Abstract
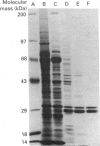
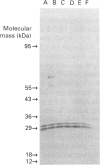
Incubation of digitonin-permeabilized bovine chromaffin cells in the absence of Ca2+ results in a loss of both cytosolic proteins and Ca(2+)-dependent secretion. Addition of these leaked proteins prevents this loss of secretory activity. We have purified a protein from an extract of bovine adrenal medulla which can partially prevent this loss of Ca(2+)-dependent secretion. Antibody against this protein inhibited the ability of leaked chromaffin-cell proteins to prevent the loss of Ca(2+)-dependent secretion. Sequence analysis showed it to have sequence identity with bovine brain 14-3-3 protein. These results demonstrate that 14-3-3 protein makes a significant contribution to the ability of leaked chromaffin-cell proteins to maintain secretory activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989 Jul 27;340(6231):313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Sontag J. M., Thiersé D., Aunis D. A reassessment of guanine nucleotide effects on catecholamine secretion from permeabilized adrenal chromaffin cells. J Biol Chem. 1989 Oct 5;264(28):16426–16434. [PubMed] [Google Scholar]
- Boston P. F., Jackson P., Kynoch P. A., Thompson R. J. Purification, properties, and immunohistochemical localisation of human brain 14-3-3 protein. J Neurochem. 1982 May;38(5):1466–1474. doi: 10.1111/j.1471-4159.1982.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Boston P. F., Jackson P., Thompson R. J. Human 14-3-3 protein: radioimmunoassay, tissue distribution, and cerebrospinal fluid levels in patients with neurological disorders. J Neurochem. 1982 May;38(5):1475–1482. doi: 10.1111/j.1471-4159.1982.tb07928.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. W., Pollard H. B. Enhancement of Ca2+-induced catecholamine release by the phorbol ester TPA in digitonin-permeabilized cultured bovine adrenal chromaffin cells. FEBS Lett. 1985 Apr 8;183(1):107–110. doi: 10.1016/0014-5793(85)80964-9. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Grasso A., Roda G., Hogue-Angeletti R. A., Moore B. W., Perez V. J. Preparation and properties of the brain specific protein 14-3-2. Brain Res. 1977 Apr 1;124(3):497–507. doi: 10.1016/0006-8993(77)90949-0. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Isobe T., Okuyama T., Yamauchi T., Fujisawa H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett. 1987 Jul 13;219(1):79–82. doi: 10.1016/0014-5793(87)81194-8. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Sugano H., Kuwano R., Sunaya T., Okuyama T., Isobe T. Widespread distribution of the 14-3-3 protein in vertebrate brains and bovine tissues: correlation with the distributions of calcium-dependent protein kinases. J Neurochem. 1991 Apr;56(4):1449–1451. doi: 10.1111/j.1471-4159.1991.tb11446.x. [DOI] [PubMed] [Google Scholar]
- Isobe T., Ichimura T., Sunaya T., Okuyama T., Takahashi N., Kuwano R., Takahashi Y. Distinct forms of the protein kinase-dependent activator of tyrosine and tryptophan hydroxylases. J Mol Biol. 1991 Jan 5;217(1):125–132. doi: 10.1016/0022-2836(91)90616-e. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Sugden D., Baker P. F. Evidence implicating protein kinase C in exocytosis from electropermeabilized bovine chromaffin cells. J Membr Biol. 1988 Aug;104(1):21–34. doi: 10.1007/BF01871899. [DOI] [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Exo1 and Exo2 proteins stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. Nature. 1992 Feb 27;355(6363):833–836. doi: 10.1038/355833a0. [DOI] [PubMed] [Google Scholar]
- Pocotte S. L., Frye R. A., Senter R. A., TerBush D. R., Lee S. A., Holz R. W. Effects of phorbol ester on catecholamine secretion and protein phosphorylation in adrenal medullary cell cultures. Proc Natl Acad Sci U S A. 1985 Feb;82(3):930–934. doi: 10.1073/pnas.82.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T., Aunis D., Bader M. F. Loss of proteins from digitonin-permeabilized adrenal chromaffin cells essential for exocytosis. J Biol Chem. 1987 Dec 5;262(34):16671–16676. [PubMed] [Google Scholar]
- Sarafian T., Pradel L. A., Henry J. P., Aunis D., Bader M. F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J Cell Biol. 1991 Sep;114(6):1135–1147. doi: 10.1083/jcb.114.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer T., Karli U. O., Gratwohl E. K., Schweizer F. E., Burger M. M. Digitonin-permeabilized cells are exocytosis competent. J Neurochem. 1987 Dec;49(6):1697–1707. doi: 10.1111/j.1471-4159.1987.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Terbush D. R., Holz R. W. Activation of protein kinase C is not required for exocytosis from bovine adrenal chromaffin cells. The effects of protein kinase C(19-31), Ca/CaM kinase II(291-317), and staurosporine. J Biol Chem. 1990 Dec 5;265(34):21179–21184. [PubMed] [Google Scholar]
- Toker A., Ellis C. A., Sellers L. A., Aitken A. Protein kinase C inhibitor proteins. Purification from sheep brain and sequence similarity to lipocortins and 14-3-3 protein. Eur J Biochem. 1990 Jul 31;191(2):421–429. doi: 10.1111/j.1432-1033.1990.tb19138.x. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]
- Wilson S. P. Regulation of chromaffin cell secretion and protein kinase C activity by chronic phorbol ester treatment. J Biol Chem. 1990 Jan 15;265(2):648–651. [PubMed] [Google Scholar]
- Wu Y. N., Wagner P. D. Calpactin-depleted cytosolic proteins restore Ca(2+)-dependent secretion to digitonin-permeabilized bovine chromaffin cells. FEBS Lett. 1991 Apr 22;282(1):197–199. doi: 10.1016/0014-5793(91)80476-j. [DOI] [PubMed] [Google Scholar]
- Wu Y. N., Wagner P. D. Effects of phosphatase inhibitors and a protein phosphatase on norepinephrine secretion by permeabilized bovine chromaffin cells. Biochim Biophys Acta. 1991 May 17;1092(3):384–390. doi: 10.1016/s0167-4889(97)90016-1. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Nakata H., Fujisawa H. A new activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+-, calmodulin-dependent protein kinase. Purification and characterization. J Biol Chem. 1981 Jun 10;256(11):5404–5409. [PubMed] [Google Scholar]