Abstract
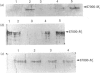
Lysosomal beta-galactosidase (beta-Gal) occurs either alone in monomeric and dimeric forms, or in a high-M(r) complex with at least two additional proteins. One is neuraminidase and the second is the protective protein, which has also been shown to possess carboxypeptidase activity. beta-Gal activity is deficient in GM1-gangliosidosis as a primary defect, and is secondarily affected in galactosialidosis (GS), where the primary defect is the absence of protective protein activity. Fibroblasts from three patients with GM1-gangliosidosis, type 1, showed markedly reduced amounts of beta-Gal cross-reacting material (CRM), and a fourth appeared to have normal levels. A patient with type 2 GM1-gangliosidosis was also found to be CRM-normal. These findings demonstrate that patients with GM1-gangliosidosis type 1 are heterogeneous with respect to the level of residual beta-Gal protein. Fibroblasts from four patients with GS were strongly CRM-positive with an anti-beta-Gal antibody, as was a sample of brain from one of these patients, suggesting that the loss of beta-Gal activity is linked to a subtler change in the primary structure of the enzyme than has been previously thought. While three GS cell lines displayed reduced carboxypeptidase activity (to 32-42% of the control), one cell line was completely devoid of activity, demonstrating that while carboxypeptidase activity is a property of the protective protein this action is distinct and separate from its protective role. On direct immunoprecipitation with anti-beta-Gal antibody, a portion of the total carboxypeptidase activity co-precipitated with beta-Gal from extracts of normal and GM1-gangliosidosis cells, consistent with the presence of the complex in these cells. However, no carboxypeptidase activity was precipitable with this antibody from GS fibroblasts, suggesting the absence of complex from these cells. To examine this further, the various forms of beta-Gal were resolved by h.p.l.c. molecular-sieve chromatography. Three forms of beta-Gal activity were resolved in normal cells: a complex, a dimer and a monomer. Residual beta-Gal activity of GS cells resolved into two of these forms, the complex and the monomer. In normal and GM1-gangliosidosis cells a portion of the total carboxypeptidase activity co-chromatographed with the complex while the bulk of the activity occurred in a single 36,000-M(r) peak. Only the low-M(r) carboxypeptidase activity was detected in GS cells. This confirms our results on immunoprecipitation indicating that portions of the beta-Gal and the carboxypeptidase activities exist outside the complex in normal, GM1-gangliosidosis and GS cells. In summary, the loss of protective protein function from GS cells results in disproportionate loss of the dimeric and monomeric forms of beta-Gal activity, but does not result in the complete degradation of the protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chitayat D., Applegarth D. A., Lewis J., Dimmick J. E., McCormick A. Q., Hall J. G. Juvenile galactosialidosis in a white male: a new variant. Am J Med Genet. 1988 Dec;31(4):887–901. doi: 10.1002/ajmg.1320310423. [DOI] [PubMed] [Google Scholar]
- D'Azzo A., Hoogeveen A., Reuser A. J., Robinson D., Galjaard H. Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4535–4539. doi: 10.1073/pnas.79.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E. P., Holzer H. Interaction of proteinases and their inhibitors from yeast. Activation of carboxypeptidase Y. Biochim Biophys Acta. 1980 Sep 9;615(1):187–198. doi: 10.1016/0005-2744(80)90022-4. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Purification, structure, and properties of hybrid beta-galactosidase proteins. J Biol Chem. 1983 Dec 10;258(23):14354–14358. [PubMed] [Google Scholar]
- Galjart N. J., Gillemans N., Harris A., van der Horst G. T., Verheijen F. W., Galjaard H., d'Azzo A. Expression of cDNA encoding the human "protective protein" associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988 Sep 9;54(6):755–764. doi: 10.1016/s0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- Galjart N. J., Gillemans N., Meijer D., d'Azzo A. Mouse "protective protein". cDNA cloning, sequence comparison, and expression. J Biol Chem. 1990 Mar 15;265(8):4678–4684. [PubMed] [Google Scholar]
- Gravel R. A., Lowden J. A., Callahan J. W., Wolfe L. S., Ng Yin Kin N. M. Infantile sialidosis: a phenocopy of type 1 GM1 gangliosidosis distinguished by genetic complementation and urinary oligosaccharides. Am J Hum Genet. 1979 Nov;31(6):669–679. [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Hata T. Action of yeast proteinase C on synthetic peptides and poly- ,L-amino acids. Biochim Biophys Acta. 1972 May 18;263(3):673–679. doi: 10.1016/0005-2795(72)90049-9. [DOI] [PubMed] [Google Scholar]
- Hoogeveen A. T., Graham-Kawashima H., d'Azzo A., Galjaard H. Processing of human beta-galactosidase in GM1-gangliosidosis and Morquio B syndrome. J Biol Chem. 1984 Feb 10;259(3):1974–1977. [PubMed] [Google Scholar]
- Hoogeveen A. T., Reuser A. J., Kroos M., Galjaard H. GM1-gangliosidosis. Defective recognition site on beta-galactosidase precursor. J Biol Chem. 1986 May 5;261(13):5702–5704. [PubMed] [Google Scholar]
- Hoogeveen A. T., Verheijen F. W., Galjaard H. The relation between human lysosomal beta-galactosidase and its protective protein. J Biol Chem. 1983 Oct 25;258(20):12143–12146. [PubMed] [Google Scholar]
- Hubbes M., D'Agrosa R. M., Callahan J. W. Human placental beta-galactosidase. Characterization of the dimer and complex forms of the enzyme. Biochem J. 1992 Aug 1;285(Pt 3):827–831. doi: 10.1042/bj2850827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. S., Mahuran D., Lowden J. A., Callahan J. W. Human placental beta-galactosidase: structural and immunological observations. Can J Biochem Cell Biol. 1984 Jun;62(6):529–534. doi: 10.1139/o84-070. [DOI] [PubMed] [Google Scholar]
- Kleijer W. J., Hoogeveen A., Verheijen F. W., Niermeijer M. F., Galjaard H., O'Brien J. S., Warner T. G. Prenatal diagnosis of sialidosis with combined neuraminidase and beta-galactosidase deficiency. Clin Genet. 1979 Jul;16(1):60–61. doi: 10.1111/j.1399-0004.1979.tb00851.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Transport of secretory and membrane glycoproteins from the rough endoplasmic reticulum to the Golgi. A rate-limiting step in protein maturation and secretion. J Biol Chem. 1988 Feb 15;263(5):2107–2110. [PubMed] [Google Scholar]
- Lowden J. A., Callahan J. W., Norman M. G., Thain M., Prichard J. S. Juvenile GM1 gangliosidosis. Occurrence with absence of two beta-galactosidase components. Arch Neurol. 1974 Sep;31(3):200–203. doi: 10.1001/archneur.1974.00490390082010. [DOI] [PubMed] [Google Scholar]
- Mahuran D. J. Characterization of human placental beta-hexosaminidase I2. Proteolytic processing intermediates of hexosaminidase A. J Biol Chem. 1990 Apr 25;265(12):6794–6799. [PubMed] [Google Scholar]
- Mahuran D. J., Neote K., Klavins M. H., Leung A., Gravel R. A. Proteolytic processing of pro-alpha and pro-beta precursors from human beta-hexosaminidase. Generation of the mature alpha and beta a beta b subunits. J Biol Chem. 1988 Apr 5;263(10):4612–4618. [PubMed] [Google Scholar]
- Mandecki W., Fowler A. V., Zabin I. Position of the lacZX90 mutation and hybridization between complete and incomplete beta-galactosidase. J Bacteriol. 1981 Aug;147(2):694–697. doi: 10.1128/jb.147.2.694-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F. Lysosomal storage diseases. Annu Rev Biochem. 1991;60:257–280. doi: 10.1146/annurev.bi.60.070191.001353. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Warner T. G. Sialidosis: delineation of subtypes by neuraminidase assay. Clin Genet. 1980 Jan;17(1):35–38. doi: 10.1111/j.1399-0004.1980.tb00111.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Klavins M. H., Willard H. F., Gravel R., Lowden J. A., Mahuran D. J. Molecular heterogeneity in the infantile and juvenile forms of Sandhoff disease (O-variant GM2 gangliosidosis). J Biol Chem. 1986 Sep 25;261(27):12680–12685. [PubMed] [Google Scholar]
- Palmeri S., Hoogeveen A. T., Verheijen F. W., Galjaard H. Galactosialidosis: molecular heterogeneity among distinct clinical phenotypes. Am J Hum Genet. 1986 Feb;38(2):137–148. [PMC free article] [PubMed] [Google Scholar]
- Strisciuglio P., Parenti G., Giudice C., Lijoi S., Hoogeveen A. T., d'Azzo A. The presence of a reduced amount of 32-kd "protective" protein is a distinct biochemical finding in late infantile galactosialidosis. Hum Genet. 1988 Nov;80(3):304–306. doi: 10.1007/BF01790104. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchemontagne J., Michaud L., Potier M. Deficient lysosomal carboxypeptidase activity in galactosialidosis. Biochem Biophys Res Commun. 1990 Apr 16;168(1):22–29. doi: 10.1016/0006-291x(90)91669-j. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Yamada T., Ariga T., Toyoshima I., Yamaguchi H., Kitahara Y., Miyatake T., Yamakawa T. Carrier detection of sialidosis with partial beta-galactosidase deficiency by the assay of lysosomal sialidase in lymphocytes. Ann Neurol. 1984 Feb;15(2):181–183. doi: 10.1002/ana.410150211. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Oshima A., Shimmoto M., Fukuhara Y., Sakuraba H., Yanagisawa N., Suzuki Y. Human beta-galactosidase gene mutations in GM1-gangliosidosis: a common mutation among Japanese adult/chronic cases. Am J Hum Genet. 1991 Aug;49(2):435–442. [PMC free article] [PubMed] [Google Scholar]
- van der Horst G. T., Galjart N. J., d'Azzo A., Galjaard H., Verheijen F. W. Identification and in vitro reconstitution of lysosomal neuraminidase from human placenta. J Biol Chem. 1989 Jan 15;264(2):1317–1322. [PubMed] [Google Scholar]