Abstract
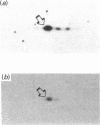
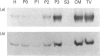
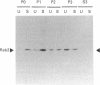
Rab proteins, which are ras-like low-molecular-mass GTP-binding proteins, are postulated to act as specific regulators of membrane trafficking in exocytosis and endocytosis. Previously, we reported a 23 kDa tubulovesicle-associated GTP-binding protein in rabbit gastric parietal cells [Basson, Goldenring, Tang, Lewis, Padfield, Jamieson & Modlin (1991) Biochem. J. 279, 43-48]. The major component of the 23 kDa protein is now identified as rab2. Rab2 was co-localized in tubulovesicle membranes from parietal cells. Consistent with GTP-binding activity (as documented before), upon maximal stimulation of parietal cells, rab2 immunoreactivity was redistributed from a 50,000 g to a 4000 g subcellular membrane fraction. The tubulovesicle-associated rab2 behaved as an integral membrane protein, since both 0.5 M-NaCl and 0.1 M-carbonate extraction failed to remove the protein from the tubulovesicle membrane. Utilizing a PCR the rab2 cDNA sequence from rabbit parietal cells was obtained, and it showed only one amino acid difference compared with the human sequence. The results of the present study provide strong evidence that parietal cells possess a rab2 protein which is tightly associated with tubulovesicle membranes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Goldenring J. R., Oddsdottir M., Zdon M. J., Zucker K. A., Lewis J. J., Modlin I. M. A micromethod for the assay of cellular secretory physiology: application to rabbit parietal cells. Anal Biochem. 1989 Nov 1;182(2):346–352. doi: 10.1016/0003-2697(89)90606-4. [DOI] [PubMed] [Google Scholar]
- Ayala J., Touchot N., Zahraoui A., Tavitian A., Prochiantz A. The product of rab2, a small GTP binding protein, increases neuronal adhesion, and neurite growth in vitro. Neuron. 1990 May;4(5):797–805. doi: 10.1016/0896-6273(90)90206-u. [DOI] [PubMed] [Google Scholar]
- Baker D., Hicke L., Rexach M., Schleyer M., Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988 Jul 29;54(3):335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Basson M. D., Goldenring J. R., Tang L. H., Lewis J. J., Padfield P., Jamieson J. D., Modlin I. M. Redistribution of 23 kDa tubulovesicle-associated GTP-binding proteins during parietal cell stimulation. Biochem J. 1991 Oct 1;279(Pt 1):43–48. doi: 10.1042/bj2790043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar R. P., Haslam R. J. Detection of 23-27 kDa GTP-binding proteins in platelets and other cells. Biochem J. 1987 Jul 15;245(2):617–620. doi: 10.1042/bj2450617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cameron R. S., Cameron P. L., Castle J. D. A common spectrum of polypeptides occurs in secretion granule membranes of different exocrine glands. J Cell Biol. 1986 Oct;103(4):1299–1313. doi: 10.1083/jcb.103.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Gorvel J. P., Stelzer E., Simons K., Gruenberg J., Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991 Oct 24;353(6346):769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Downward J. The ras superfamily of small GTP-binding proteins. Trends Biochem Sci. 1990 Dec;15(12):469–472. doi: 10.1016/0968-0004(90)90300-z. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Südhof T. C., Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991 Jan 3;349(6304):79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hanzel D. K., Urushidani T., Usinger W. R., Smolka A., Forte J. G. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol. 1989 Jun;256(6 Pt 1):G1082–G1089. doi: 10.1152/ajpgi.1989.256.6.G1082. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinase II. Methods Enzymol. 1983;99:317–331. doi: 10.1016/0076-6879(83)99067-5. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R., Lutz R. J., Cox A. D., Conroy L., Bourne J. R., Sinensky M., Balch W. E., Buss J. E., Der C. J. Isoprenoid modification of rab proteins terminating in CC or CXC motifs. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6264–6268. doi: 10.1073/pnas.88.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Maltese W. A. rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J Biol Chem. 1991 May 5;266(13):8540–8544. [PubMed] [Google Scholar]
- Mazzeo A. R., Nandi J., Levine R. A. Effects of ethanol on parietal cell membrane phospholipids and proton pump function. Am J Physiol. 1988 Jan;254(1 Pt 1):G57–G64. doi: 10.1152/ajpgi.1988.254.1.G57. [DOI] [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Gleason M. L., Orci L., Rothman J. E. Involvement of GTP-binding "G" proteins in transport through the Golgi stack. Cell. 1987 Dec 24;51(6):1053–1062. doi: 10.1016/0092-8674(87)90591-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Plutner H., Cox A. D., Pind S., Khosravi-Far R., Bourne J. R., Schwaninger R., Der C. J., Balch W. E. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991 Oct;115(1):31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushidani T., Forte J. G. Stimulation-associated redistribution of H+-K+-ATPase activity in isolated gastric glands. Am J Physiol. 1987 Apr;252(4 Pt 1):G458–G465. doi: 10.1152/ajpgi.1987.252.4.G458. [DOI] [PubMed] [Google Scholar]
- Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]