Key Teaching Points.
-
•
With the increasing use of leadless pacemakers, there is a growing need for patients to transition to cardiac resynchronization therapy (CRT). It is feasible to use pre-existing leadless pacemakers (LPM) by configuring them to communicate with new CRT systems for enhanced synchronized pacing capabilities.
-
•
The ventricular sensing response function of the current CRT system enables the detection of rhythms from LPM. Programming the CRT to VVI mode allows it to follow the paced rate of the LPM.
-
•
Using 2 different devices—the LPM and the CRT system—allows for multisite ventricular pacing, including the interventricular septum. This approach potentially leads to more physiological conduction, improving the efficiency and effectiveness of cardiac resynchronization.
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for heart failure patients with intraventricular conduction delays, facilitating improved clinical prognosis.1 However, frequent nonresponsiveness to CRT, influenced by multifactorial causes, has led to extensive efforts to resolve this challenge, with the selection of the optimal pacing site being crucial. Implantation of multiple left ventricular (LV) leads in the coronary sinus could enhance CRT outcomes by involving a larger portion of the ventricular mass. Recent studies on triventricular pacing (Tri-CRT), which introduces a third ventricular lead for simultaneous stimulation of 3 ventricular sites, have shown improved electromechanical synchrony and better echocardiographic and clinical responses.2,3
Herein, we present a patient who already had a leadless pacemaker (LPM) and required biventricular pacing with a defibrillator owing to aggravated heart failure and ventricular tachyarrhythmias, creating a Tri-CRT configuration with pre-existing LPM at the interventricular septum.
Case report
Patient information
A 67-year-old male patient with a history of long-standing persistent atrial fibrillation, hypertension, and diabetes had a normal LV ejection fraction of 51%. However, he also had severe mitral and tricuspid regurgitation. The patient was scheduled for an elective operation for mitral and tricuspid valve replacement, but experienced recurrent episodes of syncope and presyncope owing to atrial fibrillation with slow ventricular responses during the waiting period. Thus, a leadless pacemaker (Micra; Medtronic, Minneapolis, MN) was implanted at the right ventricular (RV) midseptum. After 2 months, the patient successfully underwent mitral annuloplasty, tricuspid valvuloplasty, and maze operation. The postoperative period was stable, with no immediate complications. The baseline electrocardiography (ECG) showed atrial fibrillation with ventricular paced rhythm by leadless pacemaker (VVIR mode).
One year after the surgery, the pacing burden increased to more than 80% owing to marked symptomatic bradycardia, cardiac function had deteriorated, and the patient experienced chest pain with sustained ventricular tachycardia. The LV ejection fraction decreased from 51% to 19%, but subsequently improved to 27% with optimal medical treatment, including sacubitril/valsartan, empagliflozin, carvedilol, and spironolactone. Cardiac magnetic resonance imaging showed nonischemic pattern cardiomyopathy, and baseline 12-lead ECG revealed a totally paced rhythm with LPM. Owing to the deteriorating condition of the patient, we decided to provide a CRT defibrillator (CRT-D) to improve his clinical outcomes.
Procedural information
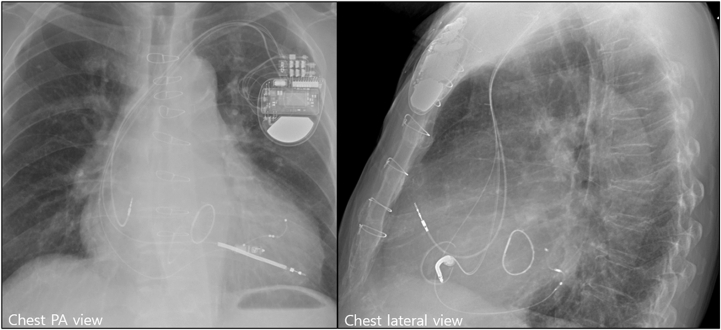
The CRT-D procedure was successfully implanted without acute complications. First, the RV defibrillation lead was fixed at the RV apex. The LV lead was then advanced into the posterolateral branch through the coronary sinus. Pacing thresholds, sensing, and impedance of the LV (1.5 V at 0.4 ms, 608 ohms) and RV lead (0.75 V at 0.4 ms, 342 ohms) were acceptable. There was no phrenic nerve capture during the 10 V pacing of the LV lead. A CRT-D generator (Cobalt XT HF MRI DTPA2QQ; Medtronic, Minneapolis, MN) was connected to the right atrial (CapSureFix Novus MRI 5076/45 cm; Medtronic), RV (Sprint Quattro Secure S 6935M/55 cm; Medtronic) and LV (Attain Performa LV lead 4898/88 cm; Medtronic) leads. The previously implanted LPM was stable at the RV midseptal wall. The chest radiography images taken after the CRT-D procedure are shown in Figure 1.
Figure 1.
Flourscopic views after cardiac resynchronization therapy defibrillator implantation with a previously implanted leadless pacemaker. PA = posteroanterior.
Optimization of CRT
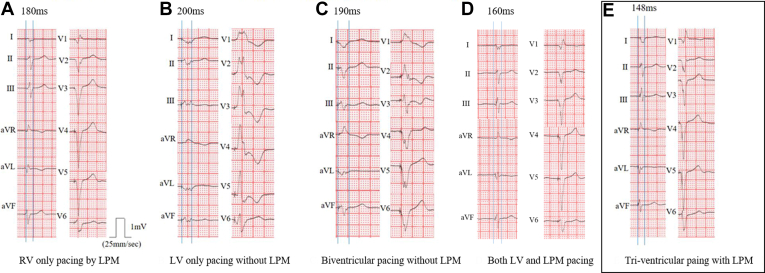
The 12-lead ECGs performed during CRT optimization are presented in Figure 2. The leadless pacemaker was programmed with VVIR mode. Before upgrading to CRT, baseline ECG with RV pacing only by LPM showed a QRS duration width of 180 ms (Figure 2A). After the LPM was switched off and optimized with LV pacing only, the QRS duration was 200 ms when the LV paced from LV1 to LV2 (Figure 2B). Next, we performed biventricular pacing optimization without LPM. When we tested biventricular pacing only with CRT, the best biventricular pacing based on CRT optimization showed a QRS duration of 190 ms when CRT-D was set to VVI mode and interventricular delay from LV to RV at 0 ms with LV paced from LV1 to LV3 (Figure 2C). When pacing was performed using only LV and LPM without RV apical pacing, the QRS duration was 160 ms (Figure 2D) Finally, Tri-CRT pacing was tested using both CRT and LPM. At that time, the LPM was in VVIR mode, and CRT was set to VVI mode to follow the ventricular rhythm from LPM through Ventricular Sensing Response (VSR). The VSR function enabled the CRT to synchronize with the baseline rhythm, which was paced by the LPM. Immediately after detecting the rhythm from the LPM, the CRT provided biventricular pacing through the VSR function. The CRT was programmed to VVI mode, not VVIR, to follow the paced rate set by the LPM. It revealed a QRS duration of 148 ms when interventricular delay from LV to RV was at 0 ms with LV paced from LV1 to LV2 (Figure 2E), which was the shortest QRS duration across various settings for CRT optimization. There was no cross-talk between CRT and LPM.
Figure 2.
QRS width in various settings for cardiac resynchronization therapy with leadless pacemaker (LPM). A: RV-only pacing by LPM. B: LV-only pacing without LPM. C: Biventricular pacing without LPM. D: Both LV and LPM pacing. LV = left ventricular; RV = right ventricular.
Clinical outcome after CRT-D upgrade
The day after the CRT-D upgrade, the transthoracic echocardiographic parameter showed improvement in LV ejection fraction from baseline 27% to 39%. At 1 month after the procedure, LV ejection fraction had improved to 44% (Supplemental Video 1). Additionally, LV end-systolic volume was 89 mL at baseline, which fell to 80 mL 1 month after the procedure. Similarly, the LV end-systolic index improved from 52 mL/m2 at baseline to 49 mL/m2 1 month after the procedure, but no significant change in LV end-diastolic volume index was observed.
Discussion
In this case, we successfully achieved Tri-CRT with both CRT-D and previously implanted LPM in a patient with severe heart failure. Triventricular pacing could achieve a narrower QRS duration compared with conventional biventricular pacing. To our knowledge, this is the first case report to demonstrate the results of Tri-CRT in a patient requiring a CRT-D upgrade from LPM.
Previously, 1.0% of individuals with de novo LPM implants underwent an upgrade to CRT.4 Given that LPM is typically fixated on the interventricular septum rather than the apex, a narrower QRS duration is expected compared with conventional RV apical pacing. Therefore, in patients requiring a CRT upgrade from an LPM, considering options for using the existing LPM in CRT optimization is important. When an additional RV defibrillation lead is required, as in our case, there could be numerous comparable situations. Through this report, we propose the potential use of septal pacing with LPM, rather than its deactivation or removal.
When upgrading from LPM to CRT, LPM has several advantages for achieving Tri-CRT. First, LPM is preferably located in the mid-RV septum owing to the possibility of cardiac perforation.5 Because the RV septum is on the way to the native conduction pathway, using LPM could help achieve more physiologic pacing compared with biventricular pacing alone. Secondly, the RV septum is located far from the LV lead, which is usually found in the lateral branch of the coronary sinus, with another RV lead at the RV apex. The distance from 3 ventricular leads increases, and the larger volume of myocardium could be recruited for pacing.6 Therefore, Tri-CRT using LPM at the interventricular septum could lead to the recruitment of a larger volume of viable myocardium. Thirdly, compared with previous methods using an additional LV lead for Tri-CRT, the approach using LPM can be beneficial in terms of procedural simplicity and reduced complications. Triventricular pacing with LPM could decrease the duration of fluoroscopy and extend the battery life of the device by eliminating the need for an extra ventricular lead in the coronary sinus. Furthermore, inserting a third ventricular lead into the lateral branch of the coronary sinus can be intricate owing to the complex anatomy of the coronary sinus, with potential phrenic nerve stimulation and issues of lead stability.7
The effectiveness and safety of Tri-CRT using standard CRT implantation techniques has been reported frequently.8 Leclercq and colleagues3 demonstrated that Tri-CRT with 1 RV and 2 LV leads was associated with higher LV ejection fraction, smaller LV end-systolic volume, and smaller LV end-systolic diameter than conventional biventricular pacing in patients with heart failure and permanent atrial fibrillation. Rogers and colleagues7 showed that Tri-CRT improved 6-minute walking distance, quality of life, LV end-systolic volume, and ejection fraction in patients with an ejection fraction of < 35% and QRS duration of ≥150 ms. However, 2 recent randomized trials reported negative results of multisite pacing. The V3 trial randomized CRT nonresponders to triple-site CRT by adding a second LV lead or control arm, indicating no change. Implantation of an additional LV lead led to several adverse events and had no benefit on quality of life and echocardiographic parameters compared with the control arms in CRT nonresponders.9 Additionally, STRIVE-HF revealed that implantation of an additional LV lead was safe but had no clinical benefit over conventional biventricular pacing in patients with left bundle branch block (LBBB) and intermediate QRS prolongation (120–150 ms).10 These negative findings could be attributed to the V3 trial’s initially including CRT nonresponders, suggesting the study population was in worse clinical condition than other study groups. Furthermore, the STRIVE-HF trial included patients with LBBB and intermediate QRS durations, for whom the CRT was recommended at a lower level (class IB). The difference in study population could therefore have derived these different outcomes.
Owing to fewer vascular or pocket-related complications and cosmetic benefits, the use of LPM is gradually increasing, and LPM also is now applicable to many aspects of treatment.11,12 As the number of patients receiving LPM increases, there will inevitably be a larger subset requiring CRT upgrades. Although biventricular pacing using LPM and LV electrodes has previously been tried and reported, this was the first utilization of LPM for Tri-CRT to overcome the limitation of conventional CRT.13,14 Careful patient follow-up is crucial, as a narrowed QRS does not ensure long-term benefits.
Conclusion
This case pertains to a patient who was pacing dependent with suspected pacing-induced cardiomyopathy. The patient did not manifest the most common indications for CRT, such as LBBB or interventricular conduction delay in heart failure patients. As such, the implications of this case should be interpreted with caution. However, patients with pronounced conduction delays could potentially benefit from multipoint pacing. Therefore, in patients with severe bradycardia who do not achieve the desired pacing effect with conventional CRT, the utilization of an LPM to facilitate ventricular septal pacing warrants consideration.
Disclosures
Jun Kim: Research fund from Boston Scientific, Medtronic, Abbott. Honororia from Boston Scientific, Medtronic, Abbott, Biosense-Webster, Novartis, Daichi-Sankyo. Other authors have no conflicts to disclose.
Acknowledgments
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2024.05.007.
Appendix. Supplementary Data
Echocardiographic four-chamber view before and after CRT implantation
References
- 1.O’Brien T., Park M.S., Youn J.C., Chung E.S. The past, present and future of cardiac resynchronization therapy. Korean Circ J. 2019;49:384–399. doi: 10.4070/kcj.2019.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zanon F., Baracca E., Pastore G., et al. Multipoint pacing by a left ventricular quadripolar lead improves the acute hemodynamic response to CRT compared with conventional biventricular pacing at any site. Heart Rhythm. 2015;12:975–981. doi: 10.1016/j.hrthm.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq C., Gadler F., Kranig W., et al. A randomized comparison of triple-site versus dual-site ventricular stimulation in patients with congestive heart failure. J Am Coll Cardiol. 2008;51:1455–1462. doi: 10.1016/j.jacc.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh A.J., Vatterott P.J., Garweg C., et al. PO-677-02 Need for cardiac resynchronization therapy upgrade with leadless pacemakers: experience with the Micra transcatheter pacemaker. Heart Rhythm. 2022;19 [Google Scholar]
- 5.Hai J.J., Fang J., Tam C.C., et al. Safety and feasibility of a midseptal implantation technique of a leadless pacemaker. Heart Rhythm. 2019;16:896–902. doi: 10.1016/j.hrthm.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Providencia R., Rogers D., Papageorgiou N., et al. Long-term results of triventricular versus biventricular pacing in heart failure: a propensity-matched comparison. JACC Clin Electrophysiol. 2016;2:825–835. doi: 10.1016/j.jacep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Rogers D.P., Lambiase P.D., Lowe M.D., Chow A.W. A randomized double-blind crossover trial of triventricular versus biventricular pacing in heart failure. Eur J Heart Fail. 2012;14:495–505. doi: 10.1093/eurjhf/hfs004. [DOI] [PubMed] [Google Scholar]
- 8.Lenarczyk R., Kowalski O., Kukulski T., et al. Triple-site biventricular pacing in patients undergoing cardiac resynchronization therapy: a feasibility study. Europace. 2007;9:762–767. doi: 10.1093/europace/eum140. [DOI] [PubMed] [Google Scholar]
- 9.Bordachar P., Gras D., Clementy N., et al. Clinical impact of an additional left ventricular lead in cardiac resynchronization therapy nonresponders: the V3 trial. Heart Rhythm. 2018;15:870–876. doi: 10.1016/j.hrthm.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 10.Gould J., Claridge S., Jackson T., et al. Standard care vs. TRIVEntricular pacing in Heart Failure (STRIVE HF): a prospective multicentre randomized controlled trial of triventricular pacing vs. conventional biventricular pacing in patients with heart failure and intermediate QRS left bundle branch block. Europace. 2022;24:796–806. doi: 10.1093/europace/euab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds D., Duray G.Z., Omar R., et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2015;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 12.Knops R.E., Reddy V.Y., Ip J.E., et al. A dual-chamber leadless pacemaker. N Engl J Med. 2023;388:2360–2370. doi: 10.1056/NEJMoa2300080. [DOI] [PubMed] [Google Scholar]
- 13.Carabelli A., Jabeur M., Jacon P., et al. European experience with a first totally leadless cardiac resynchronization therapy pacemaker system. Europace. 2020;23:740–747. doi: 10.1093/europace/euaa342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funasako M., Neuzil P., Dujka L., et al. Successful implementation of a totally leadless biventricular pacing approach. HeartRhythm Case Rep. 2020;6:153–157. doi: 10.1016/j.hrcr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic four-chamber view before and after CRT implantation