Abstract
A 66-year-old woman with a complex medical history underwent transcatheter mitral valve replacement (TMVI) owing to mitral insufficiency. Risk factors and noncompliance led to bioprosthetic valve thrombosis (BPVT) within 3 years. Emergent surgery indicated by an ad hoc heart team successfully managed the situation, showcasing challenges in BPVT management after TMVI.
Key Words: transcatheter mitral valve replacement, bioprosthetic valve thrombosis, heart team
Graphical Abstract
History of Presentation
In June 2023, a 66-year-old woman was referred from an external hospital with suspected endocarditis. Two weeks prior, she experienced thoracic pain, reduced consciousness, and acute renal failure, necessitating intubation owing to respiratory distress. After multiple unsuccessful extubation efforts, the ventilated, dialysis-dependent, septic patient was transferred to our care.
Learning Objectives
-
•
To be able to identify bioprosthetic valve thrombosis in patients with TMVI and to differentiate endocarditis.
-
•
To observe the correlation of TOE and CT imaging with the intraoperative finding in BPVT.
-
•
To recognize the need for ad hoc heart teams in decompensated patients after valve replacement.
-
•
To recognize the feasibility of surgical replacement of a degenerated, infected, or thrombosed transcatheter mitral valve.
Past Medical History
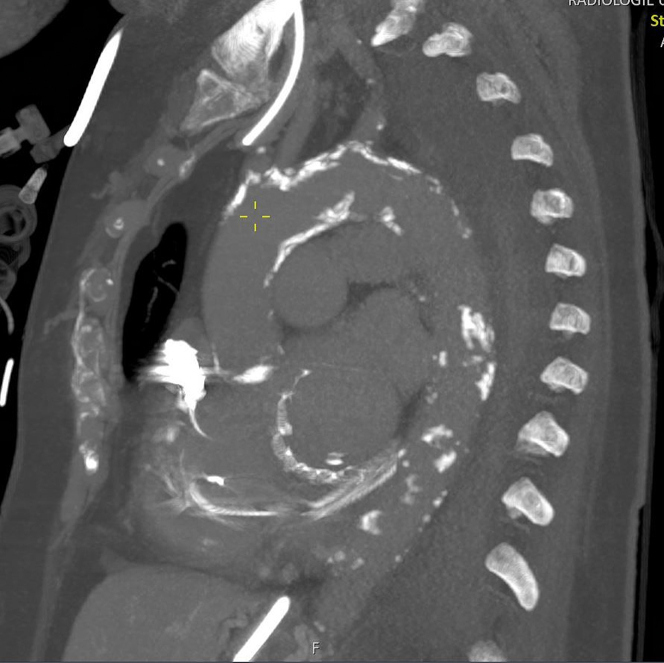
The patient first presented to our clinic in 2013 for the treatment of renal artery stenosis. She had concomitant coronary artery disease, dilated cardiomyopathy with a history of cardiac resynchronization therapy with defibrillation (CRT-D) implantation in 2008, and rheumatoid arthritis managed with immunosuppressive therapy using methotrexate (MTX). The patient presented again in 2020 with cardiac decompensation owing to severe mitral valve insufficiency caused by tethering of both leaflets. In the meantime, she had undergone treatment for pacemaker lead endocarditis in 2015. Given the high operative risk (EuroSCORE II 14%) posed by the reduced left ventricular function (LVEF, 25%), peripheral arterial occlusive disease, chronic obstructive pulmonary disease (COPD), reduced renal function, steroid and MTX therapy, and severely calcified aorta (Figure 1), a combined intervention was selected by the heart team in consultation with the patient.
Figure 1.
Aortic-Computed Tomography: Severely Calcified Aorta With Porcelain Character of the Arch and Only Small Window for Cross Clamping
The procedure took place without complications in June 2020, using a Tendyne 29S LP. Following an uneventful postoperative course, the patient was discharged on the sixth postoperative day with vitamin K antagonist (VKA) therapy and a target international normalized ratio (INR) between 2.5 and 3.5. Three months later, the patient continued to complain of exertional dyspnea. Noncompliance and suboptimal anticoagulation were suspected as the patient showed a floating structure originating from the valve ring, at the level of the former posterior leaflet with a mean gradient of 5 mm Hg (Video 1). This finding was further confirmed with a subsequent cardiac CT and most likely attributed to thrombus formations. Consequently, the patient underwent anticoagulation therapy with heparin followed by readministration of VKA. Despite multiple requests, the patient did not attend additional follow-up visits and was considered lost to follow-up.
Differential Diagnosis
The main differential diagnosis upon readmission after 3 years was endocarditis. Clinically and in terms of laboratory results, the patient exhibited signs of sepsis upon admission. However, with negative blood cultures, she only fulfilled two minor Duke criteria and did not meet a major criterion. Therefore, in addition to obtaining new blood cultures, comprehensive imaging was performed. The 6 blood culture results were all negative for bacteria and granulocytes.
Investigations
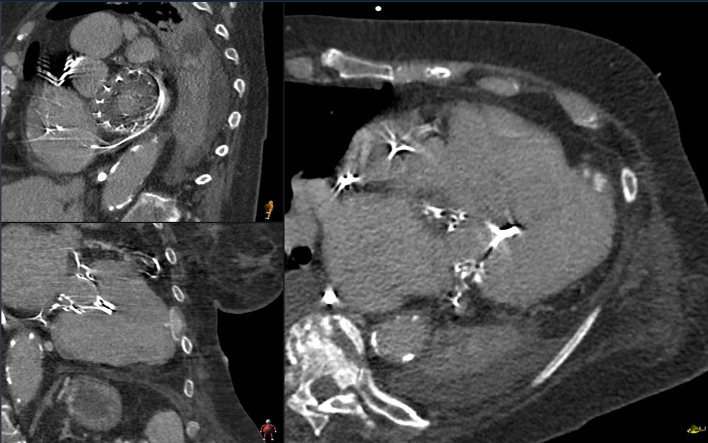
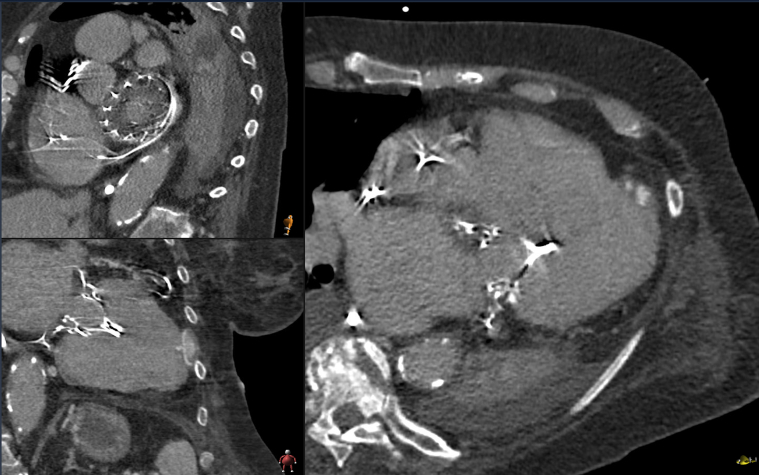
Echocardiographic examination revealed severe mitral valve stenosis resulting from extensive thrombotic deposits around the mitral valve prosthesis and severe tricuspid valve insufficiency (Video 1). Computed tomography (CT) confirmed thrombotic masses on the atrial part of the valve stent (Figure 2), while pneumonia was found as the most likely cause of the inflammation. Considering the patient’s likely noncompliance with oral anticoagulation therapy and the early postinterventional detection of floating structures on the valve prosthesis, biological prosthetic valve thrombosis (BPVT) emerged as the most probable diagnosis and presumed cause of the resulting mitral valve stenosis with subsequent congestion, causing pneumonia.
Figure 2.
Cardio-Computed Tomography
Three-dimensional multiplanar reconstruction of Tendyne Prosthesis with BPVT. The entire atrial pad is covered with a homogeneous layer several millimeters thick, which is revealed intraoperatively to be neointima with underlying fluid components.
Management
Despite the high operative risk (EuroSCORE II 74%, Society of Thoracic Surgery Adult Cardiac Surgery Database Score Operative Mortality 35%), an ad hoc heart team decided on an emergency operation (Video 2). The arterial connection to the heart-lung machine was established via the right subclavian artery to minimize manipulation of the severely calcified aorta and to reduce risk of stroke. A lead perforation and retraction of the septal leaflet was identified as the leading cause for severe tricuspid valve insufficiency (Supplemental Figure 1). After cardioplegic arrest and transseptal opening of the left atrium the entire atrial stent displayed endothelialization and was hardly distinguishable from the left atrial tissue (Supplemental Figure 2). After the endothelial layer was opened, a soft, partially liquified thrombus material was found beneath and removed carefully. Next, the atrial stent, which significantly adhered to the left atrial wall, was carefully removed using various techniques, including blunt, sharp, and electrocautery dissection. Retraction stitches were used on the opposing free edges of the atrial pad and tightened with tourniquets, allowing for the exposure and detachment of the native anterior leaflet. Subsequently, the ventricular cage of the prosthesis was reached, the tether was cut, and the entire prosthesis was removed (Supplemental Figure 3). After thorough debridement and multiple rinses of the left atrium and ventricle, a 31-mm Perimount Magna Ease prosthesis was implanted, preserving the posterior subvalvular apparatus. After complete deairing and opening of the cross-clamp, the tricuspid valve was replaced at the beating heart using a 31-mm Perimount Magna Ease prosthesis. Intraoperative echocardiographic controls confirmed preserved biventricular pump function and proper functioning of the valve prosthesis.
Discussion: Association with current guidelines/position papers/current practice
Mitral regurgitation (MR) is the most prevalent valve disease in the Western world. Different transcatheter options for MR treatment have been developed in recent years, including transcatheter mitral valve implantation (TMVI) for the treatment of patients at high surgical risk. BPVT is a serious complication after surgical and transcatheter valve implantation.
Echocardiographic features indicative of BPVT include a >50% increase in mean echo-Doppler gradient from baseline within 5 years, paroxysmal atrial fibrillation, subtherapeutic INR, increased cusp thickness, and abnormal cusp mobility.1 All criteria were met upon the patient’s emergency admission. Known patient-associated risk factors for BPVT, all of which were present in this patient, include high body mass index, female sex, atrial fibrillation, and reduced LV function.2
In TMVI prostheses, additional risk factors for BPVT arise because of the complex anatomy of the mitral valve and the design of the prostheses. The low-flow situation of the left atrium and inflow tract, in contrast to the left ventricular outflow tract, plays a significant role in the context of Virchow’s triad. This was already recognized in increased thrombosis rates of TAVR prostheses implanted in the mitral position. Particularly with the Tendyne prosthesis, the atrial stent represents large areas of thrombogenic material in the left atrium, and the bulky anchoring system alters ventricular geometry, potentially affecting ventricular flow profiles.3
In the literature, 6 of 97 patients with Tendyne prostheses experienced BPVT within the first 6 months, yet without structural valve deterioration or prosthesis dysfunction,4 resulting in the recommendation for VKA therapy with a target INR of 2.5 to 3.5.
The current American Heart Association/American Association of Thoracic Surgeons guidelines recommend VKA therapy for proven BPVT in hemodynamically stable patients without contraindications.5 The current ESC/EACTS guidelines also suggest VKA or unfractionated heparin therapy before considering reintervention.6 However, little is known about the management of TMVI dysfunction caused by BPVT. To our knowledge, this is the first report on the management of a dysfunctional Tendyne. Like initially reinitiated in this patient at 3 months follow-up, VKA therapy is indicated for functional BPVT. In cases of dysfunctional BPVT and hemodynamically unstable patients, a differentiated evaluation by an experienced heart team using transesophageal echocardiography and cardio-CT is crucial. As demonstrated in this case report, emergent surgery indicated and conducted by a dedicated heart team is technically possible and can prevent fatal consequences.
Follow-up
The patient’s postoperative course in the intensive care unit was complicated by transient dialysis-requiring renal failure. A tracheostomy was performed on the 17th postoperative day, because of anticipated long-term ventilation, and the already alert patient was transferred to a weaning clinic. The weaning process was complicated by a gastrointestinal bleeding episode but was ultimately successful. Consequently, the patient was discharged to a rehabilitation center after 2 months of hospitalization from the intervention.
Conclusions
This case report presents the successful management of a 66-year-old woman with a complex history of disease who underwent transcatheter mitral valve replacement and subsequently developed bioprosthetic valve thrombosis because of noncompliance in the presence of risk factors. Despite the increased operative risk, an ad hoc heart team indicated and successfully performed an emergency surgical procedure. The case highlights the challenges of BPVT management in complex TMVI cases and the potential of effective emergency surgical intervention when performed by an experienced heart team. This report contributes to the understanding of the management of BPVT in TMVI patients and underscores the importance of timely intervention to avert serious outcomes.
Funding Support and Author Disclosures
Dr Dohle is a consultant to Artivion, Edwards, Medira and VarmX. Dr Ruf has received consultation fees and proctor, preceptor, and speaker honoraria from Abbott Medical, Edwards Lifesciences, and TRiCares. Dr Lurz has received grants from Abbott Vascular, Edwards Lifesciences, and ReCor Medical. Dr Treede is consultant for Jena Valve Technology. Dr von Bardeleben reports advisory board activity with Abbott, Bioventrix, Boston Scientific, Edwards Lifesciences, and Medtronic and trial steering committee and lecture honoraria from Abbott Cardiovascular and Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos and figures, please see the online version of this paper.
Appendix
Transesophageal Echocardiography
Echocardiography after implantation, 3-month follow-up, after 3 years and after replacement of the transcatheter mitral valve implantation with a surgical prosthesis.
Surgical Procedure
Note the neointimal layer covering the complete atrial pad and the underlying, partially liquid thrombus masses. The video demonstrates a surgical technique in which the native anterior leaflet can be accessed and detached by circularly dissecting the edges of the atrial pad and snaring the opposite ends. The Tendyne tether at the end of the cage can be reached and cut allowing complete removal of the prosthesis.
References
- 1.Egbe A., Pislaru S., Pellikka P., et al. Bioprosthetic valve thrombosis versus structural failure. J Am Coll Cardiol. 2015;66(21):2285–2294. doi: 10.1016/j.jacc.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 2.Sachdev S., Bardia N., Nguyen L., Omar B. Bioprosthetic valve thrombosis. Cardiol Res. 2018;9(6):335–342. doi: 10.14740/cr789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascione G., Denti P. Transcatheter mitral valve replacement and thrombosis: a review. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.621258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller D., Sorajja P., Duncan A., et al. 2-Year outcomes of transcatheter mitral valve replacement in patients with severe symptomatic mitral regurgitation. J Am Coll Cardiol. 2021;78(19):1847–1859. doi: 10.1016/j.jacc.2021.08.060. [DOI] [PubMed] [Google Scholar]
- 5.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2022;43(7):561–632. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transesophageal Echocardiography
Echocardiography after implantation, 3-month follow-up, after 3 years and after replacement of the transcatheter mitral valve implantation with a surgical prosthesis.
Surgical Procedure
Note the neointimal layer covering the complete atrial pad and the underlying, partially liquid thrombus masses. The video demonstrates a surgical technique in which the native anterior leaflet can be accessed and detached by circularly dissecting the edges of the atrial pad and snaring the opposite ends. The Tendyne tether at the end of the cage can be reached and cut allowing complete removal of the prosthesis.