ABSTRACT
Rabies is a lethal zoonotic disease that threatens human health. As the only viral surface protein, the rabies virus (RABV) glycoprotein (G) induces main neutralizing antibody (Nab) responses; however, Nab titre is closely correlated with the conformation of G. Virus-like particles (VLP) formed by the co-expression of RABV G and matrix protein (M) improve retention and antigen presentation, inducing broad, durable immune responses. RABV nucleoprotein (N) can elicit humoral and cellular immune responses. Hence, we developed a series of nucleoside-modified RABV mRNA vaccines encoding wild-type G, soluble trimeric RABV G formed by an artificial trimer motif (tG-MTQ), membrane-anchored prefusion-stabilized G (preG). Furthermore, we also developed RABV VLP mRNA vaccine co-expressing preG and M to generate VLPs, and VLP/N mRNA vaccine co-expressing preG, M, and N. The RABV mRNA vaccines induced higher humoral and cellular responses than inactivated rabies vaccine, and completely protected mice against intracerebral challenge. Additionally, the IgG and Nab titres in RABV preG, VLP and VLP/N mRNA groups were significantly higher than those in G and tG-MTQ groups. A single administration of VLP or VLP/N mRNA vaccines elicited protective Nab responses, the Nab titres were significantly higher than that in inactivated rabies vaccine group at day 7. Moreover, RABV VLP and VLP/N mRNA vaccines showed superior capacities to elicit potent germinal centre, long-lived plasma cell and memory B cell responses, which linked to high titre and durable Nab responses. In summary, our data demonstrated that RABV VLP and VLP/N mRNA vaccines could be promising candidates against rabies.
KEYWORDS: Rabies virus, mRNA vaccine, glycoprotein, prefusion conformation, virus-like particle, germinal centre
Introduction
Rabies is a fatal zoonotic disease that causes nearly 59,000 deaths annually, especially in developing countries such as Africa and Asia [1]. Rabies infections can be prevented by vaccination, and inactivated rabies vaccines are widely used. However, inactivated rabies vaccines require multiple doses to induce sufficient neutralizing antibody titres and elicit full protection only in the short term [2]. Thus, a safe and effective vaccine that requires less frequent inoculations and provides long-term protection is urgently needed to prevent rabies.
Rabies is caused by the rabies virus (RABV), a negative-sense single-stranded RNA virus which genome encodes five structural proteins: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and large polymerase protein (L) [3]. RABV G, the only surface-exposed protein, is the major antigen that induces neutralizing antibodies (Nab) against RABV infection [4,5]. Therefore, RABV G is the most commonly used antigen in rabies vaccines. The unmodified rabies mRNA vaccines CV7201 and CV7202 from CureVac AG, which encode the RABV G protein, require two doses to elicit protective Nab titres in preclinical trials [6,7]. A single dose of a nucleoside-modified rabies mRNA vaccine encoding the RABV G protein induces prolonged, highly protective immune responses in mice [8]. Wan et al. utilized a core–shell structured lipopolyplex mRNA vaccine encoding RABV G that elicited potent humoral immunity in mice and dogs with a single immunization [9].
On the viral surface, RABV G is structurally heterogeneous, and the conformational epitopes that elicit Nab responses mainly exist on the trimeric form of G protein [5,10]. An enhanced antibody response was elicited when mice are immunized with the trimeric form of RABV G [11]. Therefore, we selected an artificial trimer motif (MTQ) to replace the transmembrane and endocellular domains of RABV G to form a more stable G trimer (tG-MTQ) [12]. RABV-G is a class III viral fusion protein that mediates receptor binding and membrane fusion. RABV G undergoes reversibility between pre- and post-conformation in a highly pH-dependent manner [13]. However, the main epitopes for eliciting Nab against RABV exist in the prefusion conformation (neutral pH) [13]. This structural heterogeneity may affect the generation of Nab, which usually targets quaternary epitopes in the prefusion conformation [14]. Moreover, stabilized prefusion conformation forms of the HIV Envelope glycoprotein, RSV F protein, and SARS-CoV-2 Spike protein have robust immunogenicity [15–21]. Therefore, the structure-based design of the prefusion conformation of RABV G has the potential to elicit high titres and long-term Nab.
Virus like particles (VLPs) are formed by one or several viral structural proteins that are nonreplicative, noninfective, and highly immunogenic [22,23]. VLPs are less than 200 nm in size and are easily presented by dendritic cells (DC) at injection sites to elicit an effective adaptive immune response [24]. The VLP form prolongs the retention period in lymph node follicles, contributing to presentation on follicular dendritic cells (FDC) which sufficiently actives germinal centre (GC) [23]. Several VLP vaccines have been approved for use against the human papilloma virus and Hepatitis B virus. Moreover, co-expression of RABV G and RABV M can generate RABV VLP and that RABV M is critical for viral assembly and budding [25,26]. Additionally, RABV N has B and T cell epitopes that induce humoral and cellular immune responses and protect against rabies [27,28]. Hence, RABV N is considered an alternative antigen candidate for rabies vaccines.
The design of an immunogen and vaccine platform is vital for inducing long-term, high-titre Nab. Currently, mRNA vaccines encapsulated with lipid nanoparticles (LNP) have shown robust immunogenicity, and low-dose vaccines are sufficient to elicit high Nab titres and cellular immune responses. mRNA vaccines can be rapidly manufactured and can activate GC reactions, which are required for the generation of prolonged humoral responses by eliciting long-lived plasma cells (LLPC) and memory B cells (MBC) [8,29,30]. In addition, clinically approved mRNA vaccines incorporate N1-methyl-pseudouridine which has been proved to decrease IVT mRNA innate immunogenicity and increase the immune efficacy [31].
In this study, we developed a series of nucleoside-modified mRNA vaccines encoding RABV G, soluble trimeric RABV G (tG-MTQ), and a prefusion-stabilized form of RABV G (preG). We also developed RABV VLP mRNA vaccine co-expressing preG and M to generate VLPs, and VLP/N mRNA vaccine co-expressing preG, M, and N, respectively. The immunogenicity, protective efficacy, GC responses and durability of RABV mRNA vaccines were assessed in BALB/c mice. The results demonstrated that RABV mRNA vaccines induced higher, durable humoral and cellular immune responses than inactivated rabies vaccine, and completely protected mice against intracerebral challenge. RABV mRNA vaccines, especially VLP and VLP/N mRNA vaccines showed superior capacities to elicit potent GC responses and memory B cell responses, demonstrating their potential as promising rabies vaccine candidates.
Materials and methods
Ethics statement
All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals (Jilin University, China). All the experimental procedures were reviewed and approved by the Animal Welfare and Research Ethics Committee of Jilin University (ethics number: S2021018).
Cells, viruses, protein and mice
293 T cells and BSR cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen, USA). 293 6E cells were grown in serum-free OPM-293 Medium (Cat. 81075-001, OPM Biosciences, China), supplemented with 0.1% Poloxamer 188 solution (Cat. P5556-100ML, Sigma, USA) at 100 rpm.
The G protein (Genebank: ADJ29911.1, replacing residues – 19–0 with MGWSCIILFLVATATGVHS and residues 439–524 with HHHHHH, respectively) was synthesized by GenScript Biotech Corp. (Nanjing, China).
The RABV strain CVS-11 (challenge virus standard-11) was provided by Changchun BCHT Biotechnology Co. The CVS-11 strain was used in serum neutralization and viral challenge experiments.
SPF female BALB/c mice (6–8 weeks old) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China).
mRNA production
RABV mRNA vaccines were designed based on rabies sequences of the Pasteur vaccine (PV) strain (GenBank: AAA47218.1) [32]. The rabies G gene encoded the whole length of the glycoprotein G, and the tG-MTQ gene encoded a soluble trimeric G protein with a MTQ motif (aa: GGSGGIKEEIAKIKEEQAKIKEKIAEIEKRIAEIEKRIAGGCC) added to the C-terminus of the ectodomain (amino acid – 19–435) of the G protein. And we mutated glycoprotein G sequence with H261L and H270P to generate the preG gene [14]. Matrix and nucleoprotein genes were designed based on the rabies sequences (GenBank: AAA47217.1 and BAA07689.1). The sequences were codon-optimized, synthesized by GenScript. Briefly, the linearized DNA templates of RABV mRNAs consisted of a 5’ untranslated sequences (UTR), 3’ UTR and polyA tail based on Moderna [33]. The mRNAs were produced using T7 RNA polymerase (E131; Novoprotein, China) with N1-methyl-pseudouridine triphosphate (DD4503; Vazyme, China). After transcription, cap1 structure was added via Cap1 capping system (M082; Novoprotein, China). The mRNAs were purified and stored at −80°C before use.
In vitro transfection and protein expression (IF, WB, and enzyme-linked immunosorbent assay (ELISA))
Immunofluorescence
293 T cells were transfected with RABV G, preG, or N mRNAs (1 µg), respectively, by Lipofectamine 2000 (2 µL). Then, 48 h after transfection, cells were fixed in 4% paraformaldehyde, incubated with anti-rabies virus glycoprotein antibody (ab82460; Abcam, UK), followed by FITC-conjugated goat anti-mouse IgG(H + L) (bs-0296G-FITC; Bioss, China) for 45 min. For the nucleoprotein expression assay, cells were permeabilized with 0.5% Triton X-100 after fixation and then incubated with FITC-conjugated anti-rabies Virus nucleoprotein antibody (12-0020; AbMax, China). Nuclei were stained with DAPI and images were obtained using the confocal microscope (Zeiss LSM 710, Jena, Germany).
Western blot
293 T cells were transfected with the RABV M mRNA (1 µg) by Lipofectamine 2000 (2 µL). Then, 48 h after transfection, the cells were harvested and analyzed using western blotting. The membranes were incubated with a mouse-specific antibody against RABV M (a gift from Dr. He, Academy of Military Medical Sciences) and then with a secondary antibody goat anti-mouse IgG-horseradish peroxidase (HRP) (115-035-003; Jackson ImmunoResearch Inc., West Grove, PA, USA).
293 6E cells were diluted to 1 × 106 cells/mL before transfection and 12.5 µg tG-MTQ mRNA was transfected into 10 mL 293 6E cells by Lipofectamine 2000 (25 µL). Then, 72 h post transfection, the concentrated cell supernatant was separated by 6% Native-PAGE or SDS-PAGE, and transferred onto nitrocellulose membranes. Membranes were incubated with equine-derived rabies antiserum, and followed with rabbit anti-horse IgG-HRP as a secondary antibody (bs-0308R-HRP; Bioss, China).
Sandwish enzyme-linked immunosorbent assay (ELISA)
The monoclonal antibody (mAb) 1112 recognizes the discontinuous epitopes on site II (aa: 34–42 and 198–200), and these two epitopes are linked by an S-S bridge when G protein poses trimeric form [34]. The mAb D1-25 recognizes the site III (aa: 330–338) of trimeric G protein [34]. Thus, these two commercially available mAbs are widely used in the characterization of trimeric form of G protein [14]. The sandwich ELISA was used to measure the trimeric G protein expressed in the supernatant of 293 6E cells which were transfected with tG-MTQ mRNA. Briefly, 96-well plates were coated with mAb 1112 (RDB 1112-1p500; RD Biotech, France). Then, the plates were blocked with 3% BSA at 37℃ for 1 h. Next, the concentrated cell culture supernatant was added to plates at 37°C for 1 h, followed by incubation with biotin-conjugated mAb D1-25 (MABF1967B-100UG; Merck, Germany) at 37°C for 1 h. And then, plates were incubated with HRP-conjugated streptavidin at 37°C for 1 h. Finally, plates were incubated with the TMB substrate and followed by H2SO4 to stop the reaction. The rabies virus was used as the positive control and the 293 6E cell culture supernatant was used as negative control.
VLP quantification
A structure-based prefusion conformation design is necessary for Nab production [15,20,21]. To increase prefusion conformational stability, we mutated H261 and H270P referred to previous study [14]. We co-expressed preG and matrix proteins to form VLP. The mRNAs encoding preG (12 µg) and matrix (6 µg) were co-transfected into 293 T cells with 36 µL Lipofectamine 2000 in T75 culture flask. Likewise, preG (12 µg), M (6 µg) and N (12 µg) mRNAs were co-transfected into 293 T cells with 60 µL Lipofectamine 2000 in T75 culture flask. Supernatants were collected 48 h after transfection, centrifuged at 3000 rpm for 30 min at 4°C, followed with 27,000 rpm (Beckman SW28 rotor, ∼130,000 ×g) for 4 h at 4°C. After centrifugation, the VLP pellets were resuspended in PBS and stored at 4°C.
Electron and immunoelectron microscopy
The VLP pellets were stained with 2% sodium phosphotungstate and then observed under transmission electron microscopy (TEM) (HT7800, Tokyo, Japan). The VLPs were bound to grids, incubated with mouse anti-RABV G mAb (ab1002, Abcam, UK). After incubation with gold-labeled goat anti-mouse IgG antibody, the grids were observed under TEM.
Western blotting
The VLPs collected in the supernatants of 293 T cells that co-transfected with preG, M mRNAs (RABV VLP), or co-transfected with preG, M, N mRNAs (RABV VLP/N) were analyzed by Western blotting with mouse anti-RABV G mAb (3R7-4F1, HyTest, Finland) and mouse anti-RABV M polyclonal antibodies. And 293 T cells in RABV VLP/N group were harvested, incubated with mouse anti-RABV N mAb (a gift from Changchun BCHT Biotechnology Co.) and then with a secondary antibody goat anti-mouse IgG-horseradish peroxidase (HRP) (115-035-003; Jackson ImmunoResearch Inc., West Grove, PA, USA).
LNP formulation
LNP formulations were prepared as described previously [35]. Briefly, lithium chloride-purified mRNA (0.17 mg/mL) was diluted into sodium citrate buffer (pH 4.0). The mRNA was encapsulated using a microfluidics mixer (NanoAssemblr, Canada) wherein an ethanolic lipid mixture of SM102, 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and PEG2000 (50:10:38.5:1.5 mol/mol). The encapsulated RNA-to-total lipid ratio was 3:1 (v/v) and the total lipid concentration was 12.5 mM. To ensure co-expression of preG and M in the same cell, RABV preG and M mRNAs were co-formulated in same LNP at the ratio of 2:1 to generate RABV VLP mRNA vaccine. Similarly, RABV preG, M and N mRNAs were co-formulated in one LNP at the ratio of 2:1:2 to generate RABV VLP/N mRNA vaccine [36,37]. After diafiltration with 50 × volume PBS buffer (pH 7.4) and sterile filtration with a 0.22 μm filter, mRNA-LNPs were stored at 4°C until use.
The mRNA-LNP formulation was characterized for particle diameter, polydispersity index (PDI), zeta potentials using NanoZS (Malvern, UK). The mean hydrodynamic diameter was ∼120 nm, with a PDI of 0.1–0.2.
The encapsulation efficiency was tested with RiboGreen RNA assay (R11490; Thermo Fisher Scientific, USA). Briefly, samples were incubated with RiboGreen reagent in 1 × TE buffer with or without 0.5% Triton X-100 to measure the total and the unencapsulated mRNA, respectively. Fluorescence intensities were measured at an excitation of 485 nm and emission at 528 nm. Based on the RNA standard curves with and without triton, the encapsulation efficiency was calculated by the following formula: EE% = ((total mRNA − unencapsulated mRNA)/total mRNA) x 100%. For all groups, the mRNA encapsulation efficiencies are above 85%.
Immunization procedure
BALB/c mice were immunized with mRNA formulations in a volume of 0.1 mL via intramuscular injection into the hind legs. Seven groups of mice (n = 18) were immunized twice at 14 d intervals. For rabies G, tG-MTQ, and preG groups, mice were immunized with 5 µg mRNA. For rabies preG VLP, mice were immunized with 5 µg preG and 2.5 µg matrix mRNAs. For nucleoprotein/preG VLP, mice were immunized with 5 µg preG, 2.5 µg matrix, and 5 µg nucleoprotein mRNAs. Negative control mice received the same dose of empty LNP, and a one-tenth dose of commercial inactivated rabies vaccine was used as the positive control. The commercial inactivated rabies vaccine was provided by Changchun BCHT Biotechnology Co., Ltd. (Changchun, China). The potency of the vaccine was above 2.5 IU/mL.
RABV-specific immunoglobulin titre
RABV-specific IgG in mouse serum was detected by ELISA. The immunized mouse serum was serially diluted and added to inactivated rabies vaccine-coated plates, followed by the addition of HRP-conjugated goat anti-mouse IgG, IgG2a, IgG2b, or IgG1 (ab97240-1 mg, ab97245-1 mg, ab97250-1 mg, Abcam, UK). The optical density was measured at 450 nm using an iMarkTM Microplate Reader (Bio-Rad, Hercules, CA, USA).
Serum neutralization assay
Virus-neutralizing antibody titres were determined using the rapid fluorescent focus inhibition test (RFFIT) assay, as previously described [38]. In this study, the national standard of human immunoglobulin prepared by NIFDC was used as the reference standard (37 IU/mL). Firstly, the reference standard was diluted into 1 IU/mL. Briefly, mice serum and reference standard were serially 3-fold diluted in DMEM and added 100 μL to 96-well plates. The diluted CVS-11 strain which could infect more than 80% BSR cells was added to the plates at 50 μL/well. Then, the plates were incubated at 37°C for 1 h. Next, 50 μL of 5 × 104 BSR cells were added to the plates and cultured at 37°C with 5% CO2 for 24 h. Subsequently, the cell layer was washed with PBS and fixed with 80% cold acetone for 1 h. After fixing, 1:400 FITC-conjugated Rabies virus nucleoprotein antibody (5500; Merck, Germany) was added and incubated at 37°C for 1 h. Finally, the fluorescence area was observed under a fluorescence microscope (Olympus IX70, Tokyo, Japan) and the results of neutralizing titre were calculated by the Reed and Muench formula.
Enzyme-linked immunospot (ELISpot) assay
The assay was performed using a mouse IFN-γ or IL-4 ELISpotPLUS kit (Cat. no. 3321-4HPW-10, 3311-4HPW-10; MabTech, Sweden). Briefly, the spleens were collected on day 24 and 126, respectively, and 1 × 106 splenocytes were incubated with inactivated rabies vaccine (1:100 dilution) at 37°C for 24 h. Then, the plates were incubated with detection antibody (R4-6A2-biotin; BVD6-24G2-biotin) at room temperature for 2 h. Next, 1:1000 HRP-conjugated streptavidin was added and incubated at room temperature for 1 h. After washing with PBS, plates were added with TMB substrates and developed until distinct spots emerge. Finally, reaction was stopped by washing extensively in deionized water. The number of spots were read and analyzed with an ImmunoSpot S6 analyzer (Cellular Technology Limited, USA).
Sample processing and flow cytometry
The mice were euthanized by CO2 inhalation, and both inguinal lymph nodes were harvested on day 24. The procedure details are provided in the supplementary materials.
Challenge infection
Mice were immunized twice at 14 d intervals, and six mice of each group were challenged intracerebrally (i.c.) with 50-fold LD50 CVS-11 in a volume of 30 μL at day 28 which referred to NIH test for potency testing [11,39]. All challenged mice were observed for death during 14 d post-challenge, and animal survival was calculated.
Ab-secreting cells (ASC) ELISpot
ELISpot plates (CT790-PR5, U-CyTech) were coated with rabies glycoprotein at 10 μg/mL overnight at 4°C. Bone marrow was harvested from the femur and tibia, and preincubated with IL-2 and R848 for 3 d. A biotinylated detection antibody was added, followed by streptavidin-horseradish peroxidase conjugation. The freshly prepared AEC solution was added until spots were visible, which were then emptied and both sides of the PVDF membrane were rinsed thoroughly with demineralized water. The results were analyzed using an ImmunoSpot S6 analyzer (Cellular Technology Limited, USA).
Statistical analysis
Statistical analyses were performed using one-way analysis of variance with GraphPad Prism version 9.0.0. Values are expressed as the mean ± standard error of the mean (SEM). Differences were considered statistically significant at P < 0.05.
Results
Construction and generation of nucleoside-modified RABV mRNA vaccines
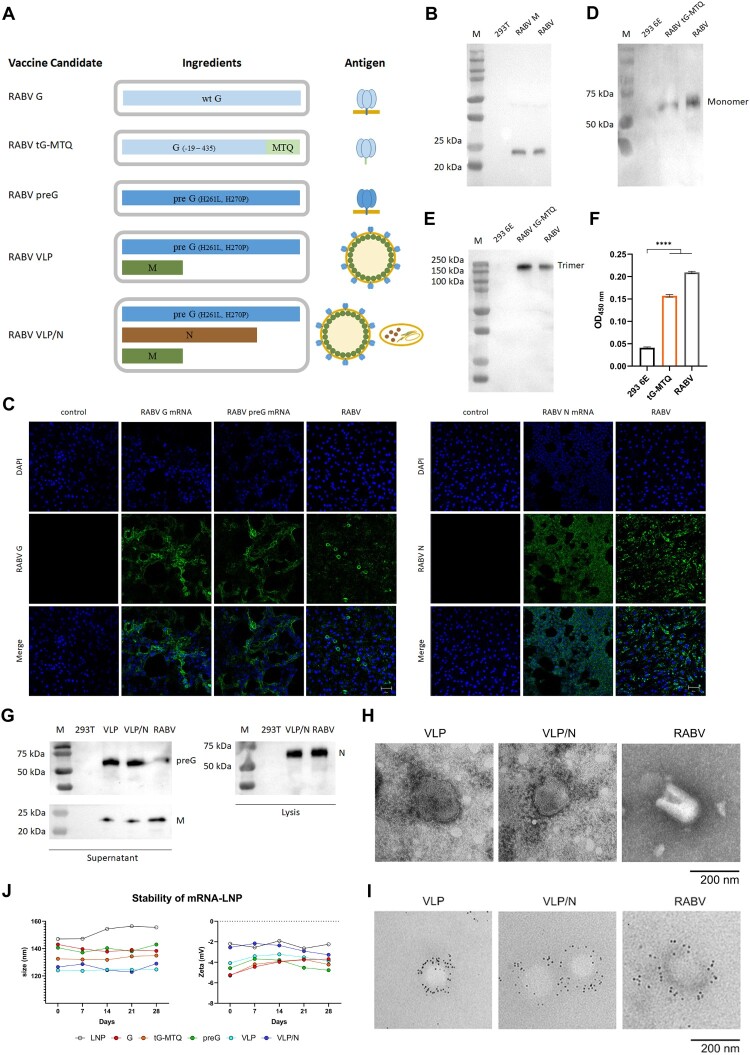
The RABV surface homotrimeric glycoprotein elicits major neutralizing antibodies; thus, it is the first choice of antigen for RABV vaccine candidates. Thus, we designed a panel of mRNA antigens to compare the immunogenicity of different forms of the RABV G protein: full-length wild-type RABV G (G), a soluble trimeric form of RABV G (tG-MTQ), a full length prefusion stabilized construct of RABV G (preG), and an RABV VLP self-assembled by co-expressing RABV preG and M protein. Additionally, we sought to enhance the design of an RABV VLP/N mRNA vaccine by adding a transcript encoding the RABV N protein (Figure 1(A)). Subsequently, we confirmed the expression of G, tG-MTQ, preG, M, and N antigen proteins by transfecting 293 T cells or 293 6E cells. Western blotting demonstrated that RABV M protein expressed effectively with right size (23 kDa) (Figure 1(B)).
Figure 1.
Design and Characterization of RABV mRNA vaccines. (A) Schematic of five mRNA candidates for rabies. RABV G contains a mRNA encoding the full length of wild type RABV G. RABV tG-MTQ contains a mRNA encoding soluble truncated trimeric form of RABV G. RABV preG contains a mRNA encoding stabilized prefusion form of RABV G. RABV VLP contains two mRNAs encoding preG and M proteins that can self-assemble into VLPs. RABV VLP/N contains three mRNAs encoding preG, M, and N proteins. (B) Western blotting of RABV M protein. Expression of RABV M proteins were probed using mouse anti-RABV M polyclonal antibodies. (C) Immunofluorescence assays of the expression of RABV G, preG, and N proteins. 293 T cells were transfected with RABV G, preG, and N mRNA for 48 h, respectively, and detected by anti-rabies virus glycoprotein antibody and FITC-rabies virus nucleoprotein antibody. Scar bar: 50 μm. (D and E) Expression of the trimeric form of RABV G protein (tG-MTQ) was analyzed by SDS-PAGE, Native-PAGE, and western blottings, which showed that the monomeric and trimeric forms of tG-MTQ were approximately 57 and 150 kD, respectively. (F) Expression of the trimeric form of RABV G protein (tG-MTQ) was analyzed using a sandwich ELISA which were probed with mAbs 1112 and D1-25. (G) Western blotting assays of RABV VLPs. Expression of RABV preG, M or N proteins was probed using mouse anti-RABV G mAb, mouse anti-RABV M polyclonal antibodies and mouse anti-RABV N mAb. (H) Electron microscopy of VLPs. RABV VLP and VLP/N were concentrated by ultracentrifugation and prepared to negative staining for electron microscopy. Scar bar: 200 nm. (I) Immunoelectron microscopy of VLPs. RABV VLP and VLP/N were stained with mouse anti-RABV G mAb and then incubated with gold-labeled goat anti-mouse IgG antibody. Scar bar: 200 nm. (J) Stability of mRNA-LNPs stored at 4°C. Particle size and Zeta potential of six mRNA-LNPs detected in 28 d.
RABV G and preG protein are membrane-anchored proteins, and the N protein is intracellular protein. Immunofluorescence results showed that G, preG proteins were expressed on the cellular membrane and N protein was expressed intracellularly (Figure 1(C)).
Due to the non-specific binding between equine-derived rabies antiserum and complete DMEM, the tG-MTQ expression was examined in 293 6E cells which were cultured in serum-free medium. The trimeric form of tG-MTQ protein was confirmed by western blotting analysis (Figure 1(D,E)). Furthermore, the mAb 1112 recognizes the discontinuous epitopes on site II (aa: 34–42 and 198–200), and these two epitopes are linked by an S-S bridge when G protein poses trimeric form. The mAb D1-25 recognizes the site III (aa: 330–338) of trimeric G protein. Thus, these two commercially available mAbs were used to measure the trimeric G protein expressed in the supernatant of 293 6E cells which were transfected with tG-MTQ mRNA. The result of sandwich ELISA demonstrated that tG-MTQ protein expressed in trimeric form (Figure 1(F)).
To confirm the assembly of RABV VLPs, we co-transfected with preG and M mRNAs at a molar ratio of 2:1 into 293 T cells. Likewise, preG, M and N mRNAs co-transfected 293 T cells at a molar ratio of 2:1:2. We concentrated the VLPs in the supernatant by ultracentrifugation, tested them using western blotting and examined them using an electron microscope. Western blotting results demonstrated that RABV VLP and VLP/N contained preG and M components, and N protein was mainly detected in 293 T cell lysate (Figure 1(G)). As shown in Figure 1(H), the enveloped RABV VLP and VLP/N particles were approximately 80–200 nm with densely arrayed surface spikes. To further verify the formation of VLPs, we performed immunoelectron microscopy (Figure 1(I)). These results demonstrated that RABV VLPs were formed and co-expression of N protein didn’t influence the assembly and budding of RABV VLPs.
We encapsulated RABV mRNAs in LNP to improve in vivo expression. To increase the efficiency of the VLP assembly, we co-packaged preG and M at a ratio of 2:1 (RABV VLP) and preG, M, and N at a ratio of 2:1:2 (RABV VLP/N) (Figure 1(A)) [36,37]. Next, the average particle size and zeta potential of mRNA-LNPs stored in PBS at 4°C were stabilized for 28 d (Figure 1(J)). Furthermore, to examine the integrity of mRNA after storing at 4°C for 28 d, we took preG mRNA-LNP as an example and found that mRNA-LNP could still express RABV preG protein in 293 T cells (Figure S1).
Based on prefusion stabilized RABV mRNA vaccines elicited robust humoral immune responses
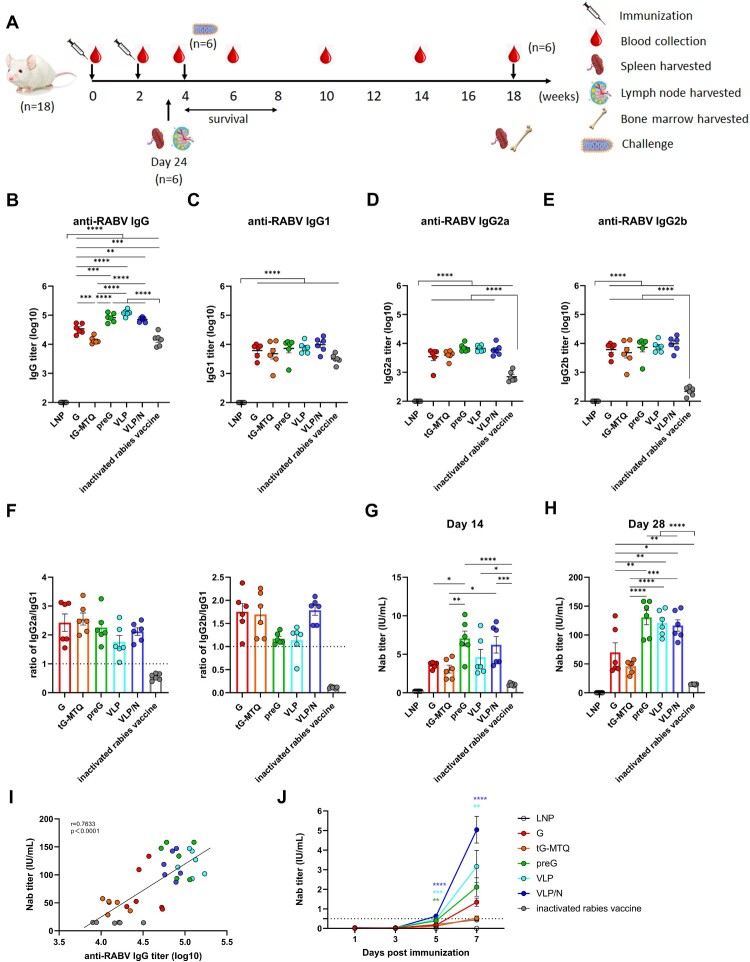
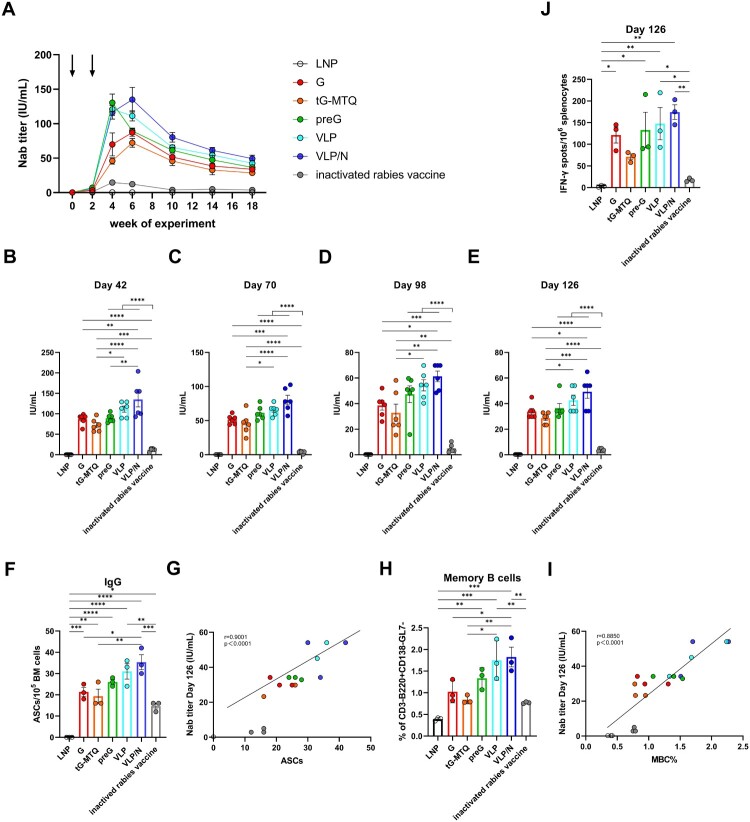
In this study, we utilized a prime-boost strategy to immunize BALB/c mice at two-week intervals to assess the humoral immune responses elicited by different RABV mRNA vaccines (n = 6) (Figure 2(A)). After booster vaccination, all RABV mRNA vaccines induced RABV-specific IgG titres; G, preG, VLP, and VLP/N induced significantly higher IgG titres than the inactivated vaccine. Furthermore, the IgG titres induced by RABV in the preG, VLP, and VLP/N groups were 2.4-, 3.5-, and 2.1-fold higher, respectively, than those in the G group (Figure 2(B)). RABV-specific IgG1 was predominant in rabies-specific IgG responses in mice immunized with the inactivated vaccine (Figure 2(C–E)). In contrast, RABV vaccines induced high levels of RABV-specific IgG2a, IgG2b and IgG1 titres (Figure 2(C–E)). As shown in Figure 2(F), the ratios of IgG2a/IgG1 and IgG2b/IgG1 suggested that the RABV mRNA vaccines elicited a Th1-biased immune response.
Figure 2.
Humoral immune responses after RABV mRNA vaccination. (A) Schematic of the immunization, rabies virus challenge, and sampling strategy. Six- to eight-week-old female BALB/c mice were immunized intramuscularly twice at day 0 and 14 with RABV G, tG-MTQ, preG, VLP, and VLP/N mRNA vaccine, respectively, followed by virus challenge and tissue sample collection. (B to E) RABV-specific IgG and IgG antibody isotype (IgG1, IgG2a, and IgG2b) titres were quantified by ELISA, respectively, from serum samples collected on day 28 (n = 6). (F) The ratio of IgG2a/IgG1 and IgG2b/IgG1 were determined from the absorbance values of 100-fold diluted serum at day 28 measured at 450 nm. (G and H) Neutralizing antibody (Nab) titres of serum collected at day 14 and 28 were analyzed by Rapid Fluorescent Focus Inhibition Test (RFFIT) assay (n = 6). The dashed line at 0.5 IU/mL represents a protective titre for RABV Nab titre. (I) Spearman correlation of (B) RABV-specific IgG titres and (H) Nab titres. (J) Kinetic of Nab in mice immunized with RABV mRNA vaccines or inactivated vaccines after a single vaccination (n = 3). * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ns, no significant difference. Data represent mean ± SEM.
Nab is crucial for preventing rabies infection, and a > 0.5 IU/mL Nab titre, is considered protective. Two weeks after the first vaccination, all RABV mRNA vaccines induced higher Nab titres (> 0.5 IU/mL) in immunized mice than the inactivated vaccine (Figure 2(G)). The Nab titres of RABV mRNA vaccines increased rapidly after the second vaccination, G (range: 39–133 IU/mL), tG-MTQ (range: 29–57 IU/mL), preG (range: 91–158 IU/mL), VLP (range: 93–147 IU/mL), and VLP/N (range: 87–147 IU/mL) elicited significant higher level than that of the inactivated vaccine (range: 14–15 IU/mL) (Figure 2(H)). Interestingly, the Nab titres of G were 1.5-fold higher than those of the tG-MTQ group. In addition, preG, VLP, and VLP/N induced 1.9-, 1.7-, and 1.7-fold higher Nab titres than the G group, respectively, indicating the advantage of antigen design based on prefusion stabilized conformation in improving immune responses. Nab titres were associated with RABV-specific IgG responses (Figure 2(I)).
Moreover, the kinetics of the Nab titre response in the early period after vaccination is important for clearing rabies and preventing infection. We have evaluated the Nab titre at days 1, 3, 5 and 7 of mice after a single immunization (n = 3). As shown in Figure 2(J), VLP and VLP/N mRNA induced significantly higher Nab titres than that in inactivated rabies vaccine group. Moreover, the Nab titres of RABV mRNA groups increased at day 7 (G: 1.1–1.8 IU/mL; tG-MTQ: 0.3–0.7 IU/mL; preG: 1.3–2.9 IU/mL; VLP: 2.2–4.8 IU/mL and VLP/N: 4.0–6.3 IU/mL). In contrast, the Nab titre of mice immunized with inactivated rabies vaccine only reached 0.39–0.46 IU/mL at day 7, which was lower than protective Nab titre level (0.5 IU/mL). Taken together, RABV mRNA vaccines induced higher Nab titre and reached the level of protective Nab titre more rapidly than inactivated vaccine after a single vaccination.
RABV mRNA vaccines induced robust cellular immune responses
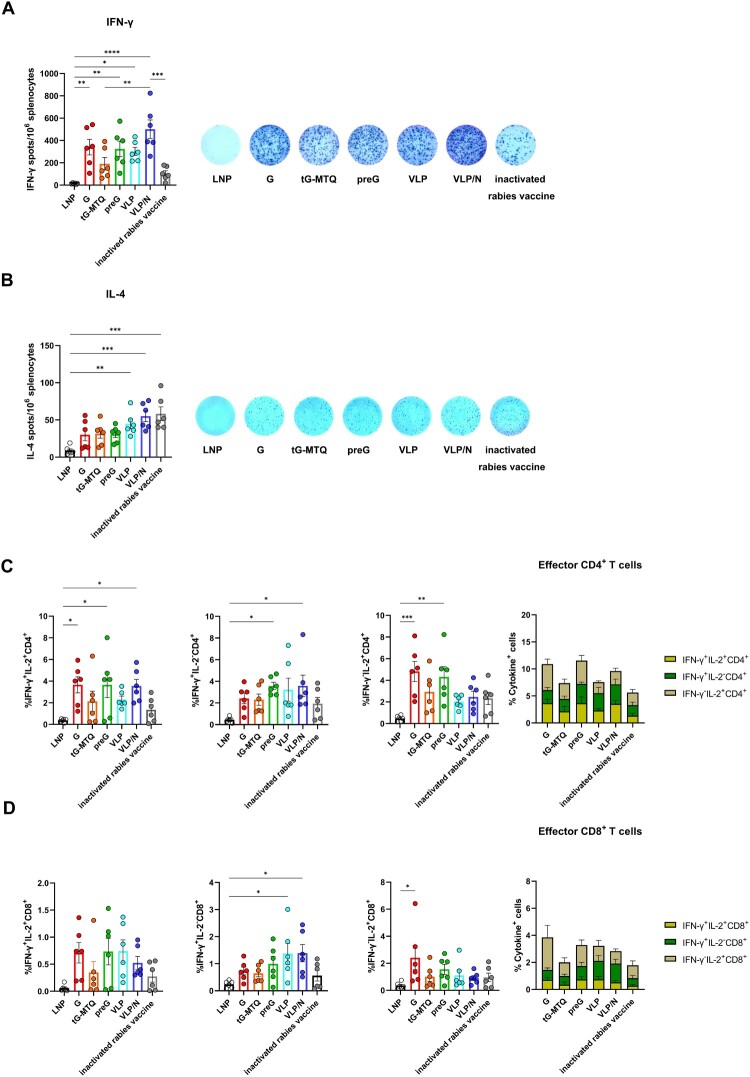
T cell responses play a key role in rabies virus clearance in the central nervous system, especially the Th1-biased immune response. Thus, we measured the RABV-specific IFN-γ and IL-4 secretion in splenocytes by ELISpot assays to evaluate T cell activation (n = 6). Our findings indicated that RABV mRNA vaccines elicit high IFN-γ secretion. VLP/N mRNA induced IFN-γ secretion at a significantly higher level than the inactivated vaccine, and higher than that of G, preG, and VLP groups but not statistically significant overall (Figure 3(A)). As shown in Figure 3(B), the VLP and VLP/N mRNA vaccines induced significantly stronger IL-4 responses than the LNP group and slightly higher levels than G, tG-MTQ, and preG. Thus, VLP and VLP/N showed relatively balanced Th1/Th2 responses compared to RABV mRNA vaccines encoding soluble and membrane-anchored immunogens.
Figure 3.
RABV mRNA vaccines induced robust cellular immune responses. (A and B) Spleen were harvested and stimulated with inactivated rabies vaccine 10 days after second immunization (n = 6). An ELISpot experiment was used to determine the ability of splenocytes to release IFN-γ (A) and IL-4 (B) after stimulation. (C and D) RABV-specific CD4+ and CD8+ T cells producing IFN-γ, IL-2 were measured by flow cytometry 10 days after second immunization. CD4+ (C) and CD8+ (D) T cells were stained for type 1 intracellular cytokine expression. * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ns, no significant difference. Data represent mean ± SEM.
Moreover, we utilized an ICS assay to measure the RABV-specific polyfunctional CD4+ and CD8+ T cells secreting IFN-γ and IL-2 (Figure S2). RABV mRNA vaccines elicited stronger IFN-γ+IL-2+, and IFN-γ+IL-2− CD4+ T responses than that of inactivated rabies vaccine (Figure 3(C)). In contrast, frequencies of RABV-specific CD8+ T cell responses were lower than those of CD4+ T cell responses as a whole. However, RABV mRNA vaccines still elicited stronger IFN-γ+IL-2+, IFN-γ+IL-2−, and IFN-γ−IL-2+ CD8+ T responses than the inactivated rabies vaccine (Figure 3(D)). These results indicated efficient RABV-specific T cell responses to RABV mRNA vaccines and better induction of CD4+ T cells compared to inactivated vaccines.
RABV mRNA vaccines protected mice against lethal rabies virus challenge
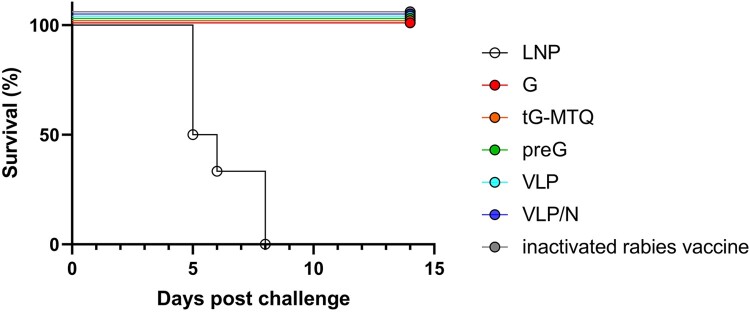
To explore the in vivo protective efficacy of the RABV mRNA vaccines, immunized BALB/c mice (n = 6) were challenged i.c. with 50-fold LD50 of the rabies virus CVS-11 strain two weeks after the second vaccination. We monitored the survival of each mouse daily for 14 d post-infection and found that all RABV mRNA vaccines provided complete protection (Figure 4).
Figure 4.
RABV mRNA vaccines protected mice from the lethal intracerebral rabies virus challenge. Immunized mice were challenged after two weeks post boost injection with 50-fold LD50 rabies virus (CVS-11 strain), and the survival curves during a 14-d observation period were illustrated by Kaplan–Meyer analysis (n = 6).
RABV VLP mRNA vaccines were superior in promoting potent GC responses by inducing tfh and GC B cells
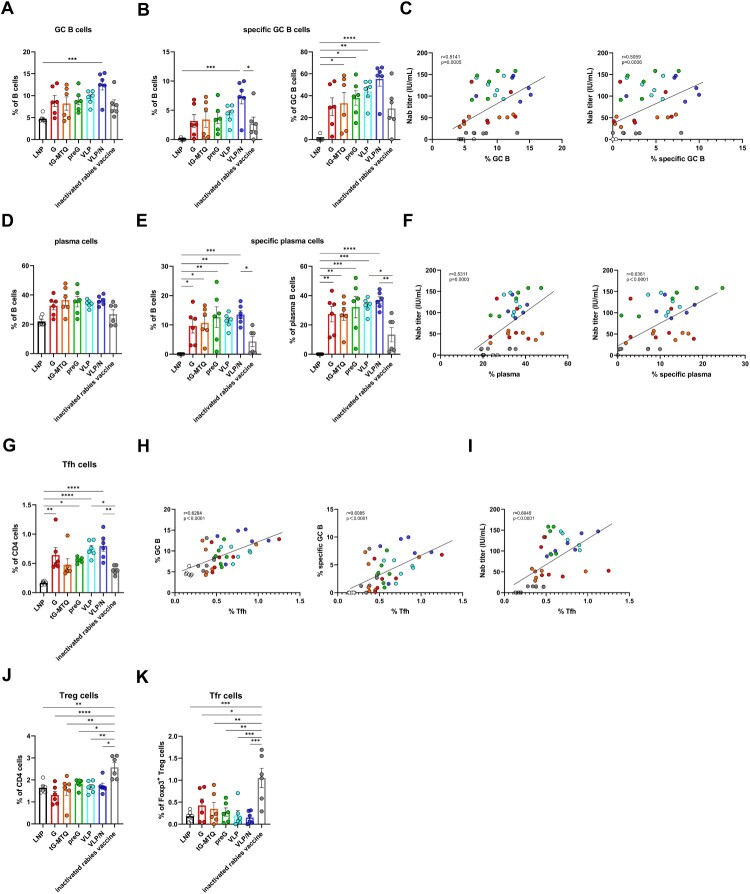
In the GC, B cells which mature with proliferation, somatic hypermutation, and affinity-selection, will produce high-affinity Nab [40–42]. To confirm the ability of RABV mRNA vaccines fostering the formation of GCs after immunization, LNs from mice immunized 10 days were directly evaluated by immunofluorescence. The formation of GCs is confirmed, which are characterized as GL-7+ cells surrounded by a network of FDC and a mantle of B220 naïve B cells. In contrast, inactivated rabies vaccine group only showed poorly formed GCs in LN from, with disrupted CD21+ FDC networks. In addition, histological analysis also showed a larger number of GCs and average colocalized area in RABV mRNA vaccine groups than those in inactivated rabies vaccine group, although no statistically significant difference was observed (Figure S3). Next, we evaluated the GC responses in lymph nodes 10 d after the second vaccination using flow cytometry (n = 6) (Figure S4). The RABV mRNA vaccines enhanced the number of GC B (CD3−B220 + IgD−GL7+) and RABV-specific GC B cells. Notably, VLP/N mRNA demonstrated a significantly higher percentage of RABV-specific GC B cells than the inactivated rabies vaccine (Figure 5(A–B) and Figure S5(A)). Correlation analysis revealed that lymph node GC B cells were significantly and positively correlated with Nab titres (Figure 5(C)). Next, we evaluated plasma cells (PC) (CD3−B220 + CD138+), which secrete large quantities of antibodies against antigens. As shown in Figure 5(D,E) and Figure S5(B), the RABV mRNA vaccines enhanced the number of plasma and RABV-specific plasma cells. RABV G, tG-MTQ, preG, VLP, and VLP/N mRNA induced 2.2-, 2.5-, 2.9-, 2.6-, and 3.1-fold higher percentage of RABV-specific plasma cells, respectively, than the inactivated rabies vaccine (Figure 5(E)). Correlation analysis also revealed that lymph node plasma cells were significantly and positively correlated with Nab titres (Figure 5(F)).
Figure 5.
RABV mRNA vaccines resulted in potent GC responses. Mice immunized i.m. with G, tG-MTQ, preG, VLP, or VLP mRNA, or inactivated rabies vaccine. Inguinal lymph nodes were collected 10 d after the second immunization (n = 6). (A) The proportion of total GC B cells (CD3−B220 + IgD−GL7+) in B cells. (B) The proportion of total RABV-specific GC B cells in B cells and total GC B cells. (C) Spearman correlation of day 24 Nab titre and lymphatic total GC B cells and RABV-specific GC B cells frequency. (D) The proportion of total plasma cells (CD3−B220 + CD138+) in B cells. (E) The proportion of total RABV-specific plasma cells in B cells and total plasma cells. (F) Spearman correlation of day 24 Nab titre and lymphatic total plasma cells and RABV-specific plasma cells frequency. (G) The proportion of total Tfh cells (CD3 + CD4 + CXCR5 + PD-1 + Foxp3−) in CD4+ T cells. (H) Correlation between lymph node Tfh cell frequency and total GC B cells or RABV-specific GC B cells frequency. (I) Correlation between day 24 Nab titre and lymph node Tfh cell frequency. (J and K) Lymph node Treg (CD3 + CD4 + Foxp3+), and Tfr (CD3 + CD4 + CXCR5 + PD-1 + Foxp3+) cell frequencies after the second immunization (n = 6). * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ns, no significant difference. Data represent mean ± SEM.
Tfh, a specialized subset of CD4+ T cells, tightly regulate GC reaction by enabling GC B cells proliferation, survival, and differentiation through co-stimulatory molecules and cytokines [42]. To examine the effects of the RABV mRNA vaccines on Tfh (CD3 + CD4 + CXCR5 + PD-1 + Foxp3−) induction, inguinal lymph nodes were analyzed by flow cytometry on day 10 after the second immunization (Figure S6). We observed that VLP and VLP/N mRNA elicited higher levels of Tfh cell induction after boost vaccination, whereas the percentage of Tfh was positively correlated with GC B cells, RABV-specific GC B cell percentage, and Nab titres (Figure 5(H,I)). Therefore, lymph node Tfh cells partially explain the potent and durable humoral immune responses induced by the RABV VLP and VLP/N mRNA vaccines. Additionally, compared with RABV mRNA vaccines, the inactivated rabies vaccine elicited remarkably higher levels of the suppressor Treg (CD3 + CD4+ Foxp3+) and Tfr (CD3 + CD4 + CXCR5 + PD-1 + Foxp3+) cells in the lymph nodes, which may restrict immune responses (Figure 5(J,K)). Taken together, these results suggest that RABV mRNA vaccines forming virus-like particle promote the generation of Tfh, GC B cells, and PCs, which would elicit a more robust and sustained humoral immune response.
RABV VLP mRNA vaccines elicited potent and sustained humoral immune responses with strong long-lived plasma cell and memory B cell responses
A long-lasting Nab titre is required for prophylactic rabies immunization. Thus, we assessed the durability of the Nab response after prime-boost vaccination. The mice in each group were bled for 126 d (n = 6), and all RABV mRNA vaccines remained stable during an 18-week period (Figure 6(A)). Notably, the Nab titre of the VLP/N mRNA group peaked four weeks after booster vaccination and ranged from 92 to 203 IU/mL, which was significantly higher than that in the G, tG-MTQ, and inactivated rabies vaccine groups during weeks 6–18 (Figure 6(B–E)). Mice immunized with the inactivated vaccine generated a lower Nab titre than those in the RABV mRNA groups, which decreased to 3.7 IU/mL at week 10. In contrast, the median Nab titres of G, tG-MTQ, preG, VLP, and VLP/N groups remained 33.8, 28.7, 36.3, 42.6, and 49.2 IU/mL, respectively, at week 18 (Figure 6(C,E)).
Figure 6.
RABV mRNA vaccination elicited sustained humoral immune responses and antigen-specific LLPC and MBC responses. (A) Kinetic of Nab titre induced by RABV mRNA vaccines in BALB/c mice. Immunization points are indicated by arrows (n = 6). (B to E) Nab titres at day 42, 70, 98, and 126 (n = 6). (F) Quantification of bone marrow (BM) RABV-specific IgG ASCs (n = 3). (G) Spearman correlation of (E) day 126 Nab titre and BM RABV-specific IgG+ ASC. (H) Flow cytometry staining of total splenic memory B cells (MBC) (n = 3). (I) Spearman correlation of (E) day 126 Nab titre and splenic MBC. (J) ELISpot assay of IFN-γ secretion in splenocytes on day 126 (n = 3). * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001; ns, no significant difference. Data represent mean ± SEM.
Some B cells are converted into LLPCs and MBCs in the lymph nodes or lymphatic parts [43,44]. LLPCs move to the bone marrow and persistently produce RABV-specific antibodies to protect the body from infection, whereas MBCs initiate rapid recall responses upon secondary exposure. To examine the LLPC and MBC responses, we compared bone marrow RABV-specific ASC elicited by RABV mRNA vaccines and inactivated rabies vaccine at four months post-immunization (n = 3). Consistent with the Nab titre data above, mice immunized with RABV mRNA vaccines had substantially higher RABV-specific IgG+ LLPC numbers than those immunized with the inactivated vaccine, and the magnitude of LLPC elicited by VLP/N was significantly higher than G and tG-MTQ groups (Figure 6(F,G)). Furthermore, MBCs were identified by flow cytometry four months post-immunization (n = 3). Notably, the magnitude of MBCs induced by VLP and VLP/N was 2.3- and 2.4-fold higher than that in the inactivated vaccine-immunized mice, which was strongly correlated with the Nab titre on day 126 (Figure 6(H–I) and Figure S7). In addition, we evaluated the antigen-specific IFN-γ and IL-4 (data not shown) activities in splenocytes by ELISpot assays at day 126 (n = 3). As shown in Figure 6(J), preG, VLP, and VLP/N mRNA induced significantly stronger IFN-γ secretions compared with the inactivated vaccine, consistent with potent Th1-biased immune responses. Taken together, these results indicate more efficient generation of long-lasting humoral and cellular immunity by the mRNA vaccine platform and the formation of virus-like particles.
Discussion
The successful of mRNA vaccines for COVID-19 pandemic has shown their benefits. First, mRNA vaccines are noninfectious and cannot be integrated into the genome. Second, the antigens can be designed rapidly and flexibly. Third, mRNA vaccine platforms can induce robust humoral and cellular immune responses. Moreover, nucleoside-modified mRNA-LNP vaccines promote GC responses and elicit potent LLPC and MBC responses, which are important for achieving durable protective humoral immune responses [29]. In addition, Mulroney et al. recently found that specific slippery sequences within mRNAs containing N1-methyl-pseudouridine can result in +1 ribosomal frameshifting and induce aberrant T-cell responses to some non-target proteins, but the potential off-target effects could be minimized or eliminated through sequence optimization [45]. We have searched the potential ribosome slippery sequences mentioned in the study by Mulroney et al. and have not found the slippery sequences in RABV G, tG-MTQ, preG and N mRNA [45]. There was a U*U*U*U* slippery site in RABV M mRNA sequence but a + 1 frame stop codon was closely present downstream of the slippery site. Thus, we think that run-off transcripts would not be produced during IVT with N1-methyl-pseudouridine.
RABV G is the only surface-exposed glycoprotein and the main target for eliciting Nab. Therefore, RABV G is widely used as an antigen in vaccines. RABV G protein assembles as a homotrimer that mediates receptor binding and membrane fusion. Several studies have demonstrated that the trimeric form of RABV G is more immunogenic than the monomeric form [11,46,47]. Moreover, RABV G is a class III viral fusion protein that can reversibly transition between a prefusion form (at neutral pH) and a postfusion form (at acidic pH), which causes structural heterogeneity and low immunogenicity. In addition, RABV N could represent a vaccine candidate because it can elicit T- and B-cell responses to produce Nab and protect against the lethal RABV challenge.
VLP-based vaccines have the advantages of a similar native viral structure, lack of a viral genome, and highly repetitive antigenic epitopes that are effective in cross-linking the B cell receptor to activate B cells. VLP vaccines are efficiently taken up by DCs, which process and present antigen fragments on MHC I and MHC II molecules to lymphocytes. Furthermore, VLP can be retained at lymph node FDC to improve GC reactions.
In this study, we first designed the RABV G protein as a vaccine antigen (G), previously tested in preclinical trials and phase I and II clinical trials [7,32,48]. Aiming to enhance both potency and durability of Nab response, we employed different designs to elicit immune responses, including tG-MTQ, preG, VLP and VLP/N. Our results showed that the G, preG, VLP, and VLP/N groups induced significantly higher RABV-specific IgG and Nab titres than the tG-MTQ and inactivated rabies vaccine. Unfortunately, the immunogenicity of RABV mRNA encoding trimeric form of G is lower than that of the wild type G, similar results have been reported [38]. This is probably because soluble antigens are more difficult to process and present by APCs than membrane-anchored antigens, or because the trimeric structure of tG-MTQ is unstable, or because the trimeric structure of tG-MTQ is different from that of native-like RABV G trimers. Interactions between transmembrane domain and W121 likely stabilize RABV G trimers and prevent G from shifting into alternate conformations, thus we will utilize different lengths of truncated G protein to form trimer in future study [49]. In addition, trimeric RABV G, which is preceded by an N-terminal CD5 signal peptide, followed by a 7 aa long linker (LIGGGGI) and an artificial GCN4-based trimerization domain, showed higher immunogenicity and protective efficacy than monomeric RABV G [11]. This flexible linker might play an important role in adjusting the angle of the RABV G monomer to form a native-like RABV G trimer. Further tests using other trimeric motifs, such as Trimer-tag, are needed to form soluble RABV G proteins as immunogens [50,51]. Next, RABV G mRNA vaccine elicited Nab titres of 69.85 IU/mL with two-dose immunizations. In comparison, preG, VLP, and VLP/N induced median Nab titres of 130.4, 121, and 116.5 IU/mL, respectively, after two injections. This result shows that antigen design based on prefusion stabilized form of RABV G (preG) is superior to the wild type (G) in displaying antigen epitopes and eliciting Nab, which is in accordance with results obtained from SARS-CoV-2 S-2P and RSV pre-F, which are based on the prefusion conformation [15,17,18]. Currently approved SARS-CoV-2 mRNA vaccines encoding a prefusion stabilized form of spike protein are highly effective in preventing more than 90% of symptomatic and severe SARS-CoV-2 infections [19]. Two RSV vaccines named Arexvy and Abrysvo, encoding RSV will be approved in 2023 [20,21]. Our study also demonstrated the importance of the prefusion conformation as a vaccine immunogen.
One of the key factors preventing rabies infection is the kinetics of Nab production during the early phase. As shown in Figure 2(J), VLP and VLP/N mRNA induced significantly higher Nab titres than that in inactivated rabies vaccine group at day 7 after a single immunization (VLP: 2.2–4.8 IU/mL; VLP/N: 4.0–6.3 IU/mL). In contrast, the Nab titre of mice immunized with inactivated rabies vaccine only reached 0.39–0.46 IU/mL at day 7, which was lower than protective Nab titre level (0.5 IU/mL). Thus, RABV mRNA vaccines can induce more rapid humoral responses than inactivated rabies vaccine to prevent infection. However, mRNA vaccines require time to express antigens in the cytoplasm, indicating that the vaccine alone is not sufficient, as in some cases, the disease develops before the vaccine takes effect [2,52]. Hence, in future studies, we will consider utilizing immunoglobulins or RABV-specific mAbs in conjunction with mRNA vaccines for post-exposure prophylaxis.
T-cell immunity responses are essential for eliminating RABV viruses. IgG subclasses assay and IFN-γ ELISpot results showed that RABV mRNA vaccines elicited stronger Th1-biased responses than inactivated rabies vaccine in BALB/c mice. All RABV mRNA vaccines activated potent RABV-specific CD4+ and CD8+ T cell responses and particularly elevated production of Th1 cytokines, in line with results from previous mRNA vaccines [8,9,32,48]. Moreover, RABV VLP and VLP/N induced higher levels of IL-4 secretion than G, tG-MTQ, and preG, displaying a slight Th2-biased response. Four months after the second immunization, mice immunized with RABV mRNA vaccines could still stimulate IFN-γ secretion. Therefore, RABV mRNA vaccines elicit sustained cellular immune responses. Furthermore, the significantly enhanced Nab titre and T-cell immune responses induced by RABV mRNA vaccines in mice provided complete protection against lethal challenge from the 50 LD50 dose of the RABV CVS-11 strain.
RABV M protein is a structural protein which plays a crucial role in virus assembly, morphogenesis and budding during RABV replication. Although M protein is less likely to induce neutralizing antibodies or specific cellular immune responses, whether single mRNA vaccine of M protein could act as adjuvant to improve the immune response (e.g. G protein-specific Nabs) of mRNA vaccine expressing G protein is worth further investigation. In addition, illustrating the possible contribution of single mRNA vaccine of N protein to G protein-specific immune response elicited by G protein-expressing mRNA vaccine would also be interesting. Several studies have demonstrated that the N protein has various B-cell and T-cell epitopes and could induce protective immunity against peripheral challenge with rabies virus [27,28]. Also, N protein is a major target for Tfh cells [28]. Yin et al. found N protein expressed in silkworm pupae induced N protein-specific IgG antibodies and protective immunity against rabies virus [53]. In brief, it would be interesting to illustrate the possible contribution of single mRNA vaccine expressing N or M protein to G-specific immune response (e.g. G protein-specific Nabs) of mRNA vaccine expressing G protein.
Prolonged and effective Nab titres require GC reactions that are tightly regulated by Tfh cells, enabling the proliferation, survival, and differentiation of GC B cells through the delivery of co-stimulatory molecules and cytokines. We quantitatively and qualitatively evaluated the GC responses to RABV mRNA vaccines and inactivated rabies vaccine. First, we found that RABV mRNA vaccines had a superior capacity to elicit potent total and RABV-specific GC B and plasma B cell responses compared with inactivated rabies vaccine. The VLP/N mRNA vaccine induced significantly higher RABV-specific GC B and plasma B cell responses than the inactivated vaccine. Next, we observed that G, preG, VLP, and VLP/N elicited significantly more Tfh cells than LNP. The levels of Tfh cells in VLP and VLP/N were remarkably higher than those in the inactivated vaccine, approximately 1.9- and 2.1-fold, respectively. We also observed that RABV mRNA vaccines induced lower levels of Tregs and Tfr cells in LNs than the inactivated vaccine, which may prolong immune responses. Our results are consistent with those of multiple studies demonstrating that mRNA vaccines trigger a GC response [54–56]. Notably, VLP and VLP/N elicited more robust Tfh and GC B cell responses due to the expansion of surface antigens to engage B cells in LNs more effectively.
Additionally, durable and high-level humoral immune responses are the key to preventing rabies reinfection. In our study, the Nab titres were monitored for 18 weeks post vaccination, and RABV mRNA vaccines peaked from week 4–6 and remained at high levels during the ensuing period. The Nab titre-decreasing kinetics of the VLP and VLP/N groups were slower than those of the G, tG-MTQ, and pre-G groups. In contrast, the inactivated rabies vaccine group peaked 4 weeks after the second immunization and dropped to 3.7 IU/mL at week 10, lasting until week 18. Bai et al. found that a dose of a nucleoside-modified rabies vaccine induced a more potent and long-term Nab than the inactivated vaccine [8]. LLPCs and MBCs maintain long-lasting immunity and are important components of the humoral response to rabies. Our B-cell ELISpot and flow cytometry analyses confirmed that RBAV mRNA vaccines elicited strong LLPC and MBC responses; VLP and VLP/N were significantly higher than those of the inactivated rabies vaccine. It is worth noting that, the immune protective efficacy in this study were not further evaluated at a time that was longer than 2 weeks after boost injection due to high Nab titre and cellular responses in mice.
In summary, we evaluated the mRNA vaccines expressing multiple forms of RABV G in BALB/c mice. The mRNA vaccines encoding prefusion stabilized forms of the RABV G protein elicited robust IgG and Nab titres compared to G, tG-MTQ, and inactivated rabies vaccines. VLP and VLP/N had a superior capacity to activate Tfh and GC B cells in LNs and generate LLPCs and MBCs, contributing to the production of higher and more sustained Nab than the G tG-MTQ and preG groups. The median Nab titre of VLP and VLP/N were 42.6 and 49.2 IU/mL at week 18, respectively, which was significantly higher than the protective Nab titre and that induced by the inactivated vaccine (3.7 IU/mL). However, our study has several limitations, future studies will need to increase the efficiency of VLP assembly and budding, and will utilize LN targeted-delivery LNP. First, we will attempt to insert ESCRT- and ALIX-binding regions into the RABV G cytoplasmic tail to improve the efficiency of VLP assembly and budding from cells [57]. Second, we will utilize comb-structured RABV mRNA vaccines to elevate immunogenicity [58]. Third, we will utilize modified LNP for targeted-delivery of mRNAs to LNs, thus reducing side effects and increasing the immune response [59,60]. In summary, our results provide a proof-of-concept of developing novel RABV mRNA vaccines encoding RABV VLP or VLP/N that can provide a safe, rapid, effective, and long-term protection against RABV infection.
Supplementary Material
Acknowledgement
We thank Yanchun Wu and Song Jin from the BCHT Biotechnology Co. for assistance with animal experiments.
Funding Statement
This study was supported by the National Natural Science Foundation of China [Grant Number 32170944] and the Jilin Province Science and Technology Development Plan [Grant Number 20240305081YY, 20220402044GH].
Author contributions
Conceptualization: J.L. and C.J.; Investigation: J.L., W.S., and C.J.; Methodology and experiments: J.L., J.S., X.D., W.L., Y.W., Z.W., H.P. and Y.Z.; Funding acquisition and supervision: W.S. and C. J.; Writing original draft: J.L.; Writing editing: J.L. and C.J. All authors reviewed the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Minghui R, Stone M, Semedo MH, et al. New global strategic plan to eliminate dog-mediated rabies by 2030. Lancet Glob Health. 2018;6(8):e828–e829. doi: 10.1016/S2214-109X(18)30302-4 [DOI] [PubMed] [Google Scholar]
- 2.WHO Publication . Rabies vaccines: WHO position paper–recommendations. Vaccine. 2010;28(44):7140–7142. doi: 10.1016/j.vaccine.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 3.Blanchard T. Rabies and other lyssavirus diseases. Lancet. 2004;363(9424):1906–1907. author reply 1907. doi: 10.1016/S0140-6736(04)16367-8 [DOI] [PubMed] [Google Scholar]
- 4.Sasaki M, Anindita PD, Ito N, et al. The role of heparan sulfate proteoglycans as an attachment factor for rabies virus entry and infection. J Infect Dis. 2018;217(11):1740–1749. doi: 10.1093/infdis/jiy081 [DOI] [PubMed] [Google Scholar]
- 5.Astray RM, Jorge SA, Pereira CA.. Rabies vaccine development by expression of recombinant viral glycoprotein. Arch Virol. 2017;162(2):323–332. doi: 10.1007/s00705-016-3128-9 [DOI] [PubMed] [Google Scholar]
- 6.Alberer M, Gnad-Vogt U, Hong HS, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390(10101):1511–1520. doi: 10.1016/S0140-6736(17)31665-3 [DOI] [PubMed] [Google Scholar]
- 7.Aldrich C, Leroux-Roels I, Huang KB, et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine. 2021;39(8):1310–1318. doi: 10.1016/j.vaccine.2020.12.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai S, Yang T, Zhu C, et al. A single vaccination of nucleoside-modified rabies mRNA vaccine induces prolonged highly protective immune responses in mice. Front Immunol. 2022;13:1099991. doi: 10.3389/fimmu.2022.1099991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan J, Yang J, Wang Z, et al. A single immunization with core-shell structured lipopolyplex mRNA vaccine against rabies induces potent humoral immunity in mice and dogs. Emerg Microbes Infect. 2023;12:2270081. doi: 10.1080/22221751.2023.2270081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaudin Y, Ruigrok RW, Tuffereau C, et al. Rabies virus glycoprotein is a trimer. Virology. 1992;187(2):627–632. doi: 10.1016/0042-6822(92)90465-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koraka P, Bosch BJ, Cox M, et al. A recombinant rabies vaccine expressing the trimeric form of the glycoprotein confers enhanced immunogenicity and protection in outbred mice. Vaccine. 2014;32(36):4644–4650. doi: 10.1016/j.vaccine.2014.06.058 [DOI] [PubMed] [Google Scholar]
- 12.Wang JY, Song WT, Li Y, et al. Improved expression of secretory and trimeric proteins in mammalian cells via the introduction of a new trimer motif and a mutant of the tPA signal sequence. Appl Microbiol Biotechnol. 2011;91(3):731–740. doi: 10.1007/s00253-011-3297-0 [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Lin S, Ye F, et al. Structural analysis of rabies virus glycoprotein reveals pH-dependent conformational changes and interactions with a neutralizing antibody. Cell Host Microbe. 2020;27(3):441–453.e447. doi: 10.1016/j.chom.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Ng WM, Fedosyuk S, English S, et al. Structure of trimeric pre-fusion rabies virus glycoprotein in complex with two protective antibodies. Cell Host Microbe. 2022;30(9):1219–1230.e1217. doi: 10.1016/j.chom.2022.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang LA, Phung E, Crank MC, et al. A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine. Sci Transl Med. 2022;14(676):eade0424. doi: 10.1126/scitranslmed.ade0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Kumar S, Allen JD, et al. HIV-1 vaccine design through minimizing envelope metastability. Sci Adv. 2018;4(11):eaau6769. doi: 10.1126/sciadv.aau6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh CL, Goldsmith JA, Schaub JM, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369(6510):1501–1505. doi: 10.1126/science.abd0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu M, Chamblee M, Zhang Y, et al. SARS-CoV-2 prefusion spike protein stabilized by six rather than two prolines is more potent for inducing antibodies that neutralize viral variants of concern. Proc Natl Acad Sci USA. 2022;119(35):e2110105119. doi: 10.1073/pnas.2110105119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 COVID-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kampmann B, Madhi SA, Munjal I, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–1464. doi: 10.1056/NEJMoa2216480 [DOI] [PubMed] [Google Scholar]
- 21.Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. doi: 10.1056/NEJMoa2209604 [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, Zinkernagel RM.. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235 [DOI] [PubMed] [Google Scholar]
- 23.Link A, Zabel F, Schnetzler Y, et al. Innate immunity mediates follicular transport of particulate but not soluble protein antigen. J Immunol. 2012;188(8):3724–3733. doi: 10.4049/jimmunol.1103312 [DOI] [PubMed] [Google Scholar]
- 24.Manolova V, Flace A, Bauer M, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984 [DOI] [PubMed] [Google Scholar]
- 25.Bernardino TC, Astray RM, Pereira CA, et al. Production of rabies VLPs in insect cells by Two monocistronic baculoviruses approach. Mol Biotechnol. 2021;63(11):1068–1080. doi: 10.1007/s12033-021-00366-z [DOI] [PubMed] [Google Scholar]
- 26.Qi Y, Kang H, Zheng X, et al. Incorporation of membrane-anchored flagellin or Escherichia coli heat-labile enterotoxin B subunit enhances the immunogenicity of rabies virus-like particles in mice and dogs. Front Microbiol. 2015;6:169. doi: 10.3389/fmicb.2015.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu ZF, Dietzschold B, Schumacher CL, et al. Rabies virus nucleoprotein expressed in and purified from insect cells is efficacious as a vaccine. Proc Natl Acad Sci USA. 1991;88(5):2001–2005. doi: 10.1073/pnas.88.5.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ertl HC, Dietzschold B, Gore M, et al. Induction of rabies virus-specific T-helper cells by synthetic peptides that carry dominant T-helper cell epitopes of the viral ribonucleoprotein. J Virol. 1989;63(7):2885–2892. doi: 10.1128/jvi.63.7.2885-2892.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardi N, Hogan MJ, Naradikian MS, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederer K, Castaño D, Gómez Atria D, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53(6):1281–1295.e1285. doi: 10.1016/j.immuni.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karikó K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi: 10.1038/mt.2008.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnee M, Vogel AB, Voss D, et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10(6):e0004746. doi: 10.1371/journal.pntd.0004746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Liu Y, Li C, et al. Recent advances and innovations in the preparation and purification of in vitro-transcribed-mRNA-based molecules. Pharmaceutics. 2023 Aug 23;15(9): 2182. doi: 10.3390/pharmaceutics15092182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volokhov DV, Fry AM, Furtak V, et al. An ELISA-based antigenicity test of rabies recombinant glycoprotein cannot predict its protective potency in vivo. Mol Cell Probes. 2022;63:101815. doi: 10.1016/j.mcp.2022.101815 [DOI] [PubMed] [Google Scholar]
- 35.Corbett KS, Edwards DK, Leist SR, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, Lu G, Tan S, et al. A COVID-19 mRNA vaccine encoding SARS-CoV-2 virus-like particles induces a strong antiviral-like immune response in mice. Cell Res. 2020;30(10):936–939. doi: 10.1038/s41422-020-00392-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang P, Narayanan E, Liu Q, et al. A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat Med. 2021;27(12):2234–2245. doi: 10.1038/s41591-021-01574-5 [DOI] [PubMed] [Google Scholar]
- 38.Li M, Fang E, Wang Y, et al. An mRNA vaccine against rabies provides strong and durable protection in mice and dogs. Front Immunol. 2023;14:1288879. doi: 10.3389/fimmu.2023.1288879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toinon A, Moreno N, Chausse H, et al. Potency test to discriminate between differentially over-inactivated rabies vaccines: agreement between the NIH assay and a G-protein based ELISA. Biologicals. 2019;60:49–54. doi: 10.1016/j.biologicals.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 40.Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen CD, Okada T, Tang HL, et al. Imaging of germinal center selection events during affinity maturation. Science. 2007;315(5811):528–531. doi: 10.1126/science.1136736 [DOI] [PubMed] [Google Scholar]
- 42.Gitlin AD, Shulman Z, Nussenzweig MC.. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509(7502):637–640. doi: 10.1038/nature13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6(10):741–750. doi: 10.1038/nri1886 [DOI] [PubMed] [Google Scholar]
- 44.Bortnick A, Chernova I, Quinn WJ III, et al. Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol. 2012;188(11):5389–5396. doi: 10.4049/jimmunol.1102808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulroney TE, Pöyry T, Yam-Puc JC, et al. N(1)-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature. 2024;625(7993):189–194. doi: 10.1038/s41586-023-06800-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HH, Yang DK, Nah JJ, et al. Comparison of the protective efficacy between single and combination of recombinant adenoviruses expressing complete and truncated glycoprotein, and nucleoprotein of the pathogenic street rabies virus in mice. Virol J. 2017;14(1):122. doi: 10.1186/s12985-017-0789-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta PK, Sharma S, Walunj SS, et al. A DNA vaccine that encodes rabies virus glycoprotein lacking transmembrane domain enhances antibody response but not protection. Acta Virol. 2006;50(2):87–92. [PubMed] [Google Scholar]
- 48.Li J, Liu Q, Liu J, et al. An mRNA-based rabies vaccine induces strong protective immune responses in mice. Virol J. 2022;19(1):184. doi: 10.1186/s12985-022-01919-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Callaway HM, Zyla D, Larrous F, et al. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci Adv. 2022;8(24):eabp9151. doi: 10.1126/sciadv.abp9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H, Su D, Zhang J, et al. Improvement of pharmacokinetic profile of TRAIL via trimer-tag enhances its antitumor activity in vivo. Sci Rep. 2017;7(1):8953. doi: 10.1038/s41598-017-09518-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buntinx E, Brochado L, Borja-Tabora C, et al. Immunogenicity of an adjuvanted SARS-CoV-2 trimeric S-protein subunit vaccine (SCB-2019) in SARS-CoV-2-naïve and exposed individuals in a phase 2/3, double-blind, randomized study. Vaccine. 2023;41(11):1875–1884. doi: 10.1016/j.vaccine.2023.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilde H, Khawplod P, Hemachudha T, et al. Postexposure treatment of rabies infection: can it be done without immunoglobulin? Clin Infect Dis. 2002;34(4):477–480. doi: 10.1086/324628 [DOI] [PubMed] [Google Scholar]
- 53.Yin X, Li Z, Li J, et al. Rabies virus nucleoprotein expressed in silkworm pupae at high-levels and evaluation of immune responses in mice. J Biotechnol. 2013;163(3):333–338. doi: 10.1016/j.jbiotec.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Alameh MG, Tombácz I, Bettini E, et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity. 2021;54(12):2877–2892.e2877. doi: 10.1016/j.immuni.2021.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mudd PA, Minervina AA, Pogorelyy MV, et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell. 2022;185(4):603–613.e615. doi: 10.1016/j.cell.2021.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597(7875):268–273. doi: 10.1038/s41586-021-03841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hoffmann MAG, Yang Z, Huey-Tubman KE, et al. ESCRT recruitment to SARS-CoV-2 spike induces virus-like particles that improve mRNA vaccines. Cell. 2023;186(11):2380–2391.e2389. doi: 10.1016/j.cell.2023.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tockary TA, Abbasi S, Matsui-Masai M, et al. Comb-structured mRNA vaccine tethered with short double-stranded RNA adjuvants maximizes cellular immunity for cancer treatment. Proc Natl Acad Sci USA. 2023;120(29):e2214320120. doi: 10.1073/pnas.2214320120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Ye Z, Huang C, et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc Natl Acad Sci USA. 2022;119(34):e2207841119. doi: 10.1073/pnas.2207841119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dasari V, McNeil LK, Beckett K, et al. Lymph node targeted multi-epitope subunit vaccine promotes effective immunity to EBV in HLA-expressing mice. Nat Commun. 2023;14(1):4371. doi: 10.1038/s41467-023-39770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.