 | 80(44.20) | ||
| Differentiation | |||
| Poor | 112(61.88) | ||
| Moderately or well | 69(38.12) | ||
| cT stage | |||
| T2 | 16(8.84) | ||
| T3 | 46(25.41) | ||
| T4 | 119(65.75) | ||
| cN stage | |||
| N0 | 36(19.89) | ||
| N1 | 35(19.34) | ||
| N2 | 53(29.28) | ||
| N3 | 57(31.49) | ||
| cTNM stage | |||
| II | 53(29.28) | ||
| III | 128(70.72) | ||
| Chemotherapy regimen | |||
| XELOX | 94(51.93) | ||
| SOX | 87(48.07) | ||
| Extent of surgical resection | |||
| Distal subtotal gastrectomy | 69(38.12) | ||
| Total gastrectomy | 92(50.83) | ||
| Proximal subtotal gastrectomy | 20(11.05) | ||
| Surgical Procedures | |||
| Laparoscopic | 120(66.30) | ||
| Laparotomy | 61(33.70) | ||
Pre-treatment SII ( ±s) ±s)
|
467.3 ± 405.8 | 75.1–2247.0 | |
Pre-treatment PNI ( ±s) ±s)
|
52.9 ± 6.1 | 36.0-68.5 | |
Post-treatment SII ( ±s) ±s)
|
197.8 ± 120.5 | 47.5-773.2 | |
Post-treatment PNI ( ±s) ±s)
|
49.0 ± 5.5 | 35.0-64.1 |
Note ECOG, Eastern Cooperative Oncology Group; SII, Systemic immune-inflammatory index; PNI, Prognostic nutritional index. ASA-PS: American society of Aneshesiologists physical status classification system
Fig. 3.
Correlation analysis between SII and PNI. (A) Pre-neoadjuvant chemotherapy; (B) Post-neoadjuvant chemotherapy
Optimal cut-off values of SII and PNI before and after neoadjuvant chemotherapy
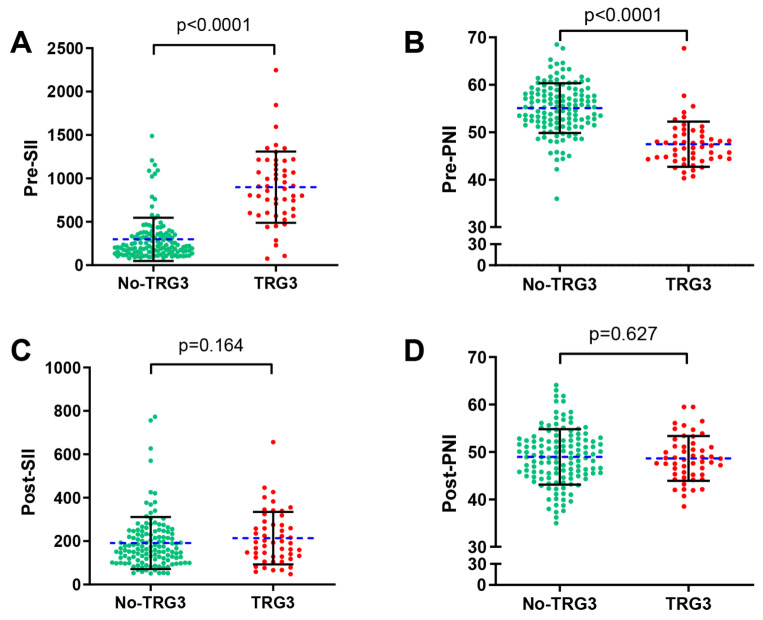
Each patients received radical surgery following two courses of neoadjuvant chemotherapy, including 11 (6.08%) with TRG0, 58 (32.04%) with TRG1, 61 (33.70%) with TRG2, and 51 (28.18%) with TRG3. The mean SII before and after neoadjuvant chemotherapy was 898.0 ± 410.5 and 214.1 ± 120.8, respectively, in 51 patients with TRG3, in addition to PNI of 47.5 ± 4.8 and 49.0 ± 5.9, respectively. Nevertheless, for the 130 non-TRG3 patients, the mean pre-neoadjuvant chemotherapy SII and PNI values were 298.3 ± 249.6 and 55.1 ± 5.2, respectively, while the mean post-treatment SII value was 191.4 ± 120.2 and PNI value was 48.6 ± 4.7. We observed that TRG3 patients had substantially greater SII compared with non-TRG3 patients before neoadjuvant chemotherapy (p<0.0001), while PNI was significantly lower (p<0.0001) (Fig. 4A-B). However, after neoadjuvant chemotherapy, there was no discernible difference between TRG3 patients and non-TRG3 patients in terms of SII and PNI (p = 0.164, 0.627) (Fig. 4C-D).
Fig. 4.
Relationship between TRG3/non-TRG 3 and the SII(A/C)/PNI(B/D). (A, B) Before neoadjuvant chemotherapy; (C, D) After neoadjuvant chemotherapy
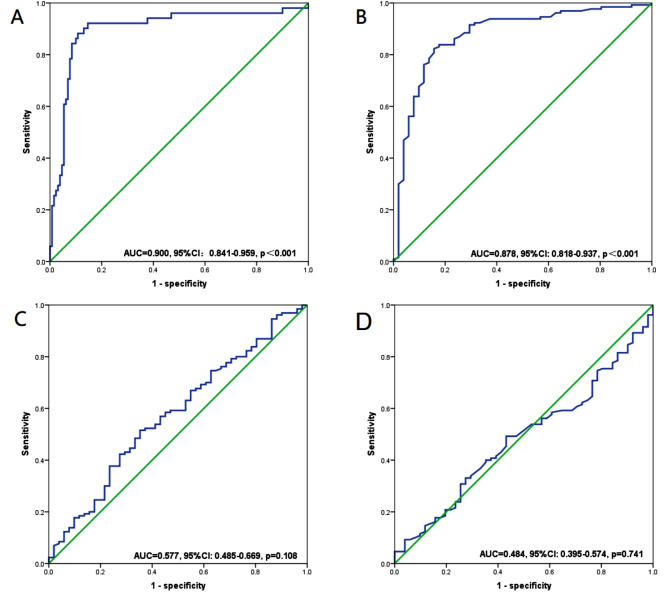
The best cut-off values for the SII and PNI continuous variables based on the maximum Yorden index were identified using receiver operating characteristic (ROC) curves. This allowed us to evaluate the SII and PNI’s predictive ability to distinguish between TRG3 and non-TRG3 patients before and after neoadjuvant chemotherapy. As results, the optimal cut-off values for SII before neoadjuvant chemotherapy was 471.5 (AUC = 0.900, 95%CI: 0.841–0.959, p < 0.001; sensitivity 88.2%, specificity 89.2%), and for PNI was 48.6 (AUC = 0.878, 95%CI: 0.818–0.937, p < 0.001; sensitivity 91.5%, specificity 70.6%) (Fig. 5A-B). Nonetheless, SII [AUC = 0.577, 95%CI: 0.485–0.669, p = 0.108; sensitivity 55.0%, specificity 77.3%] and PNI [AUC = 0.484, 95%CI: 0.395–0.574, p = 0.741; sensitivity 55.0%, specificity 77.3%] after neoadjuvant chemotherapy failed to accurately differentiate between TRG3 and non-TRG3 patients (Fig. 5C-D).
Fig. 5.
ROC curves for discriminating patients with TRG 3 and those with non-TRG 3 according to values of the SII (A/C) and PNI(B/D). (A, B) Before neoadjuvant treatment; (C, D) After neoadjuvant treatment
Relationship between clinicopathological features and SII-PNI score
By this, the pre-neoadjuvant chemotherapy SII-PNI score of 2 was defined as greater than the SII cut-off value (≥ 471.5) and less than the PNI (≤ 48.6) cut-off value, 1 score was defined as greater than the SII cut-off value or less than the PNI cut-off value, and 0 scores were defined as neither greater than the SII cut-off value nor less than the PNI cut-off value. Among these patients, 107 patients (59.17%) were of SII-PNI score 0, 41 patients (22.65%) were of SII-PNI score 1, and 33 patients (18.23%) were of SII-PNI score 2. Furthermore, we analyzed that patients with a SII-PNI score of 0 had a higher proportion of moderate-to-highly differentiated pathological types, while patients with a score of 2 tended to be poorly differentiated (P = 0.002). Interestingly, we also analyzed the relationship between SII-PNI score and other clinicopathological features, and found no significant difference (P > 0.05) (Table 2).
Table 2.
The association between clinicopathological features and SII-PNI score
| Characteristics | SII-PNI | P | ||
|---|---|---|---|---|
| 0 score (N = 107) |
1 score (N = 41) |
2 score (N = 33) |
||
| Gender | 0.959 | |||
| Male | 79(73.83%) | 31(75.61%) | 24(72.73%) | |
| Female | 28(26.17%) | 10(24.39%) | 9(27.27%) | |
| Age (years) | 0.057 | |||
| ≤ 60.0 | 62(57.94%) | 18(43.90%) | 12(36.36%) | |
| >60.0 | 45(42.06%) | 23(56.10%) | 21(63.64%) | |
| ECOG performance status | 0.765# | |||
| 0 | 96(89.72%) | 37(90.24%) | 31(93.94%) | |
| 1 | 11(10.28%) | 4(9.76%) | 2(6.06%) | |
| Tumor size (cm) | 0.315 | |||
| < 5.0 | 30(28.04%) | 16(39.02%) | 8(24.24%) | |
| ≥ 5.0 | 77(71.96%) | 25(60.98%) | 25(75.76%) | |
| Lesion site | 0.239# | |||
| Up 1/3 | 46(42.99%) | 20(48.78%) | 10(30.30%) | |
| Middle 1/3 | 12(11.21%) | 8(19.51%) | 5(15.15%) | |
| Low 1/3 | 49(45.79%) | 13(31.71%) | 18(54.55%) | |
| Differentiation | 0.002 | |||
| Poor | 55(51.40%) | 31(75.61%) | 26(78.79%) | |
| Moderately or well | 52(48.60%) | 10(24.39%) | 7(21.21%) | |
| cTNM stage | 0.079 | |||
| II | 32(29.91%) | 16(39.02%) | 5(15.15%) | |
| III | 75(70.09%) | 25(60.98%) | 28(84.85%) | |
| Chemotherapy regimen | 0.891 | |||
| XELOX | 54(50.47%) | 22(53.66%) | 18(54.55%) | |
| SOX | 53(49.53%) | 19(46.34%) | 15(45.45%) | |
Note # Fisher’s exact test
The association between neoadjuvant chemotherapy response and SII-PNI score
All patients received standard doses. We analyzed the preoperative treatment intensity for all patients. Only 4 patients (2.21%) experienced a reduction in treatment intensity, primarily due to the occurrence of grade 3 adverse reactions (based on the CTCAE ersion 5.0). These reactions manifested as nausea and vomiting, which were alleviated after nutritional support and antiemetic treatment. Additionally, 54 patients (29.83%) experienced grade 1 and 2 adverse reactions, mainly gastrointestinal reactions. These milder reactions were resolved with symptomatic treatment and did not necessitate a change in treatment intensity.
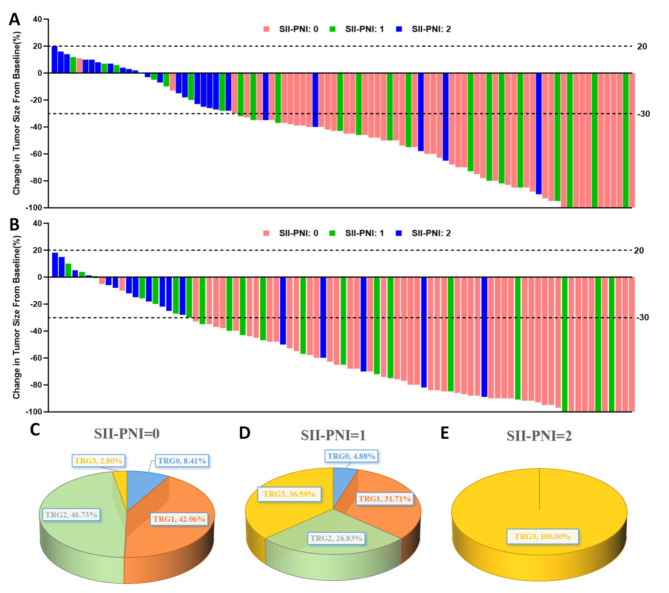
Among the 94 patients who received preoperative XELOX treatment, after 2 cycles of neoadjuvant chemotherapy and evaluation using the RECIST version 1.1, 12 patients achieved CR, 53 had PR, 29 had SD, and no patients had PD. Of the 87 patients who received the preoperative SOX regimen, evaluation after only 2 cycles of neoadjuvant chemotherapy revealed that 11 patients achieved CR, 55 had PR, and 21 had SD (Fig. 6A-B). Furthermore, we analyzed the relationship between different SII-PNI scores and CT imaging evaluations (lymph nodes with a short diameter of > 1.5 cm were evaluated as target lesions). Surprisingly, we found that patients with an SII-PNI score of 0 had the highest ORR, while patients with a score of 2 had the lowest ORR. Patients with a score of 1 had an intermediate ORR (Table 3).
Fig. 6.
Proportion of changes in imaging assessment and postoperative pathologic TRG response between different SII-PNI score groups. (A) CT imaging changes after 2 cycles of chemotherapy with the preoperative neoadjuvant chemotherapy regimen of XELOX, as assessed by RECIST version 1.1; (B) CT imaging changes after 2 cycles of chemotherapy with the preoperative neoadjuvant chemotherapy regimen of SOX, as assessed by RECIST version 1.1; (C) distribution of postoperative pathological TRG grading in patients with a SII-PNI score of 0; (D) distribution of postoperative pathological TRG grading in patients with a SII- distribution of postoperative pathologic TRG grading in patients with a PNI score of 1; (E) distribution of postoperative pathologic TRG grading in patients with a SII-PNI score of 2
Table 3.
Relationship between tumor response and the SII-PNI score
| Tumor response | SII-PNI score(%) | p-value | ||
|---|---|---|---|---|
| & RECIST version 1.1 evaluation | 0 (n = 44) | 1 (n = 29) | 2 (n = 27) | |
| ORR | 40(90.9) | 20(69.0) | 10(37.0) | <0.001 |
| DCR | 43(97.7) | 27(93.1) | 25(92.6) | 0.511 |
| # Pathologic TRG grading | ||||
| TRG3 (n = 51) | 3 (2.80) | 15 (36.59) | 33 (100.00) | <0.001 |
| Non-TRG3 (n = 130) | 104 (97.20) | 26 (63.41) | 0 (0) | |
Note SII, Systemic immune-inflammatory index; PNI, prognostic nutritional index; ORR=(CR + PR)/(CR + PR + SD + PD); DCR=(CR + PR + SD)/(CR + PR + SD + PD).CR is defined as the disappearance of all target lesions.PR is defined as a reduction of at least 30% in the sum of the target lesion diameters from the sum of the baseline diameters.PD is defined as a minimum increase in the sum of the target lesion diameters of at least 20% relative to the smallest sum of the diameters in the study.SD is defined as neither sufficient reduction to qualify for PR nor sufficient increase to qualify for PD. The target lesion was a measurable lymph node lesion with a short diameter of > 1.5 cm. The change in the sum of the diameters of all target lesions before and after neoadjuvant therapy was used to calculate the efficacy evaluation. &, evaluation of measurable lymph node lesions (short diameter > 1.5 cm) before and after neoadjuvant therapy; #, evaluation of primary lesions before and after neoadjuvant therapy
Every patient with LAGC who was enrolled in the study underwent radical resection along with 2 cycles of XELOX/SOX neoadjuvant chemotherapy without interruption. Among 181 LAGC patients who underwent radical surgical resection, the incidence of surgery-related complications was 19.89% (36/181). These complications included 18 cases (9.94%) of postoperative pneumonia, 5 cases (2.76%) of anastomotic leakage, 4 cases (2.21%) of intestinal obstruction, 4 cases (2.21%) of deep vein thrombosis of the lower limbs, 3 cases (1.66%) of abdominal bleeding, and 2 cases (1.10%) of cardiovascular and cerebrovascular diseases. According to the Clavien-Dindo surgical complication grading system, 20 of the 36 patients with complications were assessed as grade I, 13 as grade II, and 3 as grade III.
Based on the difference in SII-PNI score, we observed that 9 patients (8.41%) with a score of 0 were TRG 0, 45 (42.06%) patients achieved TRG 1, 50 (46.73%) patients achieved TRG 2, and 3 (2.80%) patients achieved TRG 3. Patients with a score of 1 were 2 (TRG 0, 4.88%), 13 (TRG 1, 31.71%), 11 (TRG 2, 26.83%) and 15 (TRG 3, 36.59%), respectively, but all patients with a score of 2 were TRG 3(Fig. 6C-D). Pathological response alterations varied significantly between groups with various SII-PNI scores (p<0.001), and the proportion of non-TRG3 increased as the score decreased (Table 3).
Relationship between SII-PNI score and prognosis
All patients were scheduled to continue receiving the original chemotherapy regimen for 6 cycles after surgery. However, 14 patients (7.73%) did not undergo adjuvant chemotherapy following radical surgery. Additionally, 20 patients (11.05%) experienced a reduction in chemotherapy intensity due to postoperative debilitation. The remaining patients received postoperative adjuvant chemotherapy according to the original regimen and standard treatment intensity.
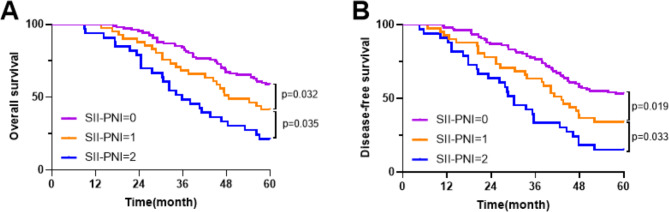
The median period was 64.2 months for all patients who were follow-up (43.2 to 94.2 months). All patients included in the research had 5-year OS and DFS rates of 48.07% and 41.99%, respectively. The 5-year OS of samples with SII-PNI scores of 2, 1, and 0 was 21.21%, 41.46%, and 58.88% according to Kaplan-Meier analysis, respectively (Fig. 7A). Patients with various SII-PNI scores had substantially varying 5-year OS (all p < 0.05). Meanwhile, patients having a SII-PNI score of 0 had a 5-year DFS of 53.27%, while the scores of 1 and 2 were 34.15% and 15.15%, respectively, and there was a statistically substantial distinction between the three groups(all p < 0.05) (Fig. 7B). Analysis of cox multivariate data revealed that tumor response (OS: HR = 3.992, 95%CI: 1.493–9.223, p = 0.003; DFS: HR = 3.709, 95%CI: 1.324–10.893, p = 0.003), postoperative adjuvant chemotherapy (OS: HR = 2.989, 95%CI: 1.290–7.874, p = 0.007; DFS: HR = 2.876, 95%CI: 1.182–6.653, p = 0.009), intensity of postoperative adjuvant chemotherapy (OS: HR = 1.755, 95%CI: 1.002–3.183, p = 0.012; DFS: HR = 1.890, 95%CI: 1.145–3.624, p = 0.015), ypTNM stage (OS: HR = 5.992, 95%CI: 2.098–13.911, p = 0.001; DFS: HR = 6.546, 95%CI: 2.242–15.923, p = 0.001), SII-PNI score (OS: HR = 4.982, 95%CI: 1.890-10.234, p = 0.001; DFS: HR = 4.763, 95%CI: 1.994–13.903, p = 0.001) and pathological type (OS: HR = 3.982, 95%CI: 2.204–8.304,p = 0.009; DFS: HR = 4.339, 95%CI: 2.330–9.874, p = 0.005) were not only the 5-year OS but also the independent risk for DFS, which were not related to neoadjuvant chemotherapy regimen(p = 0.772, 0.834) (Table 4).
Fig. 7.
Kaplan-Meier survival curves in patients with LAGC. (A) 5-year overall survival based on SII-PNI score; (B) 5-year disease-free survival based on SII-PNI score
Table 4.
Multivariate analysis of the clinicopathological characteristics for the prognosis of LAGC patients
| Clinical variables | 5-year OS multivariate analysis | 5-year DFS multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | ||
| Age (years) | 0.872 | 0.639 | |||||
| < 60 | 1.000 | reference | 1.000 | reference | |||
| ≥ 60 | 1.063 | 0.632–1.872 | 1.203 | 0.764–1.897 | |||
| Surgical Procedures | 0.892 | 0.707 | |||||
| Laparoscopic | 1.000 | reference | 1.000 | reference | |||
| Laparotomy | 1.108 | 0.772–1.893 | 1.337 | 0.598–2.436 | |||
| Surgical resection | 0.632 | 0.703 | |||||
| Distal subtotal | 1.000 | reference | 1.000 | reference | |||
| Total | 1.232 | 0.835–1.678 | 1.114 | 0.704–1.697 | |||
| Proximal subtotal | 0.995 | 0.670–1.609 | 1.095 | 0.635–1.402 | |||
| Tumour size (cm) | 0.696 | 0.019 | |||||
| < 5.0 | 1.000 | reference | 1.000 | reference | |||
| ≥ 5.0 | 1.203 | 0.892–1.980 | 1.603 | 1.023–3.524 | |||
| Differentiation | 0.009 | 0.005 | |||||
| Poor | 1.000 | reference | 1.000 | reference | |||
| Moderately or well | 3.982 | 2.204–8.304 | 4.339 | 2.330–9.874 | |||
| Postoperative complication | 0.384 | 0.673 | |||||
| No | 1.000 | reference | 1.000 | reference | |||
| Yes | 1.203 | 0.837–2.430 | 1.338 | 0.675–1.980 | |||
| Clavien-Dindo classification | 0.763 | 0.772 | |||||
| I | 1.000 | reference | 1.000 | reference | |||
| II | 1.323 | 0.743–2.490 | 1.441 | 0.802–2.502 | |||
| III | 1.743 | 0.923–2.806 | 1.609 | 0.899–2.941 | |||
| ypTNM stage | 0.001 | 0.001 | |||||
| I and II | 1.000 | reference | 1.000 | reference | |||
| III | 5.992 | 2.098–13.911 | 6.546 | 2.242–15.923 | |||
| SII-PNI score | 0.001 | 0.001 | |||||
| 0 | 1.000 | reference | 1.000 | reference | |||
| 1 | 2.073 | 1.341–7.294 | 2.348 | 1.595–6.892 | |||
| 2 | 4.982 | 1.890-10.234 | 4.763 | 1.994–13.903 | |||
| Neoadjuvant chemotherapy | 0.772 | 0.834 | |||||
| XELOX | 1.000 | reference | 1.000 | reference | |||
| SOX | 1.098 | 0.893–2.009 | 1.131 | 0.721–1.993 | |||
| Intensity of neoadjuvant chemotherapy | 0.632 | 0.555 | |||||
| Standard | 1.000 | reference | 1.000 | reference | |||
| Decline | 1.055 | 0.892–1.988 | 1.227 | 0.756–2.432 | |||
| Tumor response | 0.003 | 0.008 | |||||
| Non-TRG3 | 1.000 | reference | 1.000 | reference | |||
| TRG3 | 3.992 | 1.493–9.223 | 3.709 | 1.324–10.893 | |||
| Postoperative adjuvant chemotherapy | 0.007 | 0.009 | |||||
| Yes | 1.000 | reference | 1.000 | reference | |||
| No | 2.989 | 1.290–7.874 | 2.876 | 1.182–6.653 | |||
| Intensity of postoperative adjuvant chemotherapy | 0.012 | 0.015 | |||||
| Standard | 1.000 | reference | 1.000 | reference | |||
| Decline | 1.755 | 1.002–3.183 | 1.890 | 1.145–3.624 | |||
Note SII, Systemic immune-inflammatory index; PNI, Prognostic nutritional index
Discussion
Recently, first-line neoadjuvant chemotherapy treatment with the XELOX/SOX regimen has received increasing attention and is the main treatment for LAGC patients. In our cohort, a high proportion (28.18%) of patients were insensitive to neoadjuvant chemotherapy, but there is a lack of reliable biomarkers for predicting sensitivity to first-line chemotherapy. In the current study, we developed a unique SII-PNI score evaluation to predict potential pathological responses in LAGC patients undergoing first-line neoadjuvant chemotherapy by integrating systemic immune inflammation and nutritional status. This study is the first to suggest and support the clinical importance of SII-PNI score in LAGC patients according to what is currently known. We demonstrated a close association between SII-PNI score and pathological response after neoadjuvant chemotherapy in LAGC, and as the score increases, the less sensitive to the treatment efficacy and the higher the proportion of TRG 3. In addition, we also examined the significance of the SII-PNI score based on the SII-PNI score in LAGC following radical surgery. The findings imply that SII-PNI score is a standalone predictor to evaluate the prognosis of LAGC patients.
Numerous investigations have demonstrated that systemic inflammatory response, an independent predictive indicator for cancer, may be responsible for the emergence and development of malignant tumors [14, 15]. Many experimental studies have shown that neutrophils, platelets and lymphocytes are involved in the progression of malignant tumours [15–17]. A great deal of previous studies have used traditional indicators of inflammation in peripheral blood, such as neutrophil-to-lymphocyte ratio (NLR) or platelet-to-lymphocyte ratio (PLR), as predictors of gastric cancer recurrence or overall survival [16, 17]. The SII is a new indicator of inflammation, which is calculated by the quantity of neutrophils, platelets, and lymphocytes in peripheral blood [18]. Several studies have demonstrated that SII has a more comprehensive response to systemic inflammation than NLR and PLR and is very reliable as a prognostic indicator of several types of cancer [19–21]. Nonetheless, previous studies have demonstrated that inflammatory mediators may be involved in the development of hypoalbuminemia, which can increase the tumor-related mortality in malignant tumors, suggesting that nutritional status in patients with malignant tumors is also significantly associated with tumor progression and prognosis [35, 36]. However, there are limited findings on whether LAGC’s neoadjuvant chemotherapy response and prognosis may be predicted by using SII-PNI.
In this research, we integrated nutritional and inflammatory markers to generate the SII-PNI score, which can be a predictor has extensive clinical potential tor predict the prognosis of LAGC patients and their response to neoadjuvant treatment. Surprisingly, the SII-PNI score was closely correlated with pathological regression response to first-line neoadjuvant chemotherapy commonly used with the XELOX/SOX regimen. The SII-PNI score was 2 in 33 of 51 patients (64.71%) with TRG3 and 0 in 104 of 130 patients (80.00%) with non-TRG3 specifically. Furthermore, most TRG3 patients had a SII-PNI score of 1 or 2(94.12%), which was consistent with the previous findings [30, 31]. The above conclusions indicate that SII-PNI score is a new combined hematological predictor, which can predict pathological response of LAGC to first-line neoadjuvant treatment. Tumor cells will produce a series of inflammatory mediators associated with tumors such us tumor necrosis factor-ɑ(TNF-ɑ), interleukin-3 (IL-3) and IL-6, which will further lead to a relative rise in neutrophil and platelet numbers, a decline in lymphocyte numbers, and an increase in SII [37]. Furthermore, the decline of albumin levels during neoadjuvant chemotherapy will lead to deterioration of nutritional status and further affect the immune function of patients, leading to tumor progression [38]. Consequently, a high SII-PNI score can reflect tumor invasiveness, which illustrates that SII-PNI score is some extent affected by tumor response to chemotherapy.
We used a variety of methods to investigate the association between SII-PNI score and prognosis. Patients with low SII-PNI scores, particularly those with a score of 0, had longer 5-year OS and DFS, according to a Kaplan-Meier study. In addition, we performed Cox regression analysis of all risk factors that were considered to be possible risk factors for prognosis after neoadjuvant chemotherapy and the result showed that SII-PNI score could be consider as an independent risk factor. This indicates that when the SII-PNI score rises, patients’ prognoses are worse making them more susceptible to illness progression and recurrence. The mechanisms by which SII-PNI can predict prognosis may include the following [39–43]: 1)The higher the SII-PNI score, the higher the neutrophil and (or) platelet counts in the body, the release of reactive nitrogen, active Oxygen and elastase can promote the proliferation of tumor cells with activating the P13K-AKT signalling pathway; in addition, platelets can secrete tumor-associated growth factors, which may favorably influence the development, spread, and metastasis of tumors. It allows tumor cells to evade the patient ‘s immune system, making them difficult to be recognized, ending in tumor cell proliferation. 2) A drop in the number of lymphocytes was also suggested by the elevated SII-PNI score, which would reduce the immune regulatory function of the body and promote tumor progression. 3) The patient’s nutritional condition has gotten worse, which is reflected in the body’s decreased serum albumin level. First of the three proposed mechanisms could be tested by looking for the expressions of key genes of PI3K-AKT pathway by ELISA assay which will help either to establish or discard the mechanism.
Nonetheless, the results of the study may also have been influenced to some extent by the limitations. First off, because of the single-center prospective study and with small sample included, this study exists selection bias. Additionally, it is unable to confirm from external data that the SII-PNI score is associated with pathological response and prognosis. Secondly, this study only analyzed the clinical value of SII-PNI score in predicting pathological reaction and prognosis in LAGC receiving XELOX/SOX neoadjuvant chemotherapy regimen, while other chemotherapy regimens were not included. Therefore, we need a larger sample, prospective, multi-centre studies to demonstrate the predictive value of the parameters examined in the study.
Conclusions
In conclusion, we demonstrate that the SII-PNI score as a blood marker plays an important role to predict treatment response to XELOX/SOX neoadjuvant chemotherapy in LAGC patients and is a prognostic marker that may independently predict OS and DFS. In the future, the clinical treatment of LAGC patients may obtain a benefit from the adoption of the SII-PNI grading system as a valuable tool to guide treatment decisions. However, we need a large number of samples and different populations to reduce the limitations to further validate our results in the future.
Acknowledgements
Not applicable.
Author contributions
Authors’ contributions(I)Conception and design: Qun Zhao; (II) Administrative support: Qun Zhao; (III) Provision of study materials or patients: Jiaxuan Yang, Ping’an Ding, Peigang Yang, Yuan Tian, Honghai Guo, Lingjiao Meng; (IV) Collection and assembly of data: Haotian Wu, Ping’an Ding, Peigang Yang, Yuan Tian, Honghai Guo, Yang Liu; (V) Data analysis and interpretation: Lingjiao Meng, Ping’an Ding, Chenyu Sun, Shuya Chen; (VI) Manuscript writing: All authors; (VII) Manuscript revision and editing: Lingjiao Meng, Ping’an Ding, Jiaxuan Yang, Haotian Wu, Jiaxiang Wu; (VIII) Final approval of manuscript: All authors.
Funding
This work was supported by the Cultivating Outstanding Talents Project of Hebei Provincial Government Fund (No.2019012); Hebei public health committee county-level public hospitals suitable health technology promotion and storage project (No.2019024); Hebei University Science and Technology Research Project (No.ZD2019139).
Data availability
The participant data with identifiers used to support the findings of this study were supplied by Qun Zhao under license and so cannot be made freely available. Requests for access to these data should be made to Qun Zhao, zhaoqun@hebmu.edu.cn.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (approval number: 20111214029) and informed consent was performed for all the study subjects. All the authors have followed the applicable ethical standards to maintain the research integrity without any duplication, fraud or plagiarism issues.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping’an Ding, Jiaxuan Yang, Jiaxiang Wu and Haotian Wu contributed equally to this work.
Contributor Information
Lingjiao Meng, Email: ljmeng@hebmu.edu.cn.
Qun Zhao, Email: zhaoqun@hebmu.edu.cn.
References
- 1.Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71(3):264–79. 10.3322/caac.21657. 10.3322/caac.21657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin 2016 Mar-Apr;66(2):115–32. 10.3322/caac.21338 [DOI] [PubMed]
- 3.Jiang L, Yang KH, Guan QL, Chen Y, Zhao P, Tian JH. Survival benefit of neoadjuvant chemotherapy for resectable cancer of the gastric and gastroesophageal junction: a meta-analysis. J Clin Gastroenterol. 2015 May-Jun;49(5):387–94. 10.1097/MCG.0000000000000212. [DOI] [PubMed]
- 4.Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G. Chinese laparoscopic gastrointestinal surgery study (CLASS) Group. Effect of laparoscopic vs Open Distal Gastrectomy on 3-Year disease-free survival in patients with locally advanced gastric Cancer: the CLASS-01 Randomized Clinical Trial. JAMA. 2019;321(20):1983–92. 10.1001/jama.2019.5359. 10.1001/jama.2019.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Liu H, Hu Y, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Yu J, Zheng C, Liu F, Li Z, Zhao G, Zhang J, Chen P, Li G. Chinese laparoscopic gastrointestinal surgery study (CLASS) Group. Laparoscopic vs Open Distal Gastrectomy for locally advanced gastric Cancer: five-year outcomes from the CLASS-01 Randomized Clinical Trial. JAMA Surg. 2022;157(1):9–17. 10.1001/jamasurg.2021.5104. 10.1001/jamasurg.2021.5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coccolini F, Nardi M, Montori G, Ceresoli M, Celotti A, Cascinu S, Fugazzola P, Tomasoni M, Glehen O, Catena F, Yonemura Y, Ansaloni L. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg. 2018;51:120–7. 10.1016/j.ijsu.2018.01.008. 10.1016/j.ijsu.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 7.Kanhere H, Goel R, Finlay B, Trochsler M, Maddern G. Radical gastrectomy: still the cornerstone of curative treatment for gastric Cancer in the Perioperative Chemotherapy Era-A Single Institute Experience over a Decade. Int J Surg Oncol. 2018;2018:9371492. 10.1155/2018/9371492. 10.1155/2018/9371492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Chen L, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Qi C, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Wang X, Yuan Y, E JY, Ying X, Yao C, Shen L, Ji J. RESOLVE study group. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22(8):1081–92. 10.1016/S1470-2045(21)00297-7. 10.1016/S1470-2045(21)00297-7 [DOI] [PubMed] [Google Scholar]
- 9.Xue K, Ying X, Bu Z, Wu A, Li Z, Tang L, Zhang L, Zhang Y, Li Z, Ji J. Oxaliplatin plus S-1 or capecitabine as neoadjuvant or adjuvant chemotherapy for locally advanced gastric cancer with D2 lymphadenectomy: 5-year follow-up results of a phase II-III randomized trial. Chin J Cancer Res. 2018;30(5):516–25. 10.21147/j.issn.1000-9604.2018.05.05. 10.21147/j.issn.1000-9604.2018.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Qu A, Sun Y, Zhang J, Wei B, Cui Y, Liu X, Tian W, Li Y. Neoadjuvant chemoradiotherapy plus postoperative adjuvant XELOX chemotherapy versus postoperative adjuvant chemotherapy with XELOX regimen for local advanced gastric cancer-A randomized, controlled study. Br J Radiol. 2021;94(1124):20201088. 10.1259/bjr.20201088. 10.1259/bjr.20201088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Q, Lian C, Huo Z, Li M, Liu Y, Fan L, Tan B, Zhao X, Zhang Z, Wang D, Liu Y, Guo H, Yang P, Tian Y, Li Y. The efficacy and safety of neoadjuvant chemotherapy on patients with advanced gastric cancer: a multicenter randomized clinical trial. Cancer Med. 2020;9(16):5731–45. 10.1002/cam4.3224. 10.1002/cam4.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41(8):747–95. 10.1002/cac2.12193. 10.1002/cac2.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikoma N, Estrella JS, Blum Murphy M, Das P, Minsky BD, Mansfield P, Ajani JA, Badgwell BD. Tumor Regression Grade in Gastric Cancer after Preoperative Therapy. J Gastrointest Surg. 2021;25(6):1380–7. 10.1007/s11605-020-04688-2. 10.1007/s11605-020-04688-2 [DOI] [PubMed] [Google Scholar]
- 14.Rihawi K, Ricci AD, Rizzo A, Brocchi S, Marasco G, Pastore LV, Llimpe FLR, Golfieri R, Renzulli M. Tumor-Associated macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational implications. Int J Mol Sci. 2021;22(8):3805. 10.3390/ijms22083805. 10.3390/ijms22083805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu L, Wang M, Cui X, Mo J, Yuan L, Mao F, Zhang K, Ng DM, Chen P, Wang D. Clinical significance of peripheral blood-derived inflammation markers in advanced gastric cancer after radical resection. BMC Surg. 2020;20(1):219. 10.1186/s12893-020-00884-8. 10.1186/s12893-020-00884-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong W, Zhao L, Dong Z, Dou Y, Liu Y, Ma C, Qu X. After neoadjuvant chemotherapy platelet/lymphocyte ratios negatively correlate with prognosis in gastric cancer patients. J Clin Lab Anal. 2018;32(5):e22364. 10.1002/jcla.22364. 10.1002/jcla.22364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Zhang G, Liu X, Liu X, Chen C, Yu H, Huang X, Zhang Q, Yu J. Blood neutrophil-lymphocyte ratio predicts survival for stages III-IV gastric cancer treated with neoadjuvant chemotherapy. World J Surg Oncol. 2013;11:112. 10.1186/1477-7819-11-112. 10.1186/1477-7819-11-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, Zhang X, Wang WM, Qiu SJ, Zhou J, Fan J. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22. 10.1158/1078-0432.CCR-14-0442. 10.1158/1078-0432.CCR-14-0442 [DOI] [PubMed] [Google Scholar]
- 19.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–72. 10.3748/wjg.v23.i34.6261. 10.3748/wjg.v23.i34.6261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, Liu L. Prognostic value of preoperative systemic Immune-inflammation index in patients with cervical Cancer. Sci Rep. 2019;9(1):3284. 10.1038/s41598-019-39150-0. 10.1038/s41598-019-39150-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Lu Y, Zhang S, Huang Y. Systemic Immune-inflammation index is Superior to Neutrophil to lymphocyte ratio in Prognostic Assessment of breast Cancer patients undergoing Neoadjuvant Chemotherapy. Biomed Res Int. 2020;2020:7961568. 10.1155/2020/7961568. PMID: 33381583; PMCID: PMC7762645. 10.1155/2020/7961568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. 2020;24(5):2993–3021. 10.1111/jcmm.14934. 10.1111/jcmm.14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Jiang Y, Zheng X, Pan B, Xiang H, Zheng M. Pretreatment systemic Immune-inflammation index can predict response to Neoadjuvant Chemotherapy in Cervical Cancer at stages IB2-IIB. Pathol Oncol Res. 2022;28:1610294. 10.3389/pore.2022.1610294. 10.3389/pore.2022.1610294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen L, Yan Y, Zhu L, Cong X, Li S, Song S, Song H, Xue Y. Systemic immune-inflammation index as a useful prognostic indicator predicts survival in patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Cancer Manag Res. 2017;9:849–67. 10.2147/CMAR.S151026. 10.2147/CMAR.S151026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coutu BG, Johnson KC, Bhirud A, Baine MJ, Zhen W, Zhang C, Trujillo KP, Bennion NR. Systemic Immune-Inflammatory Index Association with Survival in patients undergoing trimodality therapy for Lung Cancer. Oncology. 2022;100(5):247–56. 10.1159/000520989. 10.1159/000520989 [DOI] [PubMed] [Google Scholar]
- 26.Okadome K, Baba Y, Yagi T, Kiyozumi Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. Prognostic Nutritional Index, Tumor-infiltrating lymphocytes, and prognosis in patients with esophageal Cancer. Ann Surg. 2020;271(4):693–700. 10.1097/SLA.0000000000002985. 10.1097/SLA.0000000000002985 [DOI] [PubMed] [Google Scholar]
- 27.Li S, Tian G, Chen Z, Zhuang Y, Li G. Prognostic role of the Prognostic Nutritional Index in Pancreatic Cancer: a Meta-analysis. Nutr Cancer. 2019;71(2):207–13. 10.1080/01635581.2018.1559930. 10.1080/01635581.2018.1559930 [DOI] [PubMed] [Google Scholar]
- 28.Xishan Z, Ye Z, Feiyan M, Liang X, Shikai W. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep. 2020;10(1):17373. 10.1038/s41598-020-74525-8. 10.1038/s41598-020-74525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayasaka K, Shiono S, Suzuki K, Endoh M, Okada Y. Postoperative prognostic nutritional index as a prognostic factor after non-small cell lung cancer surgery. Gen Thorac Cardiovasc Surg. 2020;68(10):1163–71. 10.1007/s11748-020-01366-7. 10.1007/s11748-020-01366-7 [DOI] [PubMed] [Google Scholar]
- 30.Ding P, Yang P, Sun C, Tian Y, Guo H, Liu Y, Li Y, Zhao Q. Predictive effect of systemic Immune-inflammation index combined with Prognostic Nutrition Index score on efficacy and prognosis of Neoadjuvant Intraperitoneal and systemic Paclitaxel Combined with Apatinib Conversion Therapy in gastric Cancer patients with positive peritoneal lavage cytology: a prospective study. Front Oncol. 2022;11:791912. 10.3389/fonc.2021.791912. 10.3389/fonc.2021.791912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, Liu Y, Liu P, Li Y, Zhao Q. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. 2022;22(1):121. 10.1186/s12876-022-02199-9. 10.1186/s12876-022-02199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5. [PubMed] [Google Scholar]
- 33.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond). 2019;39(1):10. 10.1186/s40880-019-0349-9. 10.1186/s40880-019-0349-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi CJ, Berlin J, Branton PA et al. Protocol for the examination of specimens from patients with carcinoma of the stomach[EB/OL]. [2018–12– 03]. https://cap.objects.frb.
- 35.Magzal F, Sela S, Szuchman-Sapir A, Tamir S, Michelis R, Kristal B. In-vivo oxidized albumin- a pro-inflammatory agent in hypoalbuminemia. PLoS ONE. 2017;12(5):e0177799. 10.1371/journal.pone.0177799. 10.1371/journal.pone.0177799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004 Nov-Dec;17(6):432–7. 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed]
- 37.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121(11):2373–80. 10.1002/ijc.23173. 10.1002/ijc.23173 [DOI] [PubMed] [Google Scholar]
- 38.Jabłońska B, Pawlicki K, Mrowiec S. Associations between Nutritional and Immune Status and clinicopathologic factors in patients with pancreatic Cancer: a comprehensive analysis. Cancers (Basel). 2021;13(20):5041. 10.3390/cancers13205041. 10.3390/cancers13205041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonio N, Bønnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, Schmidt H, Feng Y, Martin P. The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J. 2015;34(17):2219–36. 10.15252/embj.201490147. 10.15252/embj.201490147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka Y, Ito S, Isobe K. Vancomycin-sensitive bacteria trigger development of colitis-associated colon cancer by attracting neutrophils. Sci Rep. 2016;6:23920. 10.1038/srep23920. 10.1038/srep23920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, Stolz DB, Land SR, Marconcini LA, Kliment CR, Jenkins KM, Beaulieu KA, Mouded M, Frank SJ, Wong KK, Shapiro SD. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23. 10.1038/nm.2084. 10.1038/nm.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol. 2010;5:47. 10.1186/1748-717X-5-47. 10.1186/1748-717X-5-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Migita K, Matsumoto S, Wakatsuki K, Kunishige T, Nakade H, Miyao S, Sho M. Effect of oral nutritional supplementation on the Prognostic Nutritional Index in Gastric Cancer patients. Nutr Cancer. 2021;73(11–12):2420–7. 10.1080/01635581.2020.1826990. 10.1080/01635581.2020.1826990 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The participant data with identifiers used to support the findings of this study were supplied by Qun Zhao under license and so cannot be made freely available. Requests for access to these data should be made to Qun Zhao, zhaoqun@hebmu.edu.cn.