Abstract
Background
Situs inversus (SI) is a rare congenital anomaly in which systemic organs and vessels are positioned in a mirror image of their normal positions. An interesting issue regarding individuals with such a condition is whether they also have reversed brain asymmetries. Most of studies on this issue indicate that, similarly to many people with normal visceral alignment, patients with SI have a left hemispheric dominance for language functions.
Case presentation
We report a rare occurrence of anomalous cerebral dominance for language in a patient with complete situs inversus. The right-handed patient developed aphasia after carotid stenting, and brain magnetic resonance imaging showed cerebral infarction in the right parietal lobe.
Conclusion
Anomalous cerebral dominance for language and visceral situs inversus in our patient both may result from a single, genetically coded atypicality of developmental gradient.
Keywords: Situs inversus, Carotid stenting, Language dominance, Brain asymmetries
Introduction
Numerous studies have shown that the left and right hemispheres of the human brain have functional and anatomical differences [1]. The most well-known functional asymmetry is the left hemisphere dominance of language, which is found in 97% of right-handers and two-thirds of left-handers [2]. As for anatomical asymmetries in the brain, Geschwind and Levitsky [1] were the first to report that the left temporal lobe is larger in 65% of adult brains, and many studies have since supported the left temporal lobe asymmetry [3]. Visceral organs are also arranged asymmetrically about the midline: the heart is situated on the left side and the liver on the right side [2]. However, in about 1 in 10,000 people, all organs are arranged in a mirror image of their normal positions [2]. This condition is known as situs inversus (SI) and is usually caused by an autosomal recessive gene. An interesting issue regarding such individuals is whether they have reversed brain asymmetries, which could provide a clue to elucidate the developmental mechanisms involved in brain asymmetries. However, only a few studies have focused on brain asymmetry in SI patients [4–8]. Most of these studies indicate that, similarly to many people with normal visceral alignment, patients with SI have a left hemispheric predominance. Only a single case report showed right-hemispheric dominance in a right-handed patient with partial situs inversus [5]. This patient presented with aphasia that was caused by right parieto-temporal stroke, which resulted from ischemia in the territory of the right middle cerebral artery [5]. We report a rare case of right hemispheric dominance for language in a right-handed patient with complete SI.
Case description
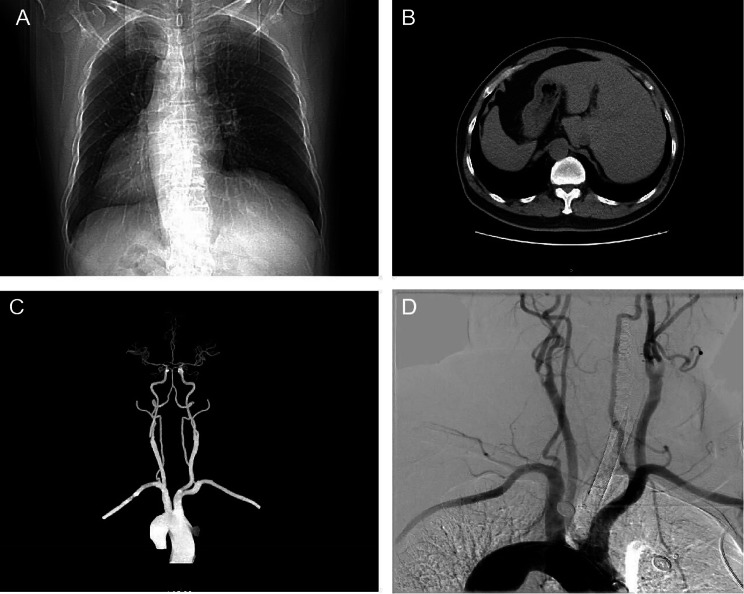
A 68-year-old right-handed man, who had a history of hypertension, was hospitalized owing to carotid stenosis found one month before. The patient’s carotid artery ultrasonography showed severe stenosis of the right carotid artery (stenosis is over 70%). Current guidelines state that medical therapy is sufficient for the treatment of carotid artery stenosis below 50% [9]. However, if stenosis is over 50% in symptomatic patients and over 70% in asymptomatic patients, surgical or endovascular methods are recommended [10]. The most common complications of carotid stenting are embolic infarcts, prolonged hypotension/bradycardia, and hyperperfusion syndrome [10]. Interestingly, during carotid ultrasonography, it was found that the heart was on the right side. Figure 1 illustrates how computed tomography (CT) and digital subtraction angiography (DSA) further confirmed the patient’s SI (Fig. 1A, B, C and D).
Fig. 1.
CT and DSA images of SI patient. (A) Chest CT image showing the heart on the right side. (B) Abdominal CT suggesting left–right viscera inversion. (C) Brain CTA image showing that the aortic arch vessels in the patient are reversed from side to side. (D) DSA image showing that the aortic arch vessels in the patient are reversed from side to side. SI: situs inversus; CT: Computed tomography; DSA: Digital subtraction angiography; CTA: Computed tomography angiography
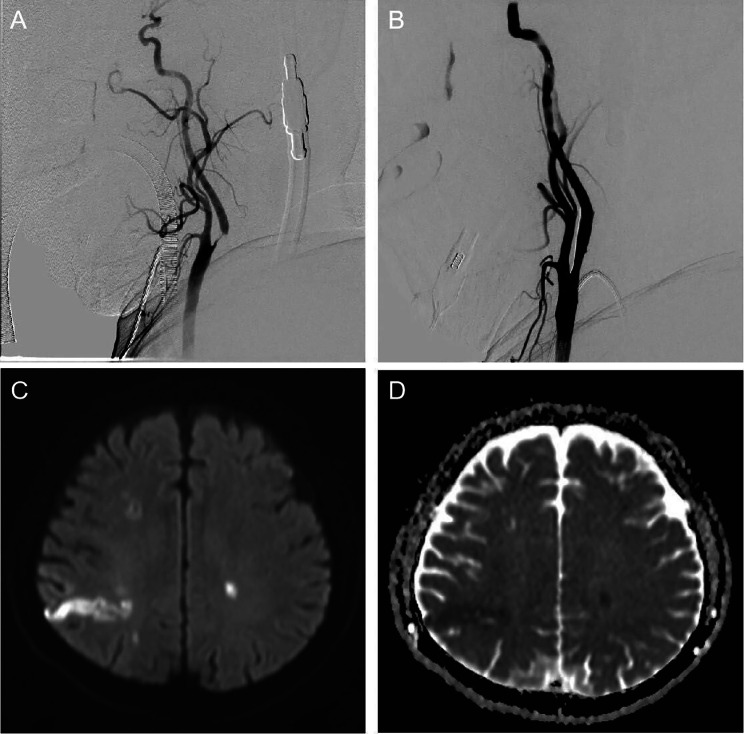
The patient underwent carotid artery stent implantation under intravenous compound inhalational anesthesia with significant improvement in carotid artery stenosis after interventional surgery (Fig. 2A and B). After waking up from anesthesia, the patient became severely aphasic, producing only a few meaningless and poorly articulated syllables (NIHSS score 4) with severe cognitive impairment (MMSE score 18). The patient immediately underwent cranial CT and carotid ultrasonography, and no cerebral hemorrhage or carotid artery occlusion was found. Immediately afterward, head magnetic resonance imaging (MRI) was performed, and acute cerebral infarction lesions were found in the right parietal lobe (Fig. 2C and D).
Fig. 2.
Comparison of preoperative and postoperative DSA images and postoperative MRI images. (A) DSA image showing severe stenosis of the right internal carotid artery. (B) Carotid artery stenosis improved significantly after carotid stenting. (C) DWI image showing high-intensity signals in the right parietal temporal lobe. (D) ADC showing low-intensity signals in the right parietal temporal lobe. DSA: Digital subtraction angiography; MRI: Magnetic resonance imaging; DWI: Diffusion Weighted Imaging; ADC: Apparent diffusion coefficient
During the first week, the patient recovered some of his verbal production abilities (NIHSS score 2) with mild cognitive impairment (MMSE score 22). He was able to make himself understood quite effectively. However, he frequently showed word-finding difficulties in his speech. After three months of follow-up, the patient’s language ability basically returned to normal with no cognitive impairment (MMSE score 28), but occasionally, he had difficulty finding words (NIHSS score 1).
Discussion
SI develops a disorder of visceral rotation during embryonic development, which is associated with mutations at a site in the parents’ genes [11]. The position of the visceral organs is diametrically opposed, but does not affect the patient’s function or daily life [11]. Whether the reverse asymmetry also exists in the brains of these patients is a question that should be investigated, but not much work has been done in this area.
Reports on functional brain asymmetry in SI patients are very limited., i.e., it remains unclarified whether there is a functional asymmetry between the bilateral cerebral hemispheres of the brain [4–8]. A case study of a patient with SI reported that a left-hemispheric stroke caused aphasia and dense right hemiplegia with no voluntary movements of the right extremities, suggesting that the patient had left-hemispheric language dominance [4]. By using anatomical and functional MRI techniques, Kennedy et al. noninvasively investigated brain asymmetries in 3 subjects with SI [6]. During language testing, all the 3 subjects exhibited greater activities on left frontal and temporal areas than on the right, indicating left-hemispheric language dominance [6]. Hence, the existing data suggest that individuals with SI do not have a reversed pattern of functional asymmetry for language, irrespective of their anatomical asymmetry of visceral organs [6]. Most of these reports indicate that patients have a left hemisphere language dominance.
There is only one case report of a right-handed patient with right hemisphere dominance and displacement of partial SI [5]. Her colon was normally situated, although the mesenteric attachments of the small intestine were reversed. The position and structure of the heart and lungs were normal, but the aortic arch was atypically situated in the sagittal plane. This pattern of visceral organization corresponds to Ivemark’s syndrome, an association of polyasplenia with partial situs inversus and cardiovascular anomalies [5]. The cause of aphasia in this patient was a right temporoparietal stroke due to ischemia of the right middle cerebral artery.
Our case report showed right hemispheric dominance in a right-handed patient with complete SI. This patient presented with aphasia that was caused by the right parietal stroke, which may have resulted from carotid plaque embolism after carotid stenting.
In this case, the patient is right-handed. Various patterns of association between cerebral dominance and disposition of internal organs have been documented, demonstrating that there is no necessay link between the asymmetries on these two levels [12]. Thus, left-handed and patients with crossed aphasia, respectively, demonstrate that anomalous dominance for manuality and language is usually not accompanied by visceral situs inversus [12]. Similarly, there is evidence that reverse visceral lateralization can be associated with normal cerebral dominance [5].
One possibility is that there is simply no relationship at all between these two kinds of asymmetries. The asymmetry in our patient would then result from the fortuitous coincidence of two exceptional abnormalities of left-right organization. On the basis of estimated frequencies of situs inversus and of crossed hemispheric dominance for language in the general population, this association would prevail in less than five individuals in one million [5].
Although most reports show that patients have a left-hemisphere language advantage, the number of cases is small (5 cases only), among which the number of stroke events is even smaller, and our case is the second one that demonstrates right-hemispheric dominance in a right-handed patient with SI. Two out of the five cases so far reported cannot be considered a small percentage.
There is an alternative explanation, linking crossed aphasia and situs inversus. Corballis and Morgan suggested that a single fundamental left-right developmental gradient, coded in the ovum, might be responsible for the usual pattern of asymmetry of both the brain and the viscera [5].
There is a further indication that situs inversus and anomalous cerebral lateralization may stem from a common origin [5]. Strictly speaking, the patient showed situs ambiguus rather than situs inversus, both in brain and in viscera. His cerebral functions were unsystematically distributed across hemispheres: Dominance for handedness was in the left hemisphere and dominance for language in the right. The strikingly rapid, albeit incomplete, improvement of his aphasia could suggest bilateral representation of language. His abdominal viscera were completely reversed (partially reversed in the case report [5]). This observation fits with the idea that in the absence of a common biasing system, all asymmetries are randomly generated as local processes [13]. There are some indications that this fundamental bias might be genetically determined. In mice, animals that are homozygous for the autosomal recessive allele iv show a random determination of their visceral situs, suggesting that the normal allele is responsible for the development of the usual visceral asymmetry [14]. In our case, the cortical infarction on the right side is larger in size, and the small punctate cerebral infarction on the left side cannot lead to severe aphasia, but it cannot be excluded that these two lesions together cause the problem. We believe that the right-sided lesion is the main cause, but of course it would be more convincing if we could do a functional brain MRI. The limitation in this article is the lack of repeated MRIs as well as functional MRIs of the brain that would demonstrate the dominant hemisphere.
Conclusion
Anomalous cerebral dominance for language and visceral situs inversus in our patient both may result from a single, genetically coded atypicality of developmental gradient.
Acknowledgements
We thank the patient and his son for their consents to publish the case report.
Abbreviations
- SI
Situs inversus
- CT
Computed tomography
- DSA
Digital subtraction angiography
- MRI
Magnetic resonance imaging
- CTA
Computed tomography angiography
Author contributions
J.Z. and G.S. designed the study and wrote the manuscript. G.N. and S.Z. critically revised the manuscript for important intellectual content. All authors have reviewed the manuscript.
Funding
We receive no funding support.
Data availability
Please contact the authors for data requests.
Declarations
Ethics approval and consent to participate
The patient has provided consent for the publication of this case report.
Consent for publication
Written informed consent was obtained from the patient and patient’s son for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor‑in‑Chief of this journal.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingmin Zhao and Guangxun Shen contributed equally to this work.
Guangxian Nan and Songji Zhao contributed equally.
Contributor Information
Guangxian Nan, Email: nangx@jlu.edu.cn.
Songji Zhao, Email: zhao-s@fmu.ac.jp.
References
- 1.Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161(3837):186–7. 10.1126/science.161.3837.186 [DOI] [PubMed] [Google Scholar]
- 2.Ihara A, et al. Neuroimaging study on brain asymmetries in situs inversus totalis. J Neurol Sci. 2010;288(1–2):72–8. 10.1016/j.jns.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 3.Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96(3):641–6. 10.1093/brain/96.3.641 [DOI] [PubMed] [Google Scholar]
- 4.Woods RP. Brain asymmetries in situs inversus. A case report and review of the literature. Arch Neurol. 1986;43(10):1083–4. 10.1001/archneur.1986.00520100087021 [DOI] [PubMed] [Google Scholar]
- 5.Cohen L, et al. Crossed aphasia with visceral situs inversus. Ann Neurol. 1993;33(2):215–8. 10.1002/ana.410330213 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy DN, et al. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53(6):1260–5. 10.1212/WNL.53.6.1260 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, et al. Dichotic listening in patients with situs inversus: brain asymmetry and situs asymmetry. Neuropsychologia. 1999;37(7):869–74. 10.1016/S0028-3932(98)00144-4 [DOI] [PubMed] [Google Scholar]
- 8.Yoshie T, et al. Successful endovascular thrombectomy for Acute M1 occlusion in a patient with Situs Inversus: a Case Report. NMC Case Rep J. 2021;8(1):355–8. 10.2176/nmccrj.cr.2020-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonati LH, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021;6(2):I. 10.1177/23969873211012121 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Ozaki S, et al. Complications and outcomes of carotid artery stenting in high-risk cases. J Stroke Cerebrovasc Dis. 2023;32(10):107329. 10.1016/j.jstrokecerebrovasdis.2023.107329 [DOI] [PubMed] [Google Scholar]
- 11.Long T, et al. Interventional treatment of basilar trunk artery aneurysms associated with situs inversus totalis: a case report. Front Surg. 2022;9:971340. 10.3389/fsurg.2022.971340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander MP, Fischette MR, Fischer RS. Crossed aphasias can be mirror image or anomalous. Case reports, review and hypothesis. Brain. 1989;112(Pt 4):953–73. 10.1093/brain/112.4.953 [DOI] [PubMed] [Google Scholar]
- 13.Brown NA, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109(1):1–9. 10.1242/dev.109.1.1 [DOI] [PubMed] [Google Scholar]
- 14.Layton WM Jr. Random determination of a developmental process: reversal of normal visceral asymmetry in the mouse. J Hered. 1976;67(6):336–8. 10.1093/oxfordjournals.jhered.a108749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the authors for data requests.