Abstract
An 82-year-old man with a secundum atrial septal defect (ASD) underwent transcatheter closure. The patient had a wide area of aortic and superior rim deficiency, with left ventricular diastolic dysfunction and moderate mitral regurgitation. These findings suggested the risk of both cardiac erosion and increased left atrial pressure after closure. To avoid cardiac erosion, a GORE® CARDIOFORM ASD (GCA) occluder (W.L. Gore & Associates, Flagstaff, AZ, USA) was considered an appropriate device in this patient. However, the possibility of excessively high left atrial pressure due to complete defect closure was a concern. Thus, we created a 4.5-mm fenestration using a surgical punch in the fabric membrane of a 44-mm GCA. The device was deployed in an appropriate position, and no significant elevation of pulmonary capillary wedge pressure was observed. One month after the closure, marked improvement in clinical symptoms and continuous flow through the fenestration were observed. This novel fenestration technique may contribute to expansion of the indications for transcatheter ASD closure in patients who require a GCA owing to an anatomically high risk of erosion accompanied by left ventricular diastolic dysfunction.
Learning objective
In elderly patients with left ventricular diastolic dysfunction, transcatheter atrial septal defect (ASD) closure is difficult because rapid resolution of an ASD shunt can cause an increase in left atrial pressure. Previous reports described the creation of a fenestration in the closure device. The use of a GORE® CARDIOFORM ASD (GCA) occluder can reduce the erosion risk; however, creating a stable fenestration is difficult. We developed a novel technique to create a stable fenestration in a GCA.
Keywords: Atrial septal defect, GORE® CARDIOFORM ASD occluder, Fenestration, Surgical aortic punch
Introduction
Transcatheter closure is recommended as the first choice for secundum atrial septal defect (ASD) [1]. Although transcatheter closure is feasible for most ASD patients, several serious complications are concerns, such as cardiac erosion and acute pulmonary congestion [1,2]. The GORE® CARDIOFORM ASD (GCA) occluder (W.L. Gore & Associates, Flagstaff, AZ, USA) is a novel device expected to reduce cardiac erosion because of soft and compliant structure [3]. Acute pulmonary congestion may occur, especially in geriatric patients with left-sided heart disorders, owing to elevation of left atrial pressure after ASD closure [1,4]. Additionally, mitral regurgitation (MR) may be increased after ASD closure, especially among geriatric patients [5]. For this reason, previous reports described the creation of a fenestration in closure device, which prevents a rapid increase in left atrial pressure [1]. However, the creation of a stable fenestration is difficult because of the fabric construction of GCA. Here, we introduced a novel technique to create a stable fenestration in a GCA in a geriatric patient with high risk for both cardiac erosion and acute pulmonary congestion.
Case report
An 82-year-old man complained of exertional dyspnea with New York Heart Association functional classification III and was diagnosed as having chronic atrial fibrillation (AF) and chronic heart failure. Chest X-ray showed cardiac dilation with a cardiothoracic ratio of 61.0% and pulmonary vascular enlargement (Fig. 1A). He underwent transthoracic echocardiography, which identified an ASD (Fig. 1B), right atrial and ventricular dilation (Fig. 1C), mild reduced left ventricular (LV) ejection fraction, LV diastolic dysfunction, and moderate MR (Fig. 1D). Despite treatment with arotinolol 10 mg, azosemide 30 mg, furosemide 20 mg, and spironolactone 25 mg, his symptoms of right-sided heart failure did not improve. It was considered that catheter ablation therapy for AF might be inefficient because of his age and left atrial dilation (left atrial diameter, 54 mm; left atrial volume index, 60 ml/m2). Thus, he was referred to our hospital for transcatheter ASD closure.
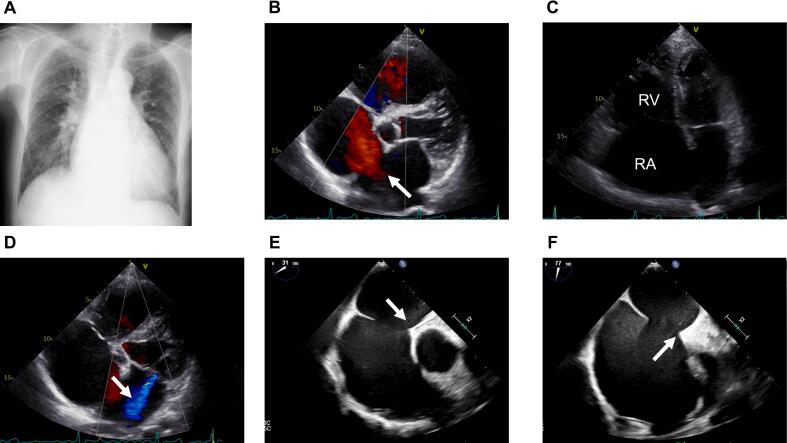
Fig. 1.
Before atrial septal defect closure. (A) Chest X-ray. (B) A 23-mm secundum atrial septal defect on transthoracic echocardiography is visible (arrow). (C) Dilated RA and RV in the apical four-chamber view. (D) Mitral regurgitation of moderate severity (arrow). (E) Insufficient aortic rim and (F) superior rim on transesophageal echocardiography (arrows).
RA, right atrium; RV, right ventricle.
Transesophageal echocardiography (TEE) demonstrated an ASD with a maximum diameter of 23 mm and a wide area of aortic and superior rim deficiency, suggesting a high risk of cardiac erosion [2] (Fig. 1E and F). Hemodynamic parameters showed a right atrial pressure of 6 mmHg, mean pulmonary artery pressure of 16 mmHg, left atrial pressure of 7 mmHg, and pulmonary vascular resistance of 1.4 Wood units. Oxygen saturation step-up was observed at the right atrium, with a QpQs of 3.92. The left atrial pressure before closure was well-controlled owing to medical therapy. On the basis of these morphological features on TEE, the 44-mm GCA was considered suitable. However, we were concerned about the risk of pulmonary congestion due to elevated left atrial pressure after closure because the patient had multiple risk factors for acute pulmonary congestion, including LV diastolic dysfunction, MR with chronic AF, and advanced age [1]. Moreover, GCA may contribute to a high rate of complete closure of ASD shunt at early phase after implantation compared with other devices [6,7]. To minimize such risks, we attempted to create a fenestration in GCA. However, creating a stable fenestration was difficult because GCA was made of polytetrafluoroethylene fabric, which differed from other devices made of metal mesh [8]. Thus, we decided to make a fenestration in GCA using a 4.5-mm sized surgical aortic punch (ACP Japan, Tokyo, Japan). The optimal ASD closure device fenestration size has not yet been established, although it was estimated to be 8 mm in a previous hemodynamic study of heart failure management [9]. To minimize the influence on the structure of GCA, we planned our procedural strategy to first create a 4.5-mm fenestration on the GCA and deploy the device under pulmonary capillary wedge pressure (PCWP) continuous monitoring (Fig. 2A and B). Should neither notable increased PCWP nor MR be observed on TEE, we planned to detach and implant the device. If either PCWP or MR were to increase remarkably, we would retrieve the device, create an additional fenestration on the same device, and re-implant it. If PCWP or MR elevation could not be controlled after this, we would consider abandoning ASD closure in this patient. The location of the fenestration was carefully selected to avoid damaging the wire frame of the device. The fenestrated device was successfully deployed with a standard technique under TEE guidance. After implantation, continuous left-to-right interatrial shunting throughout the cardiac circle was observed on TEE (Fig. 2C). PCWP was not greatly elevated, being measured as 8 mmHg before implantation and 9 mmHg afterwards. MR observed by TEE also did not remarkably deteriorate, so we considered that one 4.5-mm fenestration was sufficient in this patient. We detached and implanted the fenestrated device and completed the procedure. Antiplatelet therapy was not administered because of bleeding risk, as the patient had already taken anticoagulant therapy in the form of apixaban for AF with a CHA2DS2-VASc score of 3.
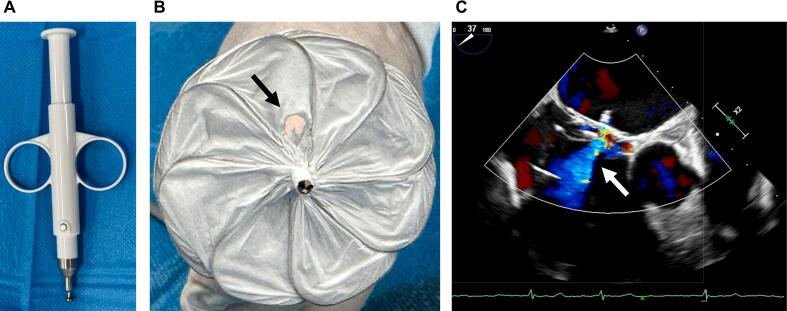
Fig. 2.
(A) A surgical aortic punch (ACP Japan, Tokyo, Japan) was used to create a 4.5-mm fenestration. (B) Fenestration on the GORE® CARDIOFORM ASD atrial septal defect occluder device (arrow). (C) Interatrial shunting through the fenestration after implantation on transesophageal echocardiography (arrow).
One month after closure, the patient's symptoms had improved dramatically to New York Heart Association functional classification II, and chest X-ray showed improved cardiac dilation with a cardiothoracic ratio of 57.4% (Fig. 3A). Transthoracic echocardiography confirmed that left-to-right interatrial fenestration flow was preserved (Fig. 3B), and right atrial and ventricular dilation had improved (Fig. 3C). MR did not notably worsen (Fig. 3D).
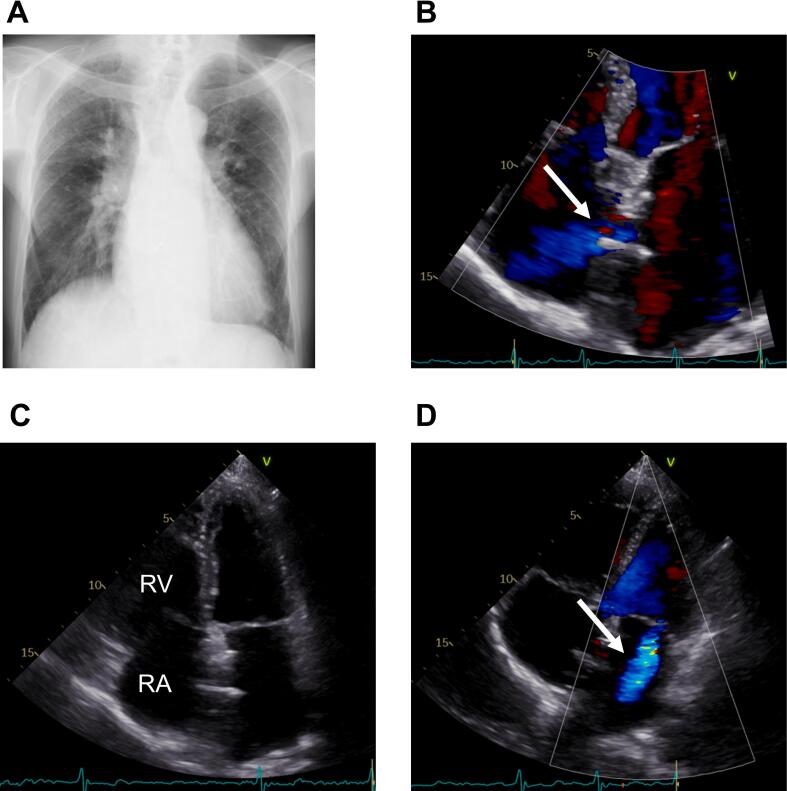
Fig. 3.
One month after atrial septal defect closure. (A) Chest X-ray. (B) Preserved left-to-right interatrial shunt flow through the fenestration on the device. (C) Improved RA and RV dilation are visible. (D) Mitral regurgitation did not notably worsen (arrow).
RA, right atrium; RV, right ventricle.
Discussion
We described transcatheter closure of an ASD with a GCA that was fenestrated using a surgical aortic punch. The patient was geriatric and had high risks of both cardiac erosion and acute pulmonary congestion. As a result, the fenestration prevented PCWP elevation and maintained left-to-right interatrial shunt flow one month after the procedure. The improvement in the patient’s clinical symptoms was dramatic.
Patients with ASD are sometimes diagnosed at an advanced age because their symptoms may be mild in childhood [1,4,5]. Transcatheter ASD closure is a minimally invasive procedure with established efficacy and safety even for geriatric patients, whose operative risks for surgical repair may be high [5]. However, certain risks for procedural complications remain. ASD with a wide area of aortic rim deficiency and/or superior rim deficiency is considered a high-risk morphological feature of cardiac erosion [2]. The GCA was composed of a helical nitinol wire frame covered with expanded polytetrafluoroethylene. The device’s soft and compliant structure is expected to reduce the risk of cardiac erosion, and its efficacy and safety for ASD closure have been reported [3]. In the present case, although TEE demonstrated a wide area of aortic and superior rim deficiency, we successfully completed transcatheter ASD closure using GCA without procedural complications, including cardiac erosion.
Geriatric patients with ASD tend to have cardiovascular comorbidities, such as AF and LV diastolic dysfunction, which is associated with aging [4]. In patients with left-sided heart disorders, there is a risk of acute pulmonary congestion after ASD closure owing to the elimination of left-to-right interatrial shunting, and elevated left atrial pressure [1]. Moreover, in geriatric patients, MR may increase after ASD closure [5]. Current guidelines recommend balloon occlusion testing to evaluate the increased left-sided filling pressure [1]. However, we did not perform a balloon occlusion test in this patient because we previously reported that balloon occlusion tests tend to overestimate postoperative PCWP [10]. In the present case, we determined that a closure device with a fenestration was indicated before the closure was performed. Therefore, the closure was performed while PCWP and MR were monitored. As a result, there was no increase in PCWP or MR after placement of the fenestrated device. It is unclear if the patient’s LV diastolic dysfunction and MR will deteriorate in the long-term as he ages. If his clinical symptoms worsen, we plan to intensify his medication or consider interventions for MR.
Transcatheter closure for ASD using a fenestrated device is recommended in recent guidelines for patients with the risk of acute pulmonary congestion [1]. In particular, previous studies reported that placing the GCA tended to result in low rates of residual shunting in the early phase after implantation [7], whereas other devices, such as the Amplatzer septal occluder device (Abbott, Chicago, IL, USA) and Occlutech Figulla Flex II device (Occlutech GmbH, Jena, Germany), tended to result in gradual decreases in the rate of residual shunting [3,6]. Thus, GCA might result in more rapid elevation in left atrial pressure compared with other devices. Previously, fenestration of a Helex ASD closure device (W.L. Gore & Associates) using an 18-G needle and sheath dilator was reported [8]. However, compared with the Helex ASD closure device, the GCA has a complex structure, which may make it difficult to create a durable fenestration using only an 18-G needle and a sheath dilator. A surgical aortic punch can be used to accurately create a fenestration of the target size, which likely contributes to long-term patency.
In conclusion, we successfully performed transcatheter ASD closure using a fenestrated GCA in a geriatric patient with the risk of both cardiac erosion and elevation of left atrial pressure after transcatheter ASD closure. This novel technique may contribute to expansion of the indications for transcatheter ASD closure in patients with LV diastolic dysfunction who require a GCA owing to an anatomically high risk of erosion.
Consent statement
Written informed consent was obtained from the patient.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
This work was not supported by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Baumgartner H., De Backer J., Babu-Narayan S.V., Budts W., Chessa M., Diller G.P., Lung B., Kluin J., Lang I.M., Meijboom F., Moons P., Mulder B.J.M., Oechslin E., Roos-Hesselink J.W., Schwerzmann M., et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 2.Amin Z., Hijazi Z.M., Bass J.L., Cheatham J.P., Hellenbrand W.E., Kleinman C.S. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 3.Sommer R.J., Love B.A., Paolillo J.A., Gray R.G., Goldstein B.H., Morgan G.J., Gillespie M.J. ASSURED clinical study: new GORE® CARDIOFORM ASD occluder for transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv. 2020;95:1285–1295. doi: 10.1002/ccd.28728. [DOI] [PubMed] [Google Scholar]
- 4.Takaya Y., Akagi T., Kijima Y., Nakagawa K., Sano S., Ito H. Long-term outcome after transcatheter closure of atrial septal defect in older patients: impact of age at procedure. JACC Cardiovasc Interv. 2015;8:600–606. doi: 10.1016/j.jcin.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K., Akagi T., Taniguchi M., Kijima Y., Goto K., Kusano K.F., Itoh H., Sano S. Transcatheter closure of atrial septal defect in a geriatric population. Catheter Cardiovasc Interv. 2012;80:84–90. doi: 10.1002/ccd.23457. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama R., Takaya Y., Akagi T., Watanabe N., Miki T., Nakagawa K., Toh N., Ito H. Efficacy and safety of atrial septal defect closure using Occlutech Figulla Flex II compared with Amplatzer Septal Occluder. Heart Vessels. 2021;36:704–709. doi: 10.1007/s00380-020-01739-1. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie M.J., Javois A.J., Moore P., Forbes T., Paolillo J.A. Use of the GORE® CARDIOFORM Septal Occluder for percutaneous closure of secundum atrial septal defects: results of the multicenter U.S. IDE trial. Catheter Cardiovasc Interv. 2020;95:1296–1304. doi: 10.1002/ccd.28814. [DOI] [PubMed] [Google Scholar]
- 8.Kenny D., Cao Q.L., Hijazi Z.M. Fenestration of a Gore Helex Septal Occluder device in a patient with diastolic dysfunction of the left ventricle. Catheter Cardiovasc Interv. 2011;78:594–598. doi: 10.1002/ccd.23009. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen T.H., Søndergaard L. Transcatheter implantation of interatrial shunt devices to lower left atrial pressure in heart failure. Int J Heart Fail. 2022;4:12–23. doi: 10.36628/ijhf.2021.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa K., Akagi T., Takaya Y., Miki T., Kijima Y., Nakayama R., Toh N., Nishii N., Nakamura K., Morita H., Ito H. Temporary balloon occlusion test can overestimate the risk of acute pulmonary edema after transcatheter atrial septal defect closure. Catheter Cardiovasc Interv. 2023 doi: 10.1002/ccd.30556. [DOI] [PubMed] [Google Scholar]