Abstract
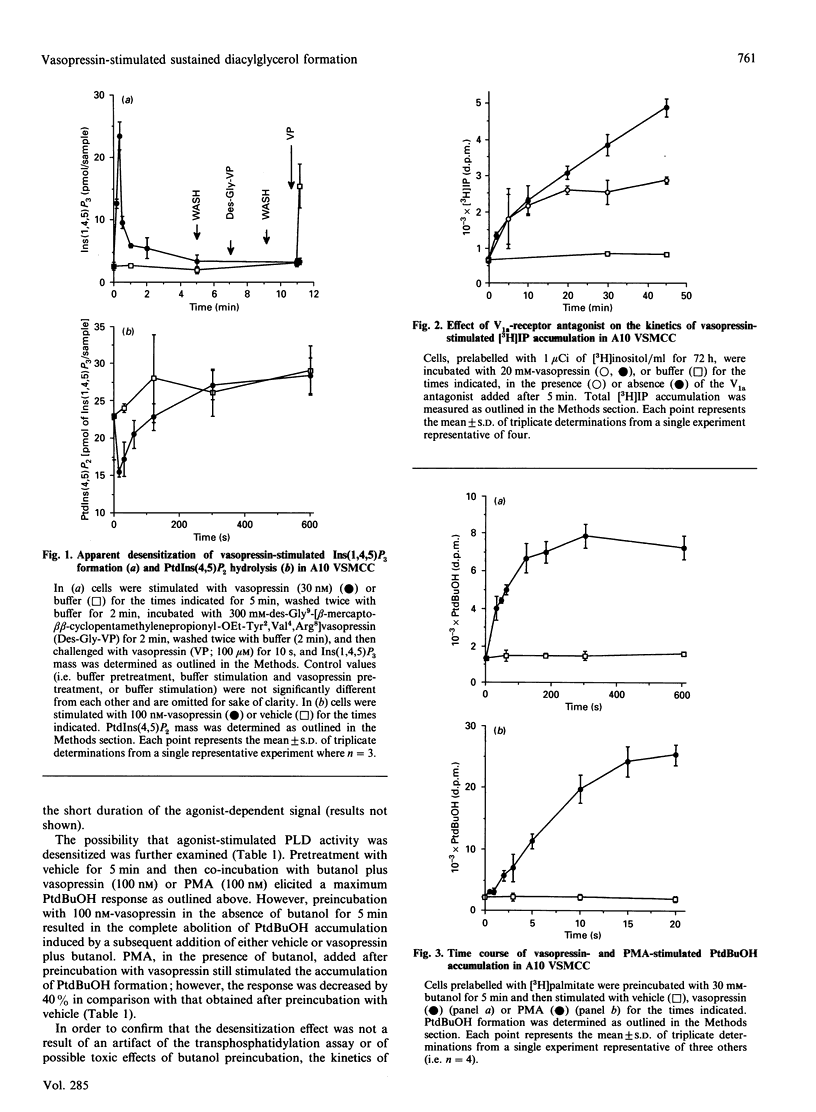
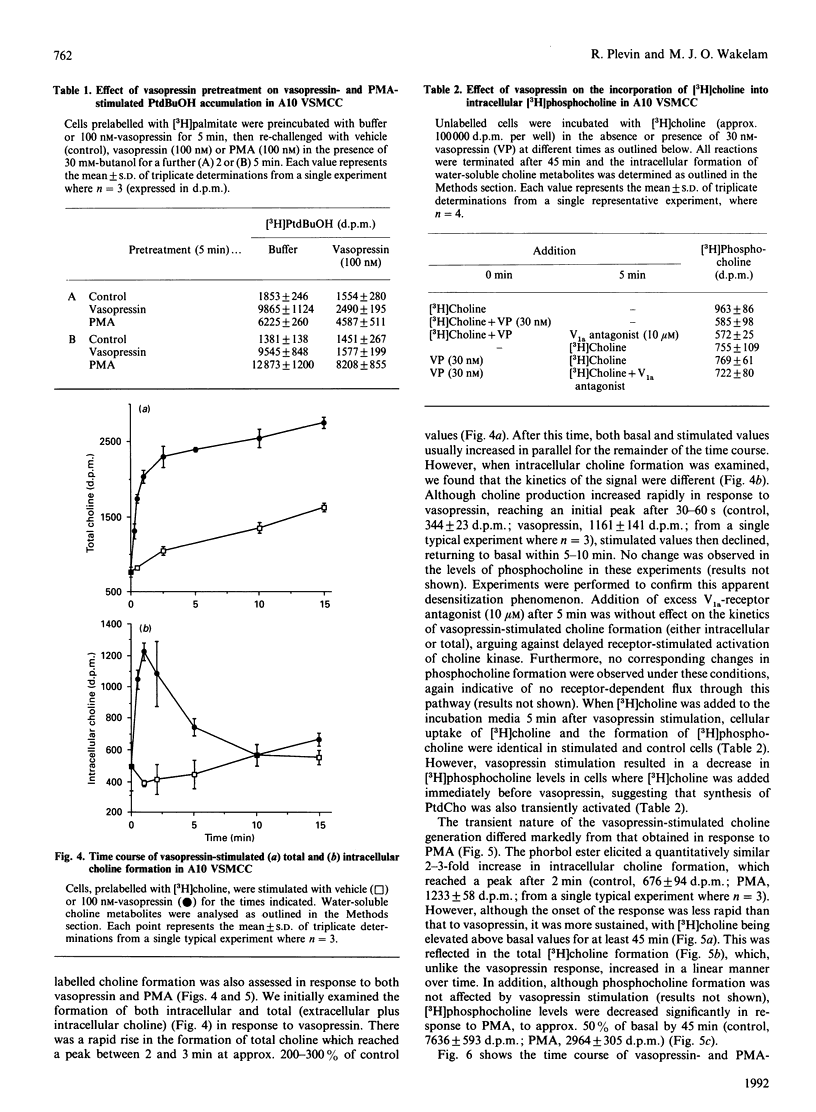
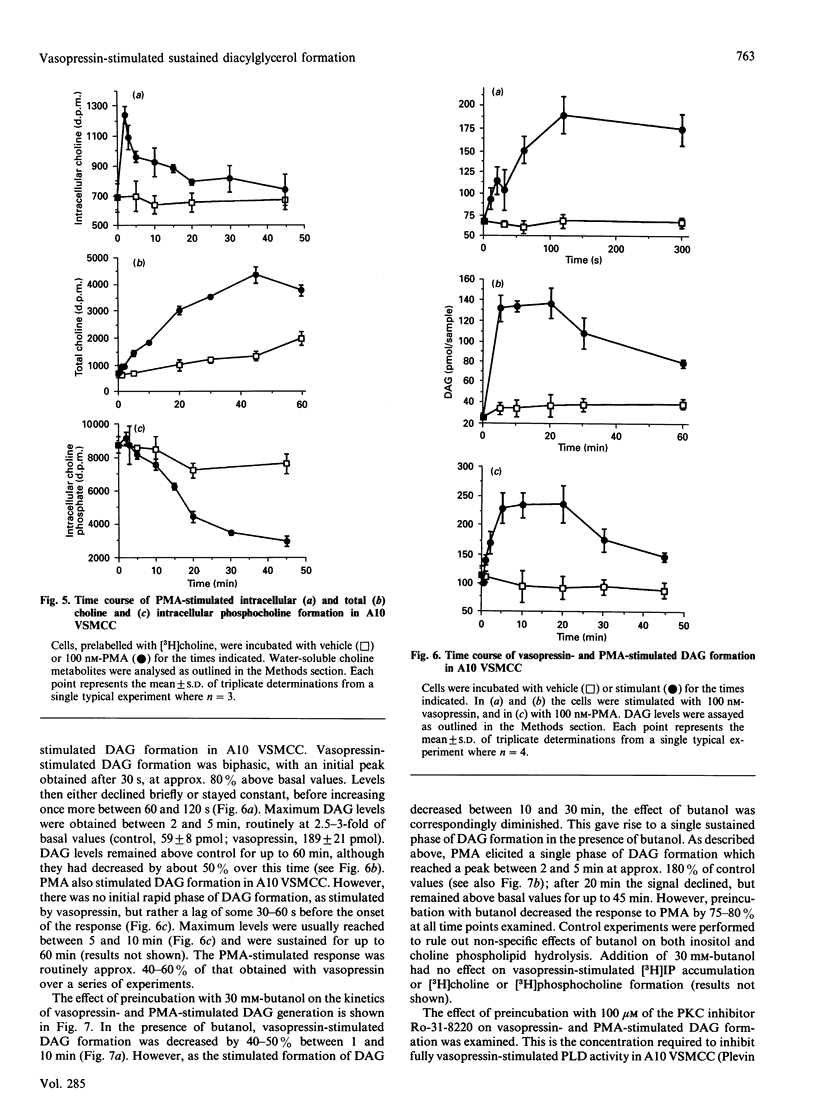
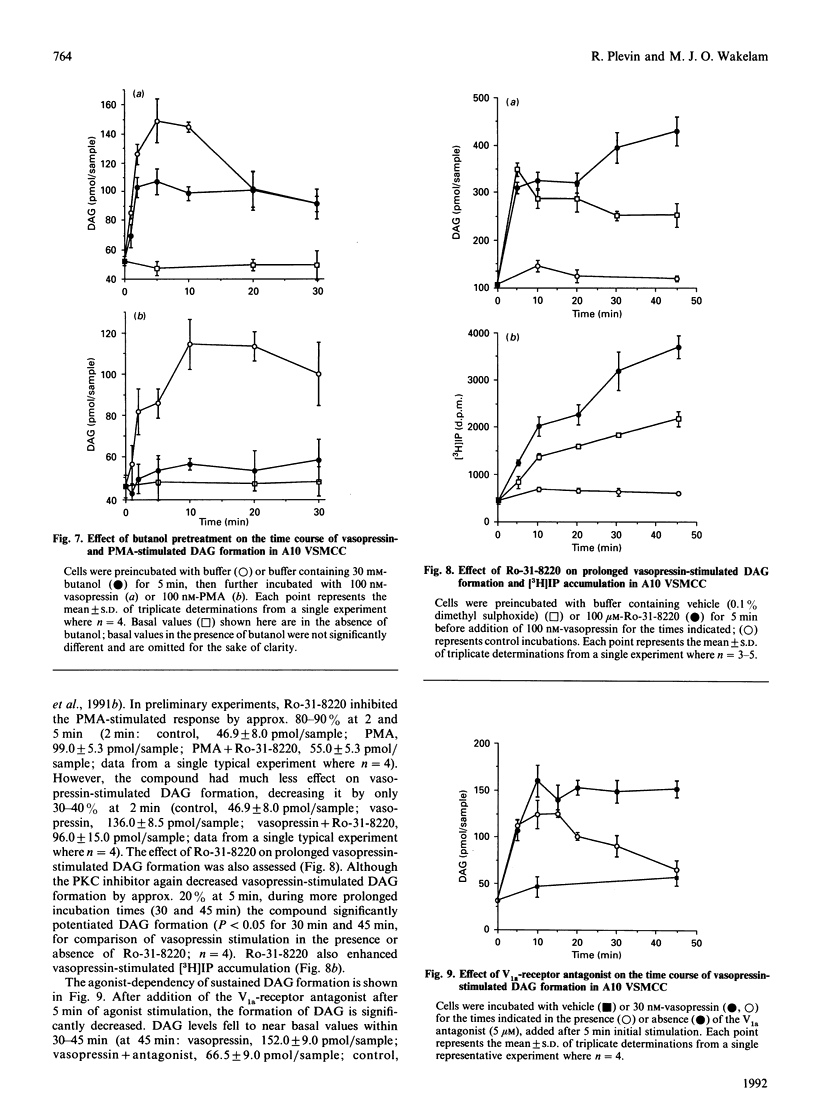
The kinetics of vasopressin-stimulated PtdIns(4,5)P2 and phosphatidylcholine (PtdCho) hydrolysis in relation to sustained diacylglycerol (DAG) formation was investigated in A10 vascular-smooth-muscle cells in culture. Vasopressin stimulated a transient increase in Ins(1,4,5)P3 mass formation, which was mirrored by a decrease in PtdIns(4,5)P2 mass levels. Vasopressin stimulated sustained accumulation of total [3H]inositol phosphates ([3H]IP) in the presence of Li+; however, this was significantly decreased by adding a vasopressin-receptor antagonist at different times after initial stimulation. Vasopressin-stimulated phospholipase D (PLD) activity was found to be a transient phenomenon lasting approx. 2 min. Experiments involving agonist preincubation with subsequent addition of butanol confirmed that vasopressin-stimulated PLD activity was desensitized. Vasopressin stimulated an increase in formation of choline, but not of phosphocholine, suggesting that PLD was the major catalytic route of PtdCho hydrolysis in this cell line. The roles of choline and inositol phospholipid hydrolysis in the prolonged phase of DAG formation was examined by comparing vasopressin-stimulated changes in DAG levels in the presence of butanol, the protein kinase C inhibitor Ro-31-8220 or a V1a-receptor antagonist. Vasopressin-stimulated DAG formation was decreased by 40-50% in the presence of butanol between 1 and 10 min; however, during more prolonged stimulation butanol was without significant effect. In cells pretreated with Ro-31-8220, vasopressin-stimulated DAG formation was decreased by approx. 30% at 2 min, but was significantly potentiated at later times. This coincided with an enhancement of vasopressin-stimulated [3H]IP accumulation. In cells exposed to the V1a-receptor antagonist 5 min after addition of vasopressin, subsequent DAG formation was significantly decreased, indicating that sustained formation of DAG, like [3H]IP accumulation, was dependent on continual agonist receptor activation. The results are discussed in terms of different phospholipid-hydrolytic pathways providing DAG generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., August J., Petrin J. M., Pai J. K. Regulation of sn-1,2-diacylglycerol second-messenger formation in thrombin-stimulated human platelets. Potentiation by protein kinase C inhibitors. Biochem J. 1990 Jul 15;269(2):465–473. doi: 10.1042/bj2690465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop W. R., Ganong B. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers by diacylglycerol kinase. Inhibition by diacylglycerol analogs in vitro and in human platelets. J Biol Chem. 1986 May 25;261(15):6993–7000. [PubMed] [Google Scholar]
- Brown K. D., Littlewood C. J., Blakeley D. M. Differential potentiation of mitogen-stimulated phosphoinositide hydrolysis in protein kinase C-depleted Swiss 3T3 cells. Biochem J. 1990 Sep 1;270(2):557–560. doi: 10.1042/bj2700557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Briscoe C. P., Wakelam M. J. The regulation of phospholipase D activity and its role in sn-1,2-diradylglycerol formation in bombesin- and phorbol 12-myristate 13-acetate-stimulated Swiss 3T3 cells. Biochem J. 1991 Dec 1;280(Pt 2):431–438. doi: 10.1042/bj2800431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Palmer S., Plevin R., Wakelam M. J. Mass measurement of inositol 1,4,5-trisphosphate and sn-1,2-diacylglycerol in bombesin-stimulated Swiss 3T3 mouse fibroblasts. Biochem J. 1990 Jan 15;265(2):617–620. doi: 10.1042/bj2650617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Analysis of the water-soluble products of phosphatidylcholine breakdown by ion-exchange chromatography. Bombesin and TPA (12-O-tetradecanoylphorbol 13-acetate) stimulate choline generation in Swiss 3T3 cells by a common mechanism. Biochem J. 1989 Oct 15;263(2):581–587. doi: 10.1042/bj2630581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. J., Wakelam M. J. Hydrolysis of phosphatidylcholine by phospholipase D is a common response to mitogens which stimulate inositol lipid hydrolysis in Swiss 3T3 fibroblasts. Biochim Biophys Acta. 1991 Apr 17;1092(2):265–272. doi: 10.1016/0167-4889(91)90166-u. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Delafontaine P., Rittenhouse S. E., Gimbrone M. A., Jr, Alexander R. W. Correlation of receptor sequestration with sustained diacylglycerol accumulation in angiotensin II-stimulated cultured vascular smooth muscle cells. J Biol Chem. 1987 Oct 25;262(30):14555–14562. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Grillone L. R., Clark M. A., Godfrey R. W., Stassen F., Crooke S. T. Vasopressin induces V1 receptors to activate phosphatidylinositol- and phosphatidylcholine-specific phospholipase C and stimulates the release of arachidonic acid by at least two pathways in the smooth muscle cell line, A-10. J Biol Chem. 1988 Feb 25;263(6):2658–2663. [PubMed] [Google Scholar]
- Haller H., Smallwood J. I., Rasmussen H. Protein kinase C translocation in intact vascular smooth muscle strips. Biochem J. 1990 Sep 1;270(2):375–381. doi: 10.1042/bj2700375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. F., Cabot M. C. Phorbol diesters stimulate the accumulation of phosphatidate, phosphatidylethanol, and diacylglycerol in three cell types. Evidence for the indirect formation of phosphatidylcholine-derived diacylglycerol by a phospholipase D pathway and direct formation of diacylglycerol by a phospholipase C pathway. J Biol Chem. 1990 Sep 5;265(25):14858–14863. [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Phosphatidylinositol 4,5-bisphosphate turnover is transient while phosphatidylinositol turnover is persistent in thyrotropin-releasing hormone-stimulated rat pituitary cells. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8540–8544. doi: 10.1073/pnas.83.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassègue B., Alexander R. W., Clark M., Griendling K. K. Angiotensin II-induced phosphatidylcholine hydrolysis in cultured vascular smooth-muscle cells. Regulation and localization. Biochem J. 1991 May 15;276(Pt 1):19–25. doi: 10.1042/bj2760019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinson E. A., Trilivas I., Brown J. H. Rapid protein kinase C-dependent activation of phospholipase D leads to delayed 1,2-diglyceride accumulation. J Biol Chem. 1990 Dec 25;265(36):22282–22287. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Ollerenshaw J., Collins P., Heagerty A. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries. Evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem. 1990 May 25;265(15):8921–8928. [PubMed] [Google Scholar]
- Orellana S., Solski P. A., Brown J. H. Guanosine 5'-O-(thiotriphosphate)-dependent inositol trisphosphate formation in membranes is inhibited by phorbol ester and protein kinase C. J Biol Chem. 1987 Feb 5;262(4):1638–1643. [PubMed] [Google Scholar]
- Palmer S., Hughes K. T., Lee D. Y., Wakelam M. J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989;1(2):147–156. doi: 10.1016/0898-6568(89)90004-1. [DOI] [PubMed] [Google Scholar]
- Paterson A., Plevin R., Wakelam M. J. Accurate measurement of sn-1,2-diradylglycerol mass in cell lipid extracts. Biochem J. 1991 Dec 15;280(Pt 3):829–830. doi: 10.1042/bj2800829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin M. S., Baldassare J. J., Raben D. M. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990 May 15;265(14):7959–7966. [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Pfeilschifter J., Bauer C. Different effects of phorbol ester on angiotensin II- and stable GTP analogue-induced activation of polyphosphoinositide phosphodiesterase in membranes isolated from rat renal mesangial cells. Biochem J. 1987 Nov 15;248(1):209–215. doi: 10.1042/bj2480209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin R., Cook S. J., Palmer S., Wakelam M. J. Multiple sources of sn-1,2-diacylglycerol in platelet-derived-growth-factor-stimulated Swiss 3T3 fibroblasts. Evidence for activation of phosphoinositidase C and phosphatidylcholine-specific phospholipase D. Biochem J. 1991 Oct 15;279(Pt 2):559–565. doi: 10.1042/bj2790559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plevin R., Palmer S., Gardner S. D., Wakelam M. J. Regulation of bombesin-stimulated inositol 1,4,5-trisphosphate generation in Swiss 3T3 fibroblasts by a guanine-nucleotide-binding protein. Biochem J. 1990 Jun 15;268(3):605–610. doi: 10.1042/bj2680605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Grzeskowiak M., Della Bianca V., Sbarbati A. De novo synthesis of diacylglycerol from glucose. A new pathway of signal transduction in human neutrophils stimulated during phagocytosis of beta-glucan particles. J Biol Chem. 1991 May 5;266(13):8034–8038. [PubMed] [Google Scholar]
- Saxena U., Klein M. G., Goldberg I. J. Metabolism of endothelial cell-bound lipoprotein lipase. Evidence for heparan sulfate proteoglycan-mediated internalization and recycling. J Biol Chem. 1990 Aug 5;265(22):12880–12886. [PubMed] [Google Scholar]
- Sunako M., Kawahara Y., Hirata K., Tsuda T., Yokoyama M., Fukuzaki H., Takai Y. Mass analysis of 1,2-diacylglycerol in cultured rabbit vascular smooth muscle cells. Comparison of stimulation by angiotensin II and endothelin. Hypertension. 1990 Jan;15(1):84–88. doi: 10.1161/01.hyp.15.1.84. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Bonser R. W., Garland L. G. Receptor-coupled phospholipase D and its inhibition. Trends Pharmacol Sci. 1991 Nov;12(11):404–408. doi: 10.1016/0165-6147(91)90617-2. [DOI] [PubMed] [Google Scholar]
- Welsh C. J., Schmeichel K., Cao H. T., Chabbott H. Vasopressin stimulates phospholipase D activity against phosphatidylcholine in vascular smooth muscle cells. Lipids. 1990 Nov;25(11):675–684. doi: 10.1007/BF02544033. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Neufeld E. J., Majerus P. W. Phosphoinositide interconversion in thrombin-stimulated human platelets. J Biol Chem. 1985 Jan 25;260(2):1046–1051. [PubMed] [Google Scholar]


