Abstract
Objective
Patients with X linked agammaglobulinemia are susceptible to enterovirus (EV) infections. Similarly, severe EV infections have been described in patients with impaired B-cell response following treatment with anti-CD20 monoclonal antibodies (mAbs), mostly in those treated for haematological malignancies. We aimed to describe severe EV infections in patients receiving anti-CD20 mAbs for immune-mediated inflammatory diseases (IMIDs).
Methods
Patients were included following a screening of data collected through the routine surveillance of EV infections coordinated by the National Reference Center and a review of the literature. Additionally, neutralising antibodies were assessed in a patient with chronic EV-A71 meningoencephalitis.
Results
Nine original and 17 previously published cases were retrieved. Meningoencephalitis (n=21/26, 81%) associated with EV-positive cerebrospinal fluid (n=20/22, 91%) was the most common manifestation. The mortality rate was high (27%). EV was the only causal agents in all reported cases. Patients received multiple anti-CD20 mAbs infusions (median 8 (5–10)), resulting in complete B-cell depletion and moderate hypogammaglobulinemia (median 4.9 g/L (4.3–6.7)), and had limited concomitant immunosuppressive treatments. Finally, in a patient with EV-A71 meningoencephalitis, a lack of B-cell response to EV was shown.
Conclusion
EV infection should be evoked in patients with IMIDs presenting with atypical organ involvement, especially meningoencephalitis. Anti-CD20 mAbs may lead to impaired B-cell response against EV, although an underlying primary immunodeficiency should systematically be discussed.
Keywords: autoimmune diseases, rituximab, antirheumatic agents, B-lymphocytes
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
The diagnostic of severe EV infection was often delayed and most often consisted of meningoencephalitis.
A drastic decrease of gammaglobulin levels was not required to present a severe infection.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
EV should be included in the initial microbiological screening panel for fever and/or atypical organ involvement, especially neurological manifestations, in patients with IMIDs due to their high mortality rate.
An underlying primary immunodeficiency should systematically be discussed.
Introduction
Enteroviruses (EV) are a common cause of self-limiting illness but can occasionally be responsible for severe organ involvement leading to organ failure, depending on the patient age, the EV type and the immune status, in particular adaptive immunity.1 Young individuals are at higher risk of lethal infections, especially during their first months of life, with a reported death rate of 11.5%, reaching 30.4% when only considering severe infections.2 3 In this case, hepatitis or coagulopathy (46%) and myocarditis (37.1%) were the two most prevalent complications followed by meningoencephalitis (11%).3 Among patients with primary immunodeficiency (PID), those with a severe or complete gammaglobulin deficiency, such as X linked agammaglobulinemia (XLA), are more prone to severe EV infections, such as chronic meningoencephalitis.4,6 In patients treated with B-cell targeting monoclonal antibodies (mAbs), a cause of acquired hypogammaglobulinemia, severe EV infections have been mainly reported in patients treated for B-cell malignancies.7 This risk has been poorly assessed in patients with immune-mediated inflammatory diseases (IMIDs), in whom EV infections may be underdiagnosed due to non-specific clinical manifestations or IMIDs mimicking organ involvement such as myocarditis or meningoencephalitis. We conducted a multicentre retrospective study combined with a literature review. We also investigated neutralising antibody response after EV infection.
Patients and methods
Original cases and definition of severe EV infections
Patients were included following a retrospective screening of the data collected through the routine surveillance of EV infections by the Enterovirus Surveillance Network (ESN) coordinated by the two laboratories of the National Reference Center (NRC) for EVs (NR Laboratories (NRLs), in Clermont-Ferrand and in Lyon) between 2016 and 2022. Briefly, hospital laboratories of the ESN distributed in all French regions voluntarily report clinical and virological data for each diagnosed EV infection. An appeal was also made by email to the ESN to catch up on certain cases not previously reported within the surveillance system. EV-positive samples are sent to the NRLs to identify the EV type. Identification was performed by sequencing of the 1D gene encoding the VP1 capsid protein, as previously described.8 9
Patients were eligible if they had a documented EV infection after at least one infusion of anti-CD20 mAbs for an IMID. Only patients with a severe infection defined as a non-self-resolving infection with organ involvement were included, given the potential lethal outcome and the need for urgent therapeutical intervention. Benign infections were not included owing to a potential bias linked to an absence of systematic screening and report, even in immunocompromised patients with IMIDs. Patients with PID who were treated with anti-CD20 mAbs for diseases other than IMIDs, such as haematological malignancies, were excluded. For each patient, we collected data prior to the EV infection (demographic, features and management of the IMID, number of anti-CD20 infusions and gammaglobulin level), related to the EV infection diagnosis (time to diagnosis, organ involvement, EV identification and type, gammaglobulin level and CD19+ B-cells count) and its management (treatments and outcomes). Outcomes were defined as survival without sequelae, survival with sequelae or death at the last evaluation.
Literature review
We searched PubMed and Embase for publications on case reports of EV infections following anti-CD20 therapy up to November 2023. The following search terms (without any filter) were used: (enterovirus or enteroviral or echovirus or coxsackievirus) AND (rituximab or ocrelizumab or obinutuzumab or ofatumumab or anti-CD20). Relevant articles were independently evaluated by GMdF and JH and selected considering the title, abstract and full text. One published case not identified in the PubMed and Embase search was identified via the NRL. Articles not related to severe EV infections and/or anti-CD20 therapy and/or patients with IMIDs were excluded. Unpublished abstracts were not included in the review.
Neutralisation assay
The immune response to EV infection was studied for one patient with AAV treated with rituximab, who suffered from a chronic meningoencephalitis associated with EV-A71 subgenotype C1. The production of neutralising antibodies to EV-A71 subgenotype C1 was studied in five serum samples collected on 21 January 22 (earliest EV-positive detection), 20 June 2022, 1 August 2022, 29 August 2022 and 25 October 2022. The test virus was a subgenotype C1 EV-A71 strain (designated C1-16) isolated from a clinical sample obtained in 2016 by the NRL (Clermont-Ferrand, France). The virus strain was propagated in rhabdomyosarcoma (RD) cell line obtained from the European Collection of Authenticated Cell Culture (Cat. No. 8511502). RD cells, used at passages between 44 and 80, were cultured in Dulbecco’s Modified Eagle Medium (DMEM) high glucose (Dutscher) containing 2 mM glutamine, 10% fetal bovine serum (FBS; Eurobio) and 1% streptomycin-penicillin. The virus stock used in neutralisation tests was prepared in RD cells and checked by sequencing the complete viral genome.10 The infection titre was determined by a titration assay based on the limiting dilution method reported earlier.11 The neutralising activity of antibodies to C1-16 EV-A71 was assessed with a live virus neutralisation assay. After heat inactivation at 56°C for 30 min, all serum samples were diluted with DMEM without FBS serially twofold from 1:4 to 1:2018. The neutralisation assay was performed with 100 viral particles dispensed in 96-well culture plate. 50 µL of diluted serum was mixed with 50 µL of virus suspension. After incubating at 37°C for 1.5 hours, an RD cell suspension containing 1.2×104 cells/well was added, and the plates were incubated at 37°C for 6 days. Each serum was tested in triplicate and in each test, batch included controls tested in duplicate: cell control, virus control (no antibody) and serum control (no virus). A positive serum control was included (polyvalent immunoglobulins (PolyIg) diluted serially twofold and tested with and without virus). PolyIg sample was kindly provided by K. Coudéré and K. Benschop from the ENPEN network. The cytopathic effect was checked daily by light microscopy. The neutralisation titre was defined as the highest dilution that exhibited >50% neutralisation of the cytopathic effect. Neutralising titres ≥1:8 were defined as indicative of preserved immunity.12 13
Statistical analyses
Data were extracted from the patients’ medical records. Qualitative variables are reported as number (%), and quantitative variables are reported as median (IQR).
Results
Patient characteristics at the time of diagnosis of EV infection
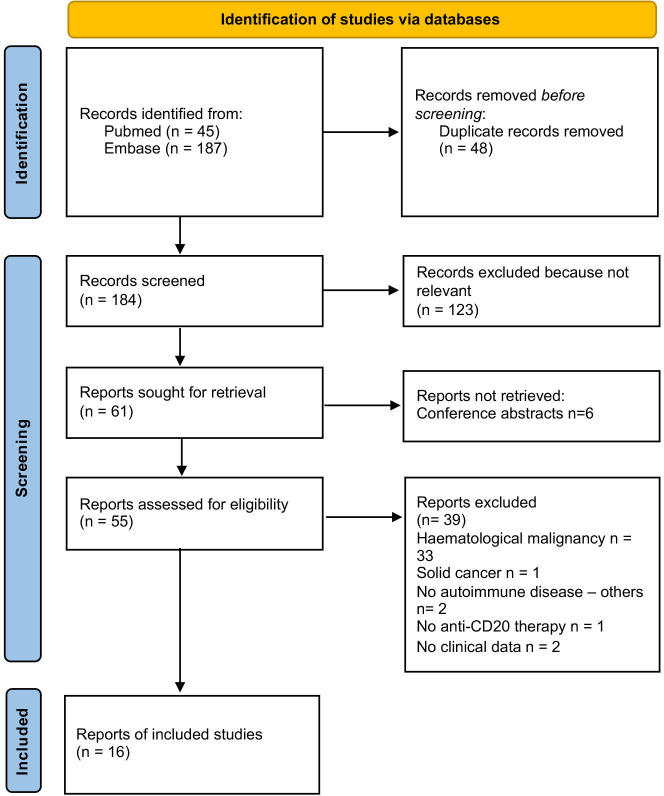
We identified a total of 26 cases of severe EV infections in patients with IMIDs, including 9 original cases and 17 published cases (flow diagram for literature review is presented in figure 1).714,29 Between 2016 and 2022, 14 013 EV infections were reported by the ESN, including 2968 in adult individuals. Of these, 26 (21.3%) out of 122 severe infections occurred in immunocompromised patients. Eleven (42.3%) patients had IMIDs treated with anti-CD20 mAbs (including two cases already published).
Figure 1. Flow chart of the study.
Patient characteristics are reported in table 1. The most common underlying IMIDs were rheumatoid arthritis (n=7, 27%), vasculitis (n=4, 15%) (three AAV and one pulmonary capillaritis) and primary autoimmune cytopenia (n=4, 15%). The median age at IMID diagnosis was 22 (15–29) years and the median disease duration before EV infection was 7 (5–12) years. No patient had a history of recurrent infections suggesting an underlying PID and only one patient had a notable history of self-resolving EV meningitis 3 years before. Only one (8%, data available for 13) patient with a history of treatment with fingolimod and dimethyl-fumarate had hypogammaglobulinemia (5.3 g/L) before anti-CD20 mAbs initiation. Patients had received a median of 1 (1–3) immunosuppressive drug before anti-CD20 mAbs initiation and one patient had undergone autologous stem cell transplant for autoimmune thrombocytopenia 20 months before rituximab initiation. Anti-CD20 mAbs consisted of rituximab infusions in 25 (96%) patients and ocrelizumab in 1 patient. Patients had received a median of 8 (5–10) anti-CD20 infusions with a median cumulated dose of 6 g (4.7–8). At the time of diagnosis of EV infection, four (18%) patients had a concomitant immunosuppressive treatment associated with anti-CD20 mAbs infusions and 4/19 (21%) had received >10 mg/day for >2 weeks of glucocorticoids within the year. Among the 19 patients with available lymphocyte phenotyping, all had a marked B-cell depletion and most (n=14/23, 61%) showed hypogammaglobulinemia (gammaglobulin or IgG <6 g/L) with a median level of gammaglobulin or IgG of 4.8 g/L (4.2–6.2) (table 1). The median decrease of gammaglobulin or IgG level following anti-CD20 mAbs treatment was 45% (n=11 (30–49)).
Table 1. Patient’s characteristics at EV infection onset.
| All casesN=26 | Original casesN=9 | Published casesN=17 | |
| Female | 19 (73%) | 6 (67%) | 13 (76%) |
| IMIDs | |||
| Rheumatoid arthritis | 7 (27%) | 3 (33%) | 4 (24%) |
| Vasculitis | 4 (15%) | 2 (22%) | 2 (12%) |
| Primary autoimmune cytopenia | 4 (15%) | 0 | 4 (24%) |
| Multiple sclerosis | 3 (12%) | 2 (22%) | 1 (6%) |
| Systemic lupus | 2 (8%) | 1 (11%) | 1 (6%) |
| Minimal change disease | 2 (8%) | 0 | 2 (12%) |
| Others* | 4 (15%) | 1 (11%) | 3 (18%) |
| Age at IMID diagnosis, years (IQR) (n=23/9/14) | 22 (15–29) | 26 (16–30) | 19 (14–24) |
| IMID duration, years (IQR) (n=23/9/14) | 7 (5–12) | 9 (8–20) | 6 (4–7) |
| Prior notable infection | 1 (4%) | 1 (11%) | 0 |
| Biological characteristics | |||
| Gammaglobulin or IgG level (g/L), median (IQR) (n=23/9/14) | 4.9 (4.3–6.7) | 5.0 (4.4–7.0) | 4.9 (4.2–6.0) |
| IgM level (g/L), median (IQR) (n=11/5/6) | 0.20 (0.12–0.45) | 0.30 (0.2–0.4) | 0.17 (0.11–0.43) |
| IgA level (g/L), median (IQR) (n=11/5/6) | 0.79 (0.50–1.07) | 1.1 (0.8–1.5) | 0.50 (0.33–0.90) |
| CD19+ B cells (/mm3), median (IQR) (n=20/8/12) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Treatments | |||
| Number of lines of treatment before anti-CD20 mAbs, median (IQR) | 1 (0–3) | 1 (0–3) | 1 (1–3) |
| Number of previous anti-CD20 mAbs infusions, median (IQR) (n=22/8/14) | 8 (5–10) | 8 (6–9) | 8 (5–10) |
| Time since last anti-CD20 mAbs infusion, months (IQR) (n=21/9/12) | 6 (3–13) | 4 (3–7) | 8 (4–14) |
| Steroid treatment (>10 mg/day for >2 weeks in the last year) | 4/19 (21%) | 3/9 (33%) | 1/10 (10%) |
| Associated immunosuppressive treatment | 4 (15%) | 1 (11%) | 3 (18%) |
| Methotrexate | 2 (8%) | 1 (11%) | 1 (6%) |
| Azathioprine | 1 (4%) | 0 | 1 (6%) |
| TNF inhibitor | 1 (4%) | 1 (11%) | 0 |
| Leflunomide | 1 (4%) | 0 | 1 (6%) |
| Hydroxychloroquine | 2 (8%) | 0 | 2 (12%) |
When data were missing, the number of cases for which the data wereas available is indicated.
Normal IgG level: 7–13 g/L; normal IgM level: 0.5–2.1 g/L; normal IgA level: 0.7–3.4 g/L.
Consisting inof: thrombotic thrombocytopenic purpura (n=1), psoriatic arthritis (n=1), anti-MOG -associated disease (n=1) and Devic’s disease (n=1).
d, day; EVenterovirusF, female; IMID, immune-mediated inflammatory diseases; IQR, interquartile range; M, male; mAbs, monoclonal antibodies; MOG, myelin oligodendrocyte glycoprotein; TNF, tumour necrosis factor
Characteristics, management and outcome of EV infections
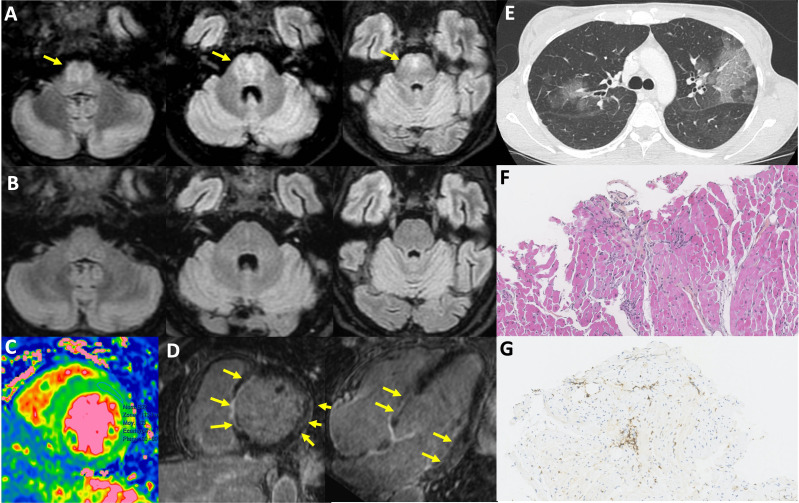
Main clinical manifestations were fever (n=19, 73%) and diverse neurological symptoms (n=21, 81%), including confusion, headaches, cognitive impairment, focal symptoms, tremor, ataxia or dysarthria (table 2). Twenty-two patients had cerebrospinal fluid (CSF) evaluation, including one without neurological symptoms: 16/20 (80%) had increased protein level (defined as >0.4 g/L, median 0.66 g/L (0.42–0.91)) and 18/20 (85%) had hypercellularity (defined as >5/mm3, median 28/mm3 (13–64)). Cerebral MRI was abnormal in 11/18 (61%). Other organ involvement included ear, nose and throat manifestations (n=12, 46%), mostly consisting of hearing loss, muscle (n=7, 27%), myocarditis (n=6, 23%), liver (n=5, 19%), skin (n=4, 15%), lung (n=2, 8%), eye (n=1, 4%) and gastrointestinal (n=1, 4%) manifestations. Organ involvements are illustrated in figure 2.
Table 2. Enteroviral infections: characteristics, management and outcome.
| All casesN=26 | Original casesN=9 | Published casesN=17 | |
| Manifestations and organ involvement | |||
| Fever | 19 (73%) | 8 (89%) | 11 (65%) |
| Neurological | 21 (81%) | 8 (89%) | 13 (76%) |
| CSF protein level (g/L), median (IQR) (n=20/9/11) | 0.66 (0.42–0.91) | 0.43 (0.40–0.70) | 0.83 (0.55–0.96) |
| CSF cellularity (/mm3), median (IQR) (n=20/8/12) | 28 (13–64) | 17 (7–26) | 35 (23–119) |
| Brain MRI abnormalities (n=18/9/9) | 11 (61%) | 4 (44%) | 7 (78%) |
| ENT | 12 (46%) | 4 (44%) | 8 (47%) |
| Muscular | 7 (27%) | 2 (22%) | 5 (29%) |
| Cardiac | 6 (23%) | 2 (22%) | 4 (24%) |
| Leading to end-stage cardiac failure | 4 (15%) | 0 | 4 (24%) |
| Liver | 5 (19%) | 2 (22%) | 3 (18%) |
| Leading to end-stage liver failure | 2 (8%) | 0 | 2 (12%) |
| Skin | 4 (15%) | 1 (11%) | 3 (18%) |
| Lung | 2 (8%) | 1 (11%) | 1 (6%) |
| Eye | 1 (4%) | 1 (11%) | 0 |
| Gut | 1 (4%) | 0 | 1 (6%) |
| EV-positive samples | |||
| Blood | 11 (42%) | 4 (44%) | 7 (41%) |
| CSF | 20 (77%) | 9 (100%) | 11 (65%) |
| Stools | 3 (12%) | 1 (11%) | 2 (12%) |
| Organ biopsy | 9 (35%) | 0 | 9 (53%) |
| Nasopharyngeal swab | 1 (4%) | 0 | 1 (6%) |
| BAL | 2 (8%) | 1 (11%) | 1 (6%) |
| EV types | |||
| EV-A71 | 6 (23%) | 4 (44%) | 2 (12%) |
| Coxsackievirus | 7 (27%) | 2 (22%) | 5 (29%) |
| Echovirus | 5 (19%) | 1 (11%) | 4 (24%) |
| Unknown | 8 (31%) | 2 (22%) | 6 (35%) |
| Treatments | |||
| IVIg | 21 (81%) | 8 (89%) | 13 (76%) |
| Steroids | 6 (23%) | 2 (22%) | 4 (24%) |
| Organ transplant | 3 (12%) | 0 | 3 (18%) |
| Others* | 6 (23%) | 0 | 6 (35%) |
| Outcomes | |||
| Survival without sequelae | 13 (50%) | 7 (78%) | 6 (35%) |
| Survival with sequelae | 6 (23%) | 1 (11%) | 5 (29%) |
| Death | 7 (27%) | 1 (11%) | 6 (35%) |
Consisting of fluoxetine (n=3), fluoxetine and pocapavir (n=1), pleconaril (n=1), acyclovir.
n=1n=1BAL, bronchoalveolar lavage; CSF, cerebrospinal fluid; ENT, ear, nose, throat; EV, enterovirus; IQR, interquartile range; IVIgintravenous immunoglobulinIVIg, intravenous immunoglobulinn=3MRI, magnetic resonance imaging
Figure 2. Organ involvement in severe enteroviral infections. (A) Brain MRI taken in June 2022 showing centro-pontine fluid-attenuated inversion recovery (FLAIR) hyperintensity without enhancement for the patient presented in figure 3; (B) brain MRI showing complete regression of abnormalities in September 2022 (same patient); (C) diffuse myocardial oedema (MRI, T2 mapping); (D) septal, basal lateral and lateral medial enhancement (MRI, late gadolinium enhancement); (E) multifocal ground glass opacities; (F and G) lymphocytic myocarditis with interstitial T cell (G:CD3+ cells) foci, dystrophic myocytes, no giant cell or eosinophilic infiltration, no myocardial necrosis.
Among 22 patients with CSF assessment, EV genome was detected in 20 (91%), 1 was not tested for EV and 1 was negative but positive on brain biopsy. Viraemia was detected in 11 (42%) patients and biopsy-proven organ involvement (heart, muscle, brain, liver or skin) was obtained in 9 (35%) patients. Two patients had negative EV genome detection in organ biopsies (cardiac and muscular) despite histological inflammation and positive EV detection in other clinical specimens. When performed, EV genome was also detected in stools, bronchoalveolar lavage fluid or nasopharyngeal swab. The median time between symptoms onset and EV identification was 1 month (0.1–6). The EV type was identified or reported for 18 cases: 6 infections (23%) were associated with EV-A71, 7 (27%) with coxsackieviruses (CVA9, B2, B3, B4 or B5) and 5 (19%) with echoviruses (E-9, 11, 18 and 25). All patients with EV-A71/C1-associated infection had neurological involvement whereas CVs were the most frequent EVs found in patients with cardiac involvement (in three out of four with available EV type).
Therapeutic management of EV infection mainly consisted of intravenous immunoglobulin (IVIg) (n=21, 81%), with highly variable doses and durations of administration. Glucocorticoids were initially used in six (23%) patients mostly because of missing diagnosis. Other treatments included fluoxetine (n=5), pocapavir (n=1), pleconaril (n=1) and acyclovir (n=1). Thirteen (50%) patients survived without sequelae, six (23%) with sequelae and seven (27%) died. Sequelae included hearing loss in two patients and heart transplant, liver transplant, cognitive–behavioural disorders or decreased visual acuity in one case each. Causes of death were infectious pneumonitis in patients with neurological involvement (n=2), fulminant myocarditis (n=2), ischaemic stroke after brain biopsy (n=1), fulminant hepatitis (n=1) and postheart transplant multiorgan failure (n=1).
Neutralising antibody response against EV in a patient with AAV
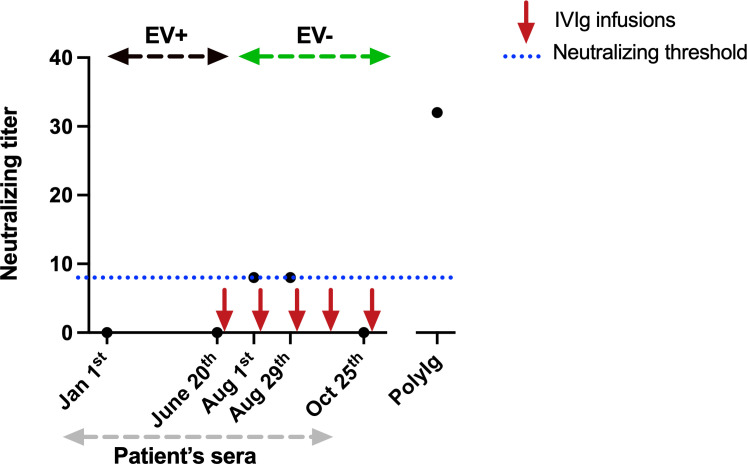
We performed neutralisation assays (figure 3) in one patient with AAV treated with repeated courses of rituximab (last infusion in December 2021). In January 2022, he progressively developed psychomotor slowing suggesting depression. In May 2022, he presented with dysarthria, walk impairment with tetra pyramidal syndrome and memory loss and was diagnosed with an EV-A71 meningoencephalitis in June 2022 (subgenotype C1, identified in blood and CSF). Brain MRI is shown in figure 2A. EV viraemia was negative in December 2021 but positive in January 2022 at the onset of neurological symptoms. He was treated with high-dose IVIg (2 g/kg/course) every month from June 2022. After IVIg initiation, the patient showed sustained negative EV RT-PCRs in blood and CSF. After three IVIg infusions, he only had mild hyper-reflexia and his brain MRI normalised (figure 2B). Neutralisation assays showed that sera from January, June and October 2022 had no neutralising activity against EV-A71 whereas the two sera from August only showed weak neutralising activity (after several IVIg infusions), suggesting an impairment to develop efficient humoral response against EV under rituximab.
Figure 3. Sera neutralisation titre overtime in one of the reported patients with chronic EV-A71 meningoencephalitis. EV, enterovirus; EV+, positive EV RT-PCR in blood and cerebrospinal fluid (CSF); EV−, negative EV RT-PCR in blood and CSF; IVIg, intravenous immunoglobulin; PolyIg, polyvalent immunoglobulins used as a positive control. Each point represents a serum sample.
Discussion
We report here 9 original and 17 previously published cases of severe EV infections in patients treated with anti-CD20 mAbs for IMIDs. Most patients developed EV infection after repeated anti-CD20 infusions despite limited concomitant immunosuppressive treatments. Meningoencephalitis with identification of EV in the CSF was the most frequent manifestation. The mortality rate was high despite identification of the EV as the causal agent in all reported cases.
Anti-CD20 mAbs lead to an impaired B-cell response as illustrated by the lack of anti-EV neutralising antibody response in one of our patients. This B-cell impairment has also been shown in vaccination response and infection susceptibility, for example, for SARS-CoV-2 infection,30 31 but severe EV infections are not typical in this setting. All the patients from this case series were diagnosed with severe EV infections in adulthood. Regardless of immune status, although EV infections, including severe cases, are mainly reported in children, adults represent around 25% of EV-infected patients each year in France.30 In patients with PID, the age of onset of severe EV infections is known to be highly variable and not limited to childhood.6 EV infections in immunocompromised adults should thus not be underestimated. Our patients also had profound B-cell depletion and frequent hypogammaglobulinemia, although not reaching the drastically low levels observed in patients with XLA, which could have had a potential impact on their susceptibility to developing EV infections. Altogether, these observations would suggest that treatment with anti-CD20 mAbs alone may not be adequate to promote EV infections but could serve as an additional factor contributing to susceptibility in predisposed patients. Therefore, a PID should be systematically discussed in these patients.
Similar to patients with primary B-cell immunodeficiency, meningoencephalitis and identification of EV in the CSF were very common in this case series,6 highlighting the neurotropism of EV. Remarkably, heart involvement was frequent in the patients compared with what has been described in B-cell PID.5 6 Among all the identified EV in this study, EV-A71, especially the emergent variant C1, and CVs B-associated infections, are both characterised by more frequent central nervous system (CNS) and cardiac involvement, respectively, similar to what is observed in immunocompetent patients, although mostly reported in young children.32 Poorly specific presentations often led to delayed EV infection diagnosis even though EV genome detection, now routinely performed in most laboratories, was frequently positive in several fluids and/or tissues. Detection of the EV genome in blood or CSF establishes with certainty the diagnosis of an ongoing EV infection. Detection of EV in throat or stool samples may increase the chances of diagnosis due to prolonged shedding of EV, although a positive result may reflect a past infection. Organ biopsies were rarely required to identify the virus and should only be performed after multiple fluids assessment.
A high mortality associated with severe EV infections was observed, especially in patients with heart involvement. However, most of the lethal infections were among published cases, thus potentially reflecting a publication bias. In addition, only half of the patients survived without sequelae. Due to the rarity of this condition, treatment strategy is not codified. IVIg were often used with variable dose and efficiency. Early treatment with high-dose IVIg could be suggested, as in XLA, owing to a plausible effect of IVIg and limited side effects (particular attention should be given to patients with heart involvement).5 Dose and frequency should then be adapted to symptoms, gammaglobulin level and EV infection diagnosis. Moreover, their effectiveness is highly dependent on the presence of sufficient levels of neutralising antibodies specific to the type of EV responsible for the infection, although not correlated with a high level of neutralising antibodies (as seen in figure 3). Some studies have also mentioned a protective effect of fluoxetine which inhibits viral replication in vitro.33 Other compassionate drugs such as pocapavir and pleconaril could be alternative options.34 Our study has limitations. Clinical data collected through the ESN might be incomplete regarding the immune status of EV-infected patients, and EV infections may not be reported as severe. This may explain the low number of patients in our study. However, all EV infections with CNS or cardiac involvement tend to be exhaustively explored limiting the number of missing data. Also, this study focused on severe EV infections and lack of denominator data. It would be interesting to estimate the prevalence of EV infections, their severity and outcomes in the growing number of patients treated with CD20 mAbs for IMIDs.VIg can be used as a first-line treatment, similar to patients with XLA with EV infections, due to its limited side effects.
In conclusion, EV genome detection should be included in the initial microbiological screening for patients with IMIDs treated with anti-CD20 mAbs who exhibit atypical and refractory organ involvement, particularly meningoencephalitis. EV infections should not only be suspected in patients with a history of recurrent infections or profound hypogammaglobulinemia. IVIg can be used as a first-line treatment, similar to patients with XLA with EV infections, due to its limited side effects. Prospective studies with systematic screening for EV infections in patients treated with IMIDs and anti-CD20 mAbs are needed to assess their incidence (overall and severe), as well as the incidence of asymptomatic viral replication and to identify associated risk factors. A systematic approach to genetic screening for an underlying immunodeficiency would help understand the pathophysiology of this rare but severe complication.
Acknowledgements
We thank for their help Dr Soumaya Sridi-Cheniti (Department of Radiology, CHU Bordeaux) and Dr Marie Jeanneau-Caron (Department of Pathology, CHU Bordeaux). We thank K Coudéré and K Benschop from the ENPEN network, who provided the polyIg sample and Jean-Luc Bailly (Auvergne University, LMGE UMR CNRS 6023, Team Epidemiology and Pathophysiology of Enterovirus Infection, Clermont-Ferrand, France) for his assistance with the serum neutralisation tests. We are grateful to all members of the EV surveillance network in Amiens (Dr Marie Louchet Ducoroy), Angers (Dr Caroline Lefeuvre, Professor Alexandra Ducancelle), Bayonne (Drs David Leyssene and Anne-Christine Jaouen), Besançon (Professor Quentin Lepiller), Bordeaux (Professor Marie-Edith Lafon and Sonia Burrel, Dr Camille Tumiotto), Bourgoin-Jallieu (Drs Tellini and Doat), Brest (Dr Léa Pilorgé and Professor Christopher Payan), Caen (Dr Cécile Schanen, Professor Astrid Vabret), Dijon (Dr Katia Balay, Professor Alexis de Rougemont), Frejus (Dr Gillon), Grenoble (Dr Sylvie Larrat), Lille (Dr Mouna Lazrek, Professor Didier Hober), Limoges (Professors Sylvie Rogez and Sophie Alain), Mantes-La-Jolie (Dr Emeline Riverain), Marseille (Drs Antoine Nougairède and Laetitia Ninove), Montpellier (Dr Vincent Foulongne, Professor Philippe Van de Perre), Nancy (Dr Véronique Vénard, Professor Evelyne Schvoerer), Nantes (Dr Marianne Coste-Burel), Nice (Dr Gonfrier), Orléans (Dr Clémence Guillaume and Jerôme Guinard), Paris—Cochin (Dr Anne-Sophie L’honneur, Professor Véronique Avettand Fenoël), Paris—Necker (Drs Hanène Abid, Marianne Burgard, Marianne Leruez-Ville), Paris—St Louis (Dr Maud Salmona, Professor Jérôme Legoff), Paris—Trousseau (Drs Kenda Saloum, Aurélie Schnuriger), Poitiers (Dr Agnès Beby-Defaux, Professor Nicolas Lévêque), Reims (Professor Laurent Andreoletti), Rennes (Dr Gisèle Lagathu, Professor Vincent Thibault), Roanne (Drs Jean-Benjamin Murat, C Brechet), Rouen (Dr Véronique Lémée, Professor Jean-Chirstophe Plantier), St Etienne (Dr Sylvie Pillet, Professor Bruno Pozzetto), Strasbourg (Dr Floriane Gallais, Professor Samira Fafi-Kremer), Suresnes (Dr Eric Farfour), Toulouse (Drs Jean-Michel Mansuy and Pauline Trémeaux, Professor Jacques Izopet), Toulon-CHI (Drs Anne-Lise Toyer and Cécile Poggi), Tours (Dr Karl Stefic, Professor Catherine Gaudy), Versailles (Dr Stéphanie Marque-Juillet), Villefranche (Dr Marine Jourdain).
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics approval: This retrospective study was approved by the Institution Review Board of the Cochin University Hospital, under the number AAA-2023-09042. This study was conducted in compliance with Good Clinical Practices and the Declaration of Helsinki principles. All patients consented to the use of their anonymised medical data.
Contributor Information
Grégoire Martin de Frémont, Email: gregoire.martin-de-fremont@aphp.fr.
Hélène Chabrolles, Email: hchabrolles@chu-clermontferrand.fr.
Audrey Mirand, Email: amirand@chu-clermontferrand.fr.
Anne Sophie L'Honneur, Email: anne-sophie.lhonneur@aphp.fr.
Nicolas Mélé, Email: N.MELE@ghu-paris.fr.
Bertrand Dunogue, Email: bertrand.dunogue@aphp.fr.
David Boutboul, Email: david.boutboul@aphp.fr.
Meryem Farhat, Email: Meryem.FARHAT@chu-lille.fr.
Eric Hachulla, Email: Eric.HACHULLA@chu-lille.fr.
Mouna Lazrek, Email: mouna.lazrek@chu-lille.fr.
Virginie Rieu, Email: vrieu@chu-clermontferrand.fr.
Alexis Mathian, Email: alexis.mathian@aphp.fr.
Helene Chaussade, Email: helene.chaussade@chu-bordeaux.fr.
Aurelie Ruet, Email: aurelie.ruet@chu-bordeaux.fr.
Sonia Burrel, Email: Sonia.burrel@chu-bordeaux.fr.
Fabienne Coury-Lucas, Email: fabienne.coury-lucas@chu-lyon.fr.
Isabelle Schuffenecker, Email: isabelle.schuffenecker@chu-lyon.fr.
Adrien Lemaignen, Email: adrien.lemaignen@univ-tours.fr.
Karl Stefic, Email: Karl.stefic@univ-tours.fr.
Maelle le Besnerais, Email: Maelle.Le-Besnerais@chu-rouen.fr.
Marion Carrette, Email: marion.carrette@chu-rouen.fr.
Luc Mouthon, Email: luc.mouthon@aphp.fr.
Veronique Avettand-Fenoel, Email: veronique.avettand@aphp.fr.
Benjamin Terrier, Email: benjamin.terrier@aphp.fr.
Jérome Hadjadj, Email: jerome.hadjadj@aphp.fr.
Data availability statement
Data are available on reasonable request.
References
- 1.Harvala H, Broberg E, Benschop K, et al. Recommendations for Enterovirus diagnostics and Characterisation within and beyond Europe. J Clin Virol. 2018;101:11–7. doi: 10.1016/j.jcv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Khetsuriani N, Lamonte A, Oberste MS, et al. Neonatal Enterovirus infections reported to the National Enterovirus surveillance system in the United States, 1983-2003. Pediatr Infect Dis J. 2006;25:889–93. doi: 10.1097/01.inf.0000237798.07462.32. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Wang H, Tang J, et al. Clinical characteristics of severe neonatal Enterovirus infection: a systematic review. BMC Pediatr. 2021;21:127. doi: 10.1186/s12887-021-02599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bearden D, Collett M, Quan PL, et al. Enteroviruses in X-linked Agammaglobulinemia: update on epidemiology and therapy∗. J Allergy Clin Immunol Pract. 2016;4:1059–65. doi: 10.1016/j.jaip.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Paccoud O, Mahlaoui N, Moshous D, et al. Current spectrum of infections in patients with X-linked Agammaglobulinemia. J Clin Immunol. 2021;41:1266–71. doi: 10.1007/s10875-021-01043-1. [DOI] [PubMed] [Google Scholar]
- 6.Halliday E, Winkelstein J, Webster ADB. Enteroviral infections in primary immunodeficiency (PID): A survey of morbidity and mortality. J Infect. 2003;46:1–8. doi: 10.1053/jinf.2002.1066. [DOI] [PubMed] [Google Scholar]
- 7.Tellez R, Lastinger AM, Hogg JP. Chronic Enteroviral Meningoencephalitis in a patient on Rituximab for the treatment of Psoriatic arthritis: A case report and brief literature review. IDCases. 2019;17:e00558. doi: 10.1016/j.idcr.2019.e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirand A, le Sage FV, Pereira B, et al. Ambulatory pediatric surveillance of hand, foot and mouth disease as signal of an outbreak of Coxsackievirus A6 infections, France, 2014-2015. Emerg Infect Dis . 2016;22:1884–93. doi: 10.3201/eid2211.160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nix WA, Oberste MS, Pallansch MA. Sensitive, Seminested PCR amplification of Vp1 sequences for direct identification of all Enterovirus Serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomba Ngangas S, Bisseux M, Jugie G, et al. Coxsackievirus A6 recombinant Subclades D3/A and D3/H were predominant in hand-foot-and-mouth disease outbreaks in the Paediatric population, France, 2010–2018. Viruses. 2022;14:1078. doi: 10.3390/v14051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailly JL, Chambon M, Peigue-Lafeuille H, et al. Activity of Glutaraldehyde at low concentrations (less than 2%) against Poliovirus and its relevance to gastrointestinal Endoscope Disinfection procedures. Appl Environ Microbiol. 1991;57:1156–60. doi: 10.1128/aem.57.4.1156-1160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergamin CS, Pérez-Hurtado E, Oliveira L, et al. Enterovirus neutralizing antibodies, monocyte toll like receptors expression and interleukin profiles are similar between non-affected and affected siblings from long-term discordant type 1 diabetes Multiplex-Sib families: the importance of HLA background. Front Endocrinol (Lausanne) 2020;11:555685. doi: 10.3389/fendo.2020.555685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyet LA, Thanh TT, Nhan LNT, et al. Neutralizing antibodies against Enteroviruses in patients with hand, foot and mouth disease. Emerg Infect Dis . 2020;26:298–306. doi: 10.3201/eid2602.190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheema S, Bunting E, Good C, et al. Balancing immunosuppression and infection: recurrent Enterovirus encephalitis in SLE. Pract Neurol. 2019;19:508–10. doi: 10.1136/practneurol-2019-002229. [DOI] [PubMed] [Google Scholar]
- 15.Cook SG, Ford AW, Lindholm DA, et al. Enteroviral Meningoencephalitis as a complication of Rituximab therapy for rheumatoid arthritis. Cureus. 2021;13:e18189. doi: 10.7759/cureus.18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diarra A, Gantois G, Lazrek M, et al. Fatal Enterovirus-related myocarditis in a patient with Devic’s syndrome treated with Rituximab. Card Fail Rev . 2021;7:e09. doi: 10.15420/cfr.2020.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein S, Thakkar R, Fong KT, et al. Compassionate-use Pocapavir and immunoglobulin therapy for treatment of Rituximab-associated Enterovirus Meningoencephalitis. J Neurovirol. 2022;28:329–34. doi: 10.1007/s13365-021-01038-z. [DOI] [PubMed] [Google Scholar]
- 18.Kapadia RK, Gill CM, Baca C, et al. Enterovirus A71 causing Meningoencephalitis and acute flaccid Myelitis in a patient receiving Rituximab. J Neuroimmunol. 2021;358:S0165-5728(21)00166-1. doi: 10.1016/j.jneuroim.2021.577639. [DOI] [PubMed] [Google Scholar]
- 19.Klingel K, Pöml P, Strunk J, et al. Lethal Enterovirus myocarditis in a patient with granulomatosis with polyangiitis following Rituximab and high-dose steroid therapy. Eur Heart J. 2021;42:2401. doi: 10.1093/eurheartj/ehab269. [DOI] [PubMed] [Google Scholar]
- 20.Levy R, Mahévas M, Galicier L, et al. Profound symptomatic Hypogammaglobulinemia: A rare late complication after Rituximab treatment for immune thrombocytopenia. Report of 3 cases and systematic review of the literature. Autoimmun Rev. 2014;13:1055–63. doi: 10.1016/j.autrev.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Luciani L, Ninove L, Zandotti C, et al. Fatal underhanded chronic Enterovirus infection associated with anti-Cd20 monotherapy for central nervous system Demyelinating disease. Mult Scler. 2021;27:320–3. doi: 10.1177/1352458520923978. [DOI] [PubMed] [Google Scholar]
- 22.Maillet F, Pineton De Chambrun M, Monzani Q, et al. Enteroviral infections in adults treated with Rituximab: A new case of chronic meningitis and Myofasciitis and literature review. Rev Med Interne. 2020;41:200–5. doi: 10.1016/j.revmed.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Palacios T, Bartelt L, Scheld W, et al. Fatal Coxsackie Meningoencephalitis in a patient with B-cell Lymphopenia and Hypogammaglobulinemia following Rituximab therapy. Ann Allergy Asthma Immunol. 2015;115:148–50. doi: 10.1016/j.anai.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quartier P, Tournilhac O, Archimbaud C, et al. Enteroviral Meningoencephalitis after Anti‐Cd20 (Rituximab) treatment. CLIN INFECT DIS. 2003;36:e47–9. doi: 10.1086/345746. [DOI] [PubMed] [Google Scholar]
- 25.Sellier-Leclerc A-L, Belli E, Guérin V, et al. Fulminant viral myocarditis after Rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol. 2013;28:1875–9. doi: 10.1007/s00467-013-2485-9. [DOI] [PubMed] [Google Scholar]
- 26.Sham L, Bitnun A, Branson H, et al. Treatment of Rituximab-associated chronic CNS Enterovirus using Ivig and fluoxetine. Neurology. 2019;92:916–8. doi: 10.1212/WNL.0000000000007468. [DOI] [PubMed] [Google Scholar]
- 27.Tekin B, Boire N, Shah K, et al. Viral Panniculitis in a patient with disseminated opportunistic Enterovirus infection. J Cutan Pathol. 2021;48:434–8. doi: 10.1111/cup.13930. [DOI] [PubMed] [Google Scholar]
- 28.Bajema KL, Simonson PD, Greninger AL, et al. Acute liver failure due to Echovirus 9 associated with persistent B-cell depletion from Rituximab. Open Forum Infect Dis. 2017;4:ofx174. doi: 10.1093/ofid/ofx174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolini LA, Canepa P, Caligiuri P, et al. Fulminant hepatitis associated with Echovirus 25 during treatment with Ocrelizumab for multiple sclerosis. JAMA Neurol. 2019;76:866–7. doi: 10.1001/jamaneurol.2019.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haidar G, Agha M, Bilderback A, et al. Prospective evaluation of Coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of Immunocompromising conditions: the COVID-19 vaccination in the immunocompromised study (COVICS) Clin Infect Dis. 2022;75:e630–44. doi: 10.1093/cid/ciac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with Rituximab: a cohort study. Lancet Rheumatol . 2021;3:e419–26. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CNR enterovirus. Clermont-Ferrand-Lyon: France French Reference Centre for enteroviruses and parechovirus. http://cnr.chu-clermontferrand.fr/CNR Available.
- 33.Bauer L, Manganaro R, Zonsics B, et al. Fluoxetine inhibits Enterovirus replication by targeting the viral 2C protein in a Stereospecific manner. ACS Infect Dis. 2019;5:1609–23. doi: 10.1021/acsinfecdis.9b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bearden D, Collett M, Quan PL, et al. Enteroviruses in X-linked Agammaglobulinemia: update on epidemiology and therapy. J Allergy Clin Immunol Pract. 2016;4:1059–65. doi: 10.1016/j.jaip.2015.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.