Abstract
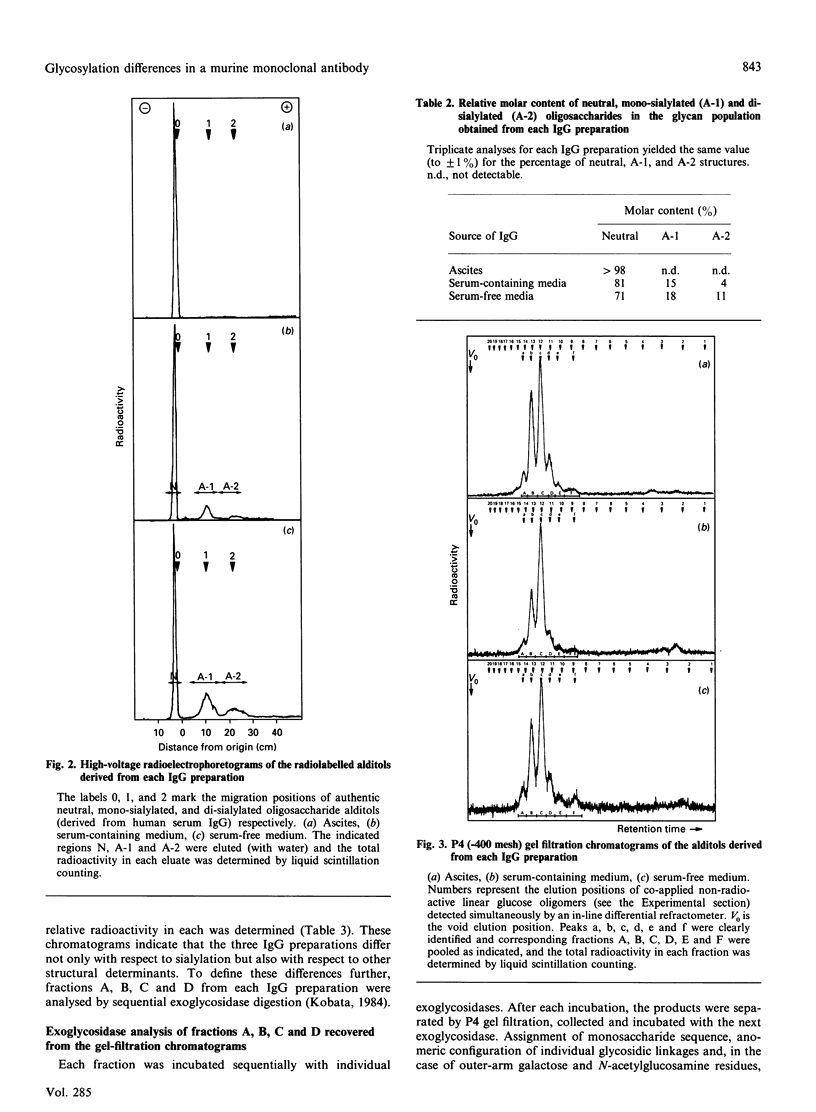
A monoclonal IgG-1 was produced by culture of a murine hybridoma (3.8.6) by three different methods, namely culture in ascites, in serum-free media and in serum-supplemented media. IgG-1 was purified to homogeneity (as judged by SDS/PAGE under reducing conditions) from each medium by ion-exchange chromatography and h.p.l.c. Protein A chromatography. Oligosaccharides were released from each IgG-1 preparation by hydrazinolysis and radiolabelled by reduction with alkaline sodium borotritide, and 'profile' analysis of the radiolabelled oligosaccharide alditols was performed by a combination of paper electrophoresis and gel-filtration chromatography. This analysis indicated clear and reproducible differences in the glycosylation patterns of the three IgG-1 preparations. Sequential exoglycosidase analysis of individual oligosaccharides derived from each IgG-1 preparation was used to define these differences. Ascites-derived material differed from serum-free-culture-derived material only with respect to the content of sialic acid. IgG-1 derived from culture in serum-containing media had an intermediate sialic acid content and a lower incidence of outer-arm galactosylation than the other two preparations. These differences in glycosylation could not be induced in any IgG-1 preparation by incubating purified IgG-1 with ascites or culture medium. It is concluded that the glycosylation pattern of a secreted monoclonal IgG is dependent on the culture method employed to obtain it.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. R., Atkinson P. H., Grimes W. J. Major carbohydrate structures at five glycosylation sites on murine IgM determined by high resolution 1H-NMR spectroscopy. Arch Biochem Biophys. 1985 Dec;243(2):605–618. doi: 10.1016/0003-9861(85)90538-7. [DOI] [PubMed] [Google Scholar]
- Arvieux J., Willis A. C., Williams A. F. MRC OX-45 antigen: a leucocyte/endothelium rat membrane glycoprotein of 45,000 molecular weight. Mol Immunol. 1986 Sep;23(9):983–990. doi: 10.1016/0161-5890(86)90129-x. [DOI] [PubMed] [Google Scholar]
- Chaplin M. F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- Curling E. M., Hayter P. M., Baines A. J., Bull A. T., Gull K., Strange P. G., Jenkins N. Recombinant human interferon-gamma. Differences in glycosylation and proteolytic processing lead to heterogeneity in batch culture. Biochem J. 1990 Dec 1;272(2):333–337. doi: 10.1042/bj2720333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goochee C. F., Monica T. Environmental effects on protein glycosylation. Biotechnology (N Y) 1990 May;8(5):421–427. doi: 10.1038/nbt0590-421. [DOI] [PubMed] [Google Scholar]
- Hymes A. J., Mullinax G. L., Mullinax F. Immunoglobulin carbohydrate requirement for formation of an IgG-IgG complex. J Biol Chem. 1979 May 10;254(9):3148–3151. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Malaise M. G., Franchimont P., Gomez F., Bouillenne C., Mahieu P. R. The spontaneous ability of normal human IgG to inhibit the Fc receptors of normal human monocytes is related to their binding capacity to lectins. Clin Immunol Immunopathol. 1987 Oct;45(1):1–16. doi: 10.1016/0090-1229(87)90106-1. [DOI] [PubMed] [Google Scholar]
- Middaugh C. R., Litman G. W. Atypical glycosylation of an IgG monoclonal cryoimmunoglobulin. J Biol Chem. 1987 Mar 15;262(8):3671–3673. [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Tse A. G., Dwek R. A., Williams A. F., Rademacher T. W. Tissue-specific N-glycosylation, site-specific oligosaccharide patterns and lentil lectin recognition of rat Thy-1. EMBO J. 1987 May;6(5):1233–1244. doi: 10.1002/j.1460-2075.1987.tb02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R., Isenberg D., Rook G., Roitt I., Dwek R., Rademacher T. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989 Apr;2(2):101–114. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Homans S. W., Parekh R. B., Dwek R. A. Immunoglobulin G as a glycoprotein. Biochem Soc Symp. 1986;51:131–148. [PubMed] [Google Scholar]
- Sheares B. T., Robbins P. W. Glycosylation of ovalbumin in a heterologous cell: analysis of oligosaccharide chains of the cloned glycoprotein in mouse L cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1993–1997. doi: 10.1073/pnas.83.7.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox H. C., Jr, Hood L. Attachment of carbohydrate to the variable region of myeloma immunoglobulin light chains. Proc Natl Acad Sci U S A. 1970 Jul;66(3):975–982. doi: 10.1073/pnas.66.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Abel C. A., Fishkin B. G., Grey H. M. Localization of the carbohydrate within the variable region of light and heavy chains of human gamma g myeloma proteins. Biochemistry. 1970 Oct 13;9(21):4217–4223. doi: 10.1021/bi00823a025. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Phillips D. C. The three-dimensional structure of the carbohydrate within the Fc fragment of immunoglobulin G. Biochem Soc Trans. 1983 Apr;11(Pt 2):130–132. [PubMed] [Google Scholar]
- Winkelhake J. L. Immunoglobulin structure and effector functions. Immunochemistry. 1978 Sep;15(9):695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]
- Yet M. G., Shao M. C., Wold F. Effects of the protein matrix on glycan processing in glycoproteins. FASEB J. 1988 Jan;2(1):22–31. doi: 10.1096/fasebj.2.1.3275561. [DOI] [PubMed] [Google Scholar]




