Abstract
Background
Age-related macular degeneration (AMD) is a leading cause of vision loss. Photobiomodulation (PBM) offers a controversial approach for managing dry AMD, aiming to halt or reverse progression through mitochondrial activity modulation. However, the efficacy and clinical relevance of PBM as a potential approach for managing dry AMD remain debated.
Methods
We systematically searched PubMed, Embase, and Cochrane databases for randomized controlled trials (RCTs) comparing PBM versus a sham in patients with dry AMD. We performed trial sequential analysis (TSA) and minimal clinically important difference (MCID) calculations to assess statistical and clinical significance applying a random-effects model with 95% confidence intervals (CI).
Results
We included three RCTs comprising 247 eyes. The pooled analysis showed that PBM significant improved BCVA (MD 1.76 letters; 95% CI: 0.04 to 3.48) and drusen volume (MD -0.12 mm³; 95% CI: -0.22 to -0.02) as compared with a sham control. However, the TSA indicated that the current sample sizes were insufficient for reliable conclusions. No significant differences were observed in GA area. The MCID analysis suggested that the statistically significant results did not translate into clinically significant benefits. In the quality assessment, all studies were deemed to have a high risk of bias.
Conclusion
This meta-analysis points limitations in the current evidence base for PBM in dry AMD treatment, with issues around small sample sizes. Statistically significant improvements do not translate into clinical benefits. The research underscores need for larger RCTs to validate PBM’s therapeutic potential for dry AMD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40942-024-00569-x.
Keywords: Age-related macular degeneration, Best-corrected visual acuity, Drusen volume, Geographic atrophy, Meta-analysis, Photobiomodulation
Background
Age-related macular degeneration (AMD) significantly impacts global visual health, particularly its advanced forms, such as geographic atrophy (GA), which leads to severe visual impairment and blindness. With population aging, the prevalence of AMD is expected to increase, highlighting the urgency for effective treatments and management strategies to mitigate its impact on quality of life and burden on healthcare systems [1].
Current therapeutic options for dry AMD are scarce and focus on lowering the progression to advanced stages such as GA, although their efficacy is often questionable [2]. Until recently, there were no treatments available specifically for GA. In 2023, the FDA approved two complement inhibitors for slowing the progression rate of GA areas [3, 4]. However, accessibility remains a major challenge. This underscores the critical need for novel therapies that can halt or ideally reverse the progression of dry AMD and GA, thereby preserving visual function.
Photobiomodulation (PBM) is a therapeutic option for dry AMD, focusing on slowing disease progression by influencing mitochondrial activity, reducing oxidative stress, and modulating inflammation through LEDs at specific wavelengths (590, 660, 850 nm) [5, 6]. Despite anecdotal reports and early studies indicating potential benefits, such as improved microperimetry outcomes for some patients, [7] its efficacy and scientific validity in preventing the progression from dry AMD to GA are subject of substantial controversy [8, 9].
Herein, we perform an updated meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of PBM versus a sham procedure in patients with dry AMD. We performed a trial sequential analysis (TSA) to evaluate if the sample was sufficient for making statistical inference [10–12] and assessed the minimum clinically important differences (MCID) calculated by pooled standard deviation (SD) to check if any statistical differences would translate to clinical significance [13, 14].
Methods
Our study was performed and reported following the Cochrane Collaboration Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement guidelines [15, 16]. The protocol was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) under protocol number CRD42024521983.
Data source and search strategy
We systematically searched PubMed, Embase, and Cochrane databases. Our search was last updated in February 2024. The search terms included “photobiomodulation” and “age-related macular degeneration”. The complete search strategy is provided in Supplemental Methods 3. All records retrieved were independently assessed by two authors, L.M.B. and T.N.O.R., and a decision regarding full-text retrieval was arbitrated by consensus between them. Full texts were reviewed by L.M.B. and T.N.O.R. and discussed regarding inclusion and exclusion criteria. References of eligible papers and systematic reviews were also searched for additional studies of interest. Conference abstracts and prospective trials were also searched.
Eligibility criteria
There was no restriction regarding publication date, status, or language. We considered studies eligible for inclusion if they [1] were RCTs; [2] directly compared PBM with sham; [3] included patients with diagnosed non-exudative AMD.
Endpoints
Our clinical outcomes of interest were: [1] last visit best corrected visual acuity (BCVA); last visit drusen volume in mm3; last visit GA area in mm2. Our pooled analyses last visit included a follow-up of at least 9 months.
Risk of bias assessment
Two independent authors (TR. and S.F.P.) assessed the risk of bias in the included RCTs using the Cochrane tool for assessing the risk of bias in randomized controlled trials (RoB-2) [17]. Disagreements were resolved through consensus.
Statistical analysis
We applied the Mantel-Haenszel random-effects model with a restricted maximum likelihood variance estimator for all outcomes. We pooled risk ratios (RR) with 95% confidence intervals (CI) for binary endpoints and mean differences (MD) with 95% CI for continuous endpoints. When needed, we extracted data using the WebPlotDigitizer tool.
We assessed heterogeneity with Cochran’s Q and I2 statistics, with p ≤ 0.10 indicating statistical significance for heterogeneity. We determined the between-study heterogeneity based on I2 values of 0%, ≤ 25%, ≤ 50%, and > 50%, indicating no observed, low, moderate, and substantial heterogeneity, respectively. All statistical analyses were performed using R version 4.3.2.
Trial Sequential Analysis
We performed TSA using the TSA software (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark) on the outcomes of BCVA, drusen volume, and GA area. We utilized a MD measure of effect and a random-effects model, setting a conventional 95% CI. The analysis incorporated a two-sided conventional boundary with 5% types I error rate. Alpha-spending boundaries were established using a two-sided boundary type, maintaining a 5% types I error rate and an 80% statistical power. The alpha and beta spending function adopted was the O’Brien-Fleming approach. In determining the required information size (RIS), we opted for an empirical method with heterogeneity correction, applying the model variance to accommodate study variability.
Minimal clinically important difference
We established the MCID for each outcome exhibiting statistical differences by calculating the pooled standard deviation (SD) and then multiplying this pooled SD by 0.5 [13, 14, 18].
Results
Study selection and characteristics
Our systematic review initially yielded 150 results. After removal of duplicates and screening based on title and abstract, 10 full-text articles were reviewed for possible inclusion. Finally, three RCTs fulfilled our inclusion criteria and were included in the analysis, [7, 19, 20] comprising a pooled population of 247 eyes, of whom 151 (61%) were randomized to the PBM group. Comprehensive details of the study selection are detailed in Fig. 1.
Fig. 1.
PRISMA flow diagram of study screening and selection. Abbreviations PBM, photobiomodulation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis
The mean age was 75.1 years. Total follow-up ranged from 9 to 13 months. All included studies were sham-controlled. Individual study characteristics are detailed in Table 1 [7, 19, 20].
Table 1.
Baseline characteristics of included studies
| Study | Study Design |
Location | No. of Patients | Eyes | Eyes PBM/Sham |
Age (SD) | Follow-up (months) |
Primary Endpoint | AREDS 1/2/3/4 |
|---|---|---|---|---|---|---|---|---|---|
| Boyer [19] | Double-masked, randomized, sham-controlled, parallel group, multicenter prospective study | USA | 100 | 148 | 93/55 |
75.4 (7.1) |
13 | BCVA | 0/19/126/0 |
| Burton [20] | Prospective, randomized, double-masked clinical trial | Europe | 44 | 53 | 34/19 |
74.1 (8.0) |
9 | BCVA | 1/11/35/6 |
| Markowitz [7] | Double-masked, randomized, sham-controlled, parallel group | Canada | 30 | 46 | 24/22 |
76 (8.3) |
12 | BCVA | 0/1/14/31 |
Abbreviations AREDS, Age-Related Eye Disease Studies; BCVA, best-corrected visual acuity; No., number; SD standard deviation, USA, United States of America
Clinical endpoints
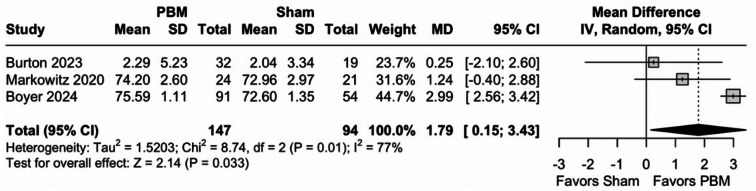
PBM showed a statistically significant improvement in BCVA over sham treatment, with a MD of 1.76 ETDRS letters among 241 eyes (95% CI [0.04; 3.48], p = 0.04) despite a high heterogeneity (I²=77%), as shown in Fig. 2. However, while statistically significant, the observed improvement did not meet the threshold for clinical relevance as defined by the MCID of 6.8 ETDRS letters. The TSA also indicated a RIS of 555 eyes for statistical inference, as the z-curve did not meet the monitoring boundary, suggesting the current sample size is insufficient, as shown in Fig. 3.
Fig. 2.
Forest plot for best corrected visual acuity (BCVA). There was a slight overall improvement favoring PBM versus sham with a mean difference of 1.76 (P = 0.04). Abbreviations CI, confidence intervals; MD, mean difference; PBM, photobiomodulation; SD, standard deviation; IV, inverse variance
Fig. 3.
Figure 3 shows a TSA for evaluating treatment efficacy in a cumulative meta-analysis. On the x-axis, the number of eyes reaches 241 across 3 studies, as shown by the blue curve. The y-axis measures the Z-score, assessing statistical deviation from the null hypothesis. The curve falls short of the required information size (555 eyes), indicated by the perpendicular line, suggesting more data is needed for a robust conclusion. The curve does not cross the monitoring boundaries, which, along with the conventional ± 1.96 Z-score boundaries, assess significance; therefore, the analysis does not conclusively favor either treatment group over the other
Anatomical endpoints
There was no significant difference between groups in GA area (73 eyes; MD -0.53 mm2; 95% CI [-1.44; 0.37]; p = 0.25; I2 = 0%), as shown in Fig. 4. However, TSA indicated that a RIS of 436 eyes would be necessary for a statistical inference, as shown in Fig. 5. Moreover, the z-curve did not reach the monitoring boundary.
Fig. 4.
Forest plot for of geographic atrophy (GA) area between Photobiomodulation (PBM) and sham treatment. The combined results yield a mean difference of -0.53, indicating no significant difference between PBM and SHAM treatments in reducing GA area (P = 0.25). Abbreviations CI, confidence intervals; MD, mean difference; PBM, photobiomodulation; SD, standard deviation; IV, inverse variance
Fig. 5.
TSA for GA area. On the x-axis, the number of eyes reaches 73 across 3 studies, as shown by the blue curve. The y-axis measures the Z-score, assessing statistical deviation from the null hypothesis. The curve falls short of the required information size (436 eyes), suggesting that more data are needed for a robust conclusion. The curve does not cross the monitoring boundaries, which, along with the conventional ± 1.96 Z-score boundaries, assess significance; therefore, the analysis does not conclusively favor either treatment group over the other. Abbreviations PBM, photobiomodulation
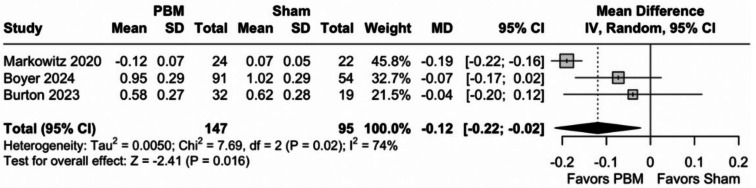
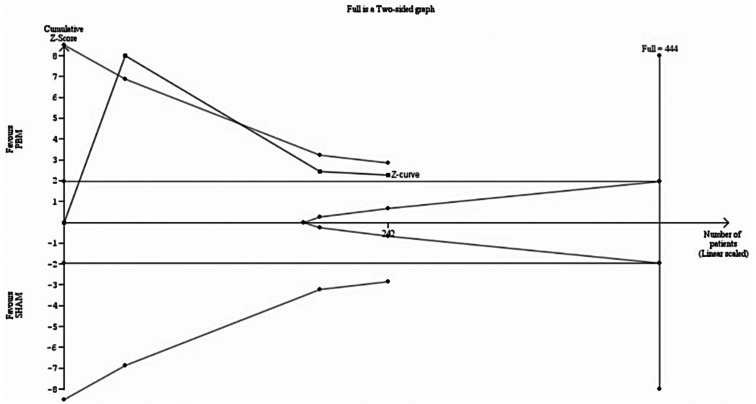
As compared with a sham procedure, PBM significantly reduced drusen volume (242 eyes; MD -0.12mm3; 95% CI [-0.22; -0.02]; p = 0.02; I2 = 74%), as shown in Fig. 6. However, the observed improvement did not meet the threshold for clinical relevance as defined by the MCID of 0.39 mm3. In addition, TSA indicated that a RIS of 444 eyes would be necessary for statistical inference, indicating insufficient sample size, as shown in Fig. 7. Moreover, the z-curve did not reach the monitoring boundary.
Fig. 6.
Forest plot for drusen volume. Results show a small mean difference of -0.12 mm³, with overall findings favoring PBM (P = 0.02). Abbreviations CI, confidence intervals; MD, mean difference; PBM, photobiomodulation; SD, standard deviation; IV, inverse variance
Fig. 7.
TSA for drusen volume. On the x-axis, the number of eyes reaches 242 across 3 studies, as shown by the blue curve’s progression. The y-axis measures the Z-score, assessing statistical deviation from the null hypothesis. The curve falls short of the required information size (444 eyes), indicated by the perpendicular line, suggesting more data are needed for statistical inference. The curve does not cross the monitoring boundaries, which, along with the conventional ± 1.96 Z-score boundaries, assess significance; therefore, the analysis does
Risk of Bias Assessment
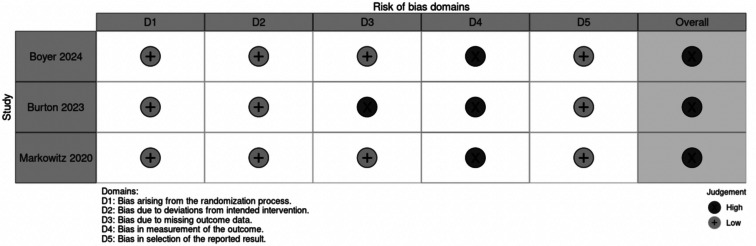
Using the Cochrane Collaboration’s RoB-2 tool, our quality assessment suggests that all three RCTs are at a high risk for bias. The primary concern was related to bias in measuring outcomes. Additionally, one of the studies experienced issues with bias due to missing outcome data attributed to disruptions caused by COVID-19 [20]. Individual RCT appraisal is detailed in Fig. 8.
Fig. 8.
Risk of bias assessment
Discussion
This meta-analysis included three RCTs with 247 eyes to assess the efficacy of PBM in patients with dry AMD. Our pooled data showed an improvement in BCVA and drusen volume in patients treated with PBM as compared with a sham with no significant difference in terms of progression of GA area. However, these statistical inferences could not be confirmed due to insufficient sample size, as indicated by the TSA. Even if the TSA was favorable, BCVA and drusen volume were not clinically significant, as they did not meet the MCID.
One may argue that the significant findings of RCTs of PBM therapy for dry AMD may not translate into clinical benefits. The largest RCT on the subject found a MD of 2.4 ETDRS letters compared with sham [19]. Nonetheless, visual acuity measurements in intermediate AMD may vary by an average of 9 ETDRS letters in patients who do not receive any treatment, much higher than the above cited MD [21]. For instance, the established MCID for photodynamic therapy in patients with neovascular membranes is 7.5 letters [22]. Of note, the FDA requires a minimum improvement of at least 15 letters for approving a pharmacological intervention in this setting [23]. Therefore, it could be contended that the benefits of PBM therapy do not meet clinical significance, which indeed was corroborated by our findings through the MCIDs results.
In addition, inadequate sample sizes limit the primary studies from definitively assessing the efficacy of PBM for dry AMD, as highlighted by previous meta-analyses that were only able to collect data from 2 studies and 96 eyes [8]. The individual trials, LIGHTSITE I and II, [7, 20] also recognized the constraints of their small cohorts. On top of the limited sample size, there are only three RCTs evaluating PBM for dry AMD, highlighting the need for additional and larger RCTs. Additionally, some might argue that the pooled sample size lacked statistical power for measuring outcomes such as the drusen volume. This issue arises because drusen size may vary, and drusen regression is a well-described phenomenon in the natural course of the disease, [24, 25] underscoring the need for larger sample sizes to draw more robust conclusions [26]. All these data and insights were corroborated by our TSA, which revealed that the existing pooled sample did not meet the required information size to make statistical inferences.
A significant challenge in evaluating treatments for dry AMD is selecting appropriate clinical endpoints. The FDA only recognizes GA volume as a valid outcome for dry AMD, whereas visual acuity and changes in drusen volume are not accepted by the regulatory agency [3]. This obstacle in finding appropriate measuring outcomes may explain the barriers that current studies on PBM face when trying to assess treatment efficacy in dry AMD. This is reflected heavily in the quality assessment, where all the studies were deemed to be at high risk of bias, consistent with evaluation of a previous meta-analysis [8]. One of the reasons for this high risk of bias was the reliance on BCVA as a measure of efficacy.
It is highly questionable whether BCVA stands as an optimal measure for treatment efficacy for drusen, since visual acuity may not be sensitive enough to detect changes in visual function in patients with intermediate AMD [27]. Another study showed lack of correlation between large drusen and BCVA [28]. Visual acuity has also shown major variations in intermediate AMD, which could potentially interfere with results. [21].
Additionally, the application of short-term drusen volume tracking as an effective endpoint for assessing efficacy in AMD has its restrictions. Studies with extended durations have demonstrated that a reduction in drusen can actually be indicative of a risk for progressing to advanced stages of AMD [3, 24, 25].
Our study has limitations. First, the small size of our pooled population may have hindered our statistical power, despite the inclusion of all studies that met eligibility criteria. Second, the absence of patient-level data precluded assessment of subgroup analyses and whether individual factors may interfere in the relative efficacy of PBM in this patient population. Finally, we could not assess the incidence of new-onset GA owing to the incomplete reporting in some of the individual studies.
Conclusion
In this meta-analysis evaluating PBM therapy for patients with dry AMD, there was a statistically significant improvement in visual acuity and drusen volumes, but not in incidence of GA. However, definitive statistical inferences are limited by an insufficient sample size, as indicated by the TSA. In addition, the significant results in terms of visual acuity and drusen volumes did not translate into clinically important benefits, as they did not meet the MCID casting doubt on PBM’s real-world efficacy. Larger RCTs with longer follow ups and more appropriate outcome measures are warranted to conclusively evaluate the role of PBM in patients with dry AMD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Rhanderson Cardoso, MD, FACC for his review of the manuscript.
Abbreviations
- AMD age
Related macular degeneration
- AREDS age
Related eye disease studies
- ARMD age
Related macular degeneration
- BCVA best
Corrected visual acuity
- CI
Confidence intervals
- ETDRS
Early treatment of diabetic retinopathy study
- FDA
Food and Drug Administration
- GA
Geographic atrophy
- MCID
Minimum clinically important differences
- MD
Mean difference
- PBM
Photobiomodulation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
Randomized controlled trial
- RIS
Required information size
- RoB-2
Risk of Bias Assessment Tool 2
- SD
Standard deviation
- TSA
Trial sequential analysis
Author contributions
L.M.B. and T.N.O.R. designed the paper, wrote the main manuscript text, interpreted the data, and prepared the figures; S.P. interpreted the data; E.A.N., F.P., L.R., M.M. substantively reviewed the manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tiago N. O. Rassi and Lucas M. Barbosa contributed equally to this work.
References
- 1.Jiang B, Jiang C, Li J, Lu P. Trends and disparities in disease burden of age-related macular degeneration from 1990 to 2019: results from the global burden of disease study 2019. Front Public Health. 2023;11:1138428. 10.3389/fpubh.2023.1138428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girmens JF, Sahel JA, Marazova K. Dry age-related macular degeneration: a currently unmet clinical need. Intractable Rare Dis Res. 2012;1(3):103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Csaky KG, Miller JML, Martin DF, Johnson MW. Drug approval for the Treatment of Geographic Atrophy: how we got Here and where we need to go. Am J Ophthalmol. 2024;263:231–9. 10.1016/j.ajo.2024.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SS, Lally DR, Hsu J, Wykoff CC, Eichenbaum D, Heier JS, et al. Avacincaptad pegol for geographic atrophy secondary to age-related macular degeneration: 18-month findings from the GATHER1 trial. Eye Lond Engl. 2023;37(17):3551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Youngblood H, Wu C, Zhang Q. Mitochondria as a target for neuroprotection: role of methylene blue and photobiomodulation. Transl Neurodegener. 2020;9(1):19. 10.1186/s40035-020-00197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bathini M, Raghushaker CR, Mahato KK. The Molecular mechanisms of Action of Photobiomodulation against neurodegenerative diseases: a systematic review. Cell Mol Neurobiol. 2022;42(4):955–71. 10.1007/s10571-020-01016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz SN, Devenyi RG, Munk MR, Croissant CL, Tedford SE, Rückert R, SHAM-CONTROLLED RANDOMIZED, SINGLE-CENTER STUDY WITH PHOTOBIOMODULATION FOR THE TREATMENT OF DRY AGE-RELATED MACULAR DEGENERATION, et al. Retina Phila Pa. 2020;40(8):1471–82. 10.1097/IAE.0000000000002632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henein C, Steel DH. Photobiomodulation for non-exudative age-related macular degeneration. Cochrane Database Syst Rev. 2021;5(5):CD013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fantaguzzi F, Tombolini B, Servillo A, Zucchiatti I, Sacconi R, Bandello F, et al. Shedding light on Photobiomodulation Therapy for Age-Related Macular Degeneration: a narrative review. Ophthalmol Ther. 2023;12(6):2903–15. 10.1007/s40123-023-00812-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39. 10.1186/s12874-017-0315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Cassai A, Tassone M, Geraldini F, Sergi M, Sella N, Boscolo A, et al. Explanation of trial sequential analysis: using a post-hoc analysis of meta-analyses published in Korean Journal of Anesthesiology. Korean J Anesthesiol. 2021;74(5):383–93. 10.4097/kja.21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah A, Smith AF. Trial sequential analysis: adding a new dimension to meta-analysis. Anaesthesia. 2020;75(1):15–20. 10.1111/anae.14705 [DOI] [PubMed] [Google Scholar]
- 13.Watt JA, Veroniki AA, Tricco AC, Straus SE. Using a distribution-based approach and systematic review methods to derive minimum clinically important differences. BMC Med Res Methodol. 2021;21(1):41. 10.1186/s12874-021-01228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–3. 10.1001/jama.2014.13128 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for systematic reviews of interventions version 6.4 (updated August 2023). Wiley; 2023. 6.4.
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 18.Draak THP, de Greef BTA, Faber CG, Merkies ISJ. The minimum clinically important difference: which direction to take. Eur J Neurol. 2019;26(6):850–5. 10.1111/ene.13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer D, Hu A, Warrow D, Xavier S, Gonzalez V, Lad E, et al. LIGHTSITE III: 13-Month Efficacy and Safety evaluation of Multiwavelength Photobiomodulation in Nonexudative (Dry) Age-Related Macular Degeneration using the Lumithera Valeda Light Delivery System. Retina Phila Pa. 2024;44(3):487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton B, Parodi MB, Jürgens I, Zanlonghi X, Hornan D, Roider J, et al. LIGHTSITE II randomized Multicenter Trial: evaluation of Multiwavelength Photobiomodulation in non-exudative age-related Macular Degeneration. Ophthalmol Ther. 2023;12(2):953–68. 10.1007/s40123-022-00640-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel PJ, Chen FK, Rubin GS, Tufail A. Intersession repeatability of visual acuity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(10):4347–52. 10.1167/iovs.08-1935 [DOI] [PubMed] [Google Scholar]
- 22.Potter MJ, Szabo SM, Li WW. Comparison of visual acuity outcomes in predominantly classic vs occult lesions in age-related macular degeneration treated with photodynamic therapy. Eye Lond Engl. 2008;22(2):194–9. [DOI] [PubMed] [Google Scholar]
- 23.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal Aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–48. 10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 24.Schlanitz FG, Baumann B, Kundi M, Sacu S, Baratsits M, Scheschy U, et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. 2017;101(2):198–203. 10.1136/bjophthalmol-2016-308422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toy BC, Krishnadev N, Indaram M, Cunningham D, Cukras CA, Chew EY, et al. Drusen regression is associated with local changes in fundus autofluorescence in intermediate age-related macular degeneration. Am J Ophthalmol. 2013;156(3):532–e5421. 10.1016/j.ajo.2013.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12):2434–41. 10.1016/j.ophtha.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forshaw TRJ, Parpounas AK, Sørensen TL. Correlation of macular sensitivity measures and visual acuity to vision-related quality of life in patients with age-related macular degeneration. BMC Ophthalmol. 2021;21(1):149. 10.1186/s12886-021-01901-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Davis MD, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report 36. JAMA Ophthalmol. 2014;132(3):272–7. 10.1001/jamaophthalmol.2013.6636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.